
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 20 October 2022
Sec. Cardiovascular Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1031451
This article is part of the Research Topic Emerging Opportunities in Congenital Cardiac Surgery View all 11 articles
 Oktavia Lilyasari1*
Oktavia Lilyasari1* Rini Istisakinah1
Rini Istisakinah1 Rina Ariani1
Rina Ariani1 Budi Rahmat2
Budi Rahmat2 Lies Dina Liastuti1
Lies Dina Liastuti1 Yovi Kurniawati1
Yovi Kurniawati1 Hary Sakti Muliawan1
Hary Sakti Muliawan1 Renan Sukmawan1
Renan Sukmawan1
Background: Pulmonary arterial hypertension secondary to atrial septal defect (ASD) is an important determinant of morbidity and mortality in defect closure. We aimed to compare perioperative outcome between preoperative borderline and low pulmonary vascular resistance index (≥4 WU.m2 and <4 WU.m2, respectively) in surgical closure of secundum atrial septal defect with concomitant pulmonary arterial hypertension.
Methods and results: This was a single-center retrospective cohort study between January 2015 and January 2020. We classified patients with low and borderline PVRI who underwent ASD closure and recorded the perioperative outcomes.
Results: We analyzed a total of 183 patients with atrial septal defect and pulmonary arterial hypertension; 92 patients with borderline PVRI and 91 patients with low PVRI. Borderline pulmonary vascular resistance index was not associated with increased risk of postoperative mortality (p = 0.621; OR0.48, 95% CI 0.04–5.48), but associated with higher risk of overall morbidity in bivariate analysis (p = 0.002; OR3.28, 95% CI 1.5–6.72). Multivariate analysis showed positive association of borderline pulmonary vascular resistance index (p = 0.045; OR2.63, 95% CI 1.02–6.77) and preoperative tricuspid valve gradient ≥64 mmHg (p = 0.034; OR2.77, 95% CI 1.08–7.13) with overall morbidity.
Conclusion: There is no difference in incidence of in-hospital mortality between preoperative borderline and low pulmonary vascular resistance index patients. However, preoperative borderline pulmonary vascular resistance index and tricuspid valve gradient ≥64 mmHg are associated with increased overall morbidity after surgical closure in secundum atrial septal defect patients with pulmonary arterial hypertension.
Atrial septal defect (ASD) is one of the most common congenital heart diseases (CHD) found with secundum ASD as the majority type. Sixty-nine percent of adult congenital heart disease (ACHD) patients in Indonesian National Cardiac Center Harapan Kita (NCCHK) are ASD patients. Due to its natural course, secundum ASD patients were often asymptomatic and diagnosed late in adulthood (1). Moreover, developing countries unexceptionally Indonesia, have their own local barriers -geographic, economic, human resources, scarcity of high-end equipment- in providing optimal medical care for such patients (2). Therefore, patients often came as “late presenters” of whom already have challenging courses.
Persistent interatrial shunt in uncorrected ASD leads to development of pulmonary arterial hypertension (PAH), and later pulmonary vascular disease (PVD). This irreversible condition denotes significant changes in pulmonary vascularization and attempt on defect closure would be hazardous (1, 3). Closure of the defect should have taken place before any irreversible changes occurred, preferably when pulmonary hypertension (PH) has not developed. Previous study showed surgical ASD closure has a favorable outcome with low mortality rate (<3%) (1). Unfortunately, preoperative PAH secondary to congenital heart disease is an important determinant for perioperative morbidity and mortality as well as long-term postoperative survival (4). Presence of PAH could lead to incidence of postoperative pulmonary hypertension crisis (PHC) that has disastrous effect with mortality rate as high as 20% (5). Given the life-threatening complications associated with pulmonary hypertensive crisis, stratifying the risk of preoperative PAH would determine the outcome after surgery.
Existing guideline stated, CHD with systemic-to-pulmonary shunts with preoperative pulmonary vascular resistance index (PVRI) < 4 WU.m2, considered as low PVRI, is appropriately safe to close. However, preoperative PVRI ≥4 WU.m2 deemed to be on grey/borderline zone where development of PVD might already occurred. Meanwhile, patients with PVRI >8 WU.m2 -even after acute vasoreactivity test- is thought to have irreversible pulmonary vascular changes and will not have favorable outcomes (6). Patients with dubious PVRI value still have the chance for defect correction with noteworthy consideration where individual patient evaluation should be performed in tertiary centers. Some of those patients received pulmonary vasodilator therapy to achieved operability criteria (treat-to-repair concept) (6, 7). Studies regarding this challenging group are still scarce, mostly are case reports with various kind of systemic-to-pulmonary shunt defect. None of the studies have showed short or long-term outcome after defect closure in borderline PVRI especially in secundum ASD patients, since these patients in developing countries tend to come as late presenter and often had develop pulmonary hypertension. The study aimed to investigate the impact of preoperative borderline PVRI compared to low PVRI on short-term outcome after surgical ASD closure in secundum ASD patient with PAH.
This was a single-center and a retrospective observational study comparing the perioperative outcome of preoperative borderline and low PVRI groups after surgical closure of secundum ASD. We identified adult (≥18 years old) patients with secundum ASD with PAH (ASD-PAH) who underwent surgical closure in our hospital between January 2015 and January 2020. Patients with ASD with other concomitant CHD, patients who underwent surgical procedures other than ASD closure and/or valve repair/replacement were excluded. We calculated the sample size for this particular study and for the 90% of statistical power, the minimal size would be 87 patients per group or 174 patients in total. The study was reviewed and approved by the Ethical Committee of Department of Cardiology and Vascular Medicine, Faculty of Medicine Universitas Indonesia, National Cardiovascular Center Harapan Kita (Ethical Approval Number: LB.02.01/VII/429/KEP.029/2020).
We collected demographic, clinical, electrocardiographic, echocardiographic, RHC, surgical and post-surgical data from medical records. Age were categorized into ≥40 and <40 year old (8). Secundum ASD was mainly diagnosed transthoracic echocardiography (TTE) and trans-esophageal echocardiography (TEE). Data of preoperative defect size was obtained with TEE when available and confirmed during procedure. The size of left ventricle (LV) was determined by end diastolic diameter (EDD) index and considered to be “small-sized” if less than 2.2 cm/m2 for male and less than 2.3 cm/m2 for female. Decrease right ventricle function defined as tricuspid annular plane systolic excursion (TAPSE) < 17 mm (9).
PAH was defined for the purpose of this study as mean pulmonary arterial pressure (mPAP) > 20 mmHg, pulmonary capillary wedge pressure < 15 mmHg, and PVR > 3 WU at rest right heart catheterization (RHC) (10). Hemodynamic parameters (Qp/Qs, PVRI, resistance ratio) were calculated using Fick method. Oxygen consumption was estimated with table from La Farge and Miettinen (11). Acute vasoreactivity test (AVT) was carried out with delivery of 100% oxygen (FiO2) via face mask for 10 min. Using data from RHC reports, all patients were recalculated for PVRI with WU.m2 as the agreed unit (6). This measure was taken due to diversified units that had been used in the past (WU or WU.m2 or WU.m-2). Borderline PVRI was defined as pulmonary vascular resistance index ≥4 WU.m2 before AVT. Low PVRI was defined as pulmonary vascular resistance index <4 WU.m2 before AVT.
Referring to our hospital clinical practice guideline, secundum ASD-PAH patients with PVRI < 8 WU.m2 before or after acute vasoreactivity test (AVT) were deemed operable. Surgical data were obtained from the surgical note. Based on previous study, aortic cross clamp (Aox) time will be classified to > 45 min and < 45 min (12). Prolonged cardiopulmonary bypass (CPB) time was defined as CPB time > 100 min (13).
Primary clinical endpoint was in-hospital mortality after surgery, defined as all-cause mortality events during post-operative period. Secondary clinical end-point was overall morbidity that includes any of pulmonary hypertension crisis event, prolonged intensive care unit stay, and/or mechanical ventilation use. Pulmonary hypertension crisis was defined as acute increase in pulmonary arterial pressure to systemic arterial pressure ratio >0.75 which was usually accompanied by an increase in central venous pressure (>20%), a decrease in blood pressure (>20%) and a decrease in oxygen saturation levels to less than 90% with signs of decreased cardiac output (5). Prolonged intensive care unit (ICU) stay was defined as period stay at ICU for more than two days (14). Twenty-four hour was used as a cutoff point to consider prolonged mechanical ventilation use (15).
Statistical analysis was performed using SPSS software for Macintosh, version 22 (IBM, New York). Categorical variables stated in frequencies and proportions. Continuous variables were expressed as means + standard deviation or medians with ranges when appropriate. For numeric variables, the differences between the two groups were analyzed with T-test or Mann-Whitney test, as appropriate. For discrete variable, the differences between two groups were analyzed with the χ2 or Fisher's exact test, as appropriate. Certain variables (age, PVRI, size defect, TAPSE, mPAP, TVG, PaO2, CPB time, AoX time) will be categorized according to agreed terms. Variables with p < 0.25 in bivariate analysis will be included in multivariate analysis using logistic regression. A two-tailed p-value of <0.05 was considered statistically significant.
During the five-year study period, surgical closure of ASD was attempted in 510 adult patients. Approximately 255 patients met the inclusion and exclusion criteria, however only 183 patients had available complete data and included in final analysis. The median preoperative PVRI was 4.3 WU.m2 (0.4–13.5 WU.m2). Ninety-two patients (51%) were classified as borderline PVRI group and 91 patients (49%) belonged to low PVRI group. After recalculation there were eleven patients with preoperative PVRI >8 WU.m2. Detailed characteristic was summarized in Figure 1.
Most of the patients were female (86.3%) with median age of 37 (18–64) years old. More than half of the population preoperative NYHA Fc II (72.7%). We found that atrial fibrillation was present in 26/183 patients (14.2%). Comparison of baseline clinical, echocardiographic and cardiac catheterization between two groups are summarized in Tables 1, 2, respectively. A distinct baseline clinical characteristic existed between two groups except for median age, gender distribution, and comorbidities. However, as variable age was dichotomized into two groups (< 40 and ≥40 years old), low PVRI group had greater proportion of patients with age ≥40 years old (48.4% vs. 32.6%; p = 0.03). Preoperative oxygen saturation (SaO2) and preoperative partial pressure of oxygen (PaO2) in borderline PVRI group were significantly lower compared to low PVRI group (p < 0.001). Phospdiesterease-5 inhibitor (Sildenafil) was the drug of choice for preoperative pulmonary vasodilator treatment (80.7% vs. Beraprost 13.8%). Combination therapy were used in 5.5% patients. Comparison between groups showed a contrasting echocardiography and cardiac catheterization features. Through basic echocardiography it showed that borderline PVRI group had significantly decreased RV function (TAPSE) with preoperative tricuspid valve gradient (TVG) that was higher than those of low PVRI group. However, there was no significant difference in defect size, LV size, and proportions of mitral regurgitation severity (p > 0.05). No difference found in the type of anesthesia used during RHC procedure.
Table 3 summarized surgical procedure and perioperative ICU care between two groups. Median defect size was 30 (15–60 mm) without significant difference between two groups (p = 0.376). Most defects were closed with pericardial patch (89.6%), the rest with direct closure and in some cases a small fenestration was created in the surgical patch. Different characteristics found regarding perioperative care. Patients in borderline PVRI group received significantly more supportive drugs (inotropic, vasopressor, and/or inodilator agent) compared to low PVRI group (p < 0.001). Similar result was recorded for perioperative pulmonary vasodilator treatment usage. Nitric oxide inhalation was used for two patients as pulmonary hypertension crisis (PHC) treatment. Inhaled Illoprost was given for 50% patients who received perioperative pulmonary vasodilator treatment. Sildenafil was given as substitute for Illoprost or as a single therapy. Median length of stay in the ICU was 1 (1–23) day, while duration of mechanical ventilation was 12 (0–548) hours. Two patients had early extubation in the operating room.
The most common perioperative complication was arrhythmias (15.3%) with new-onset atrial fibrillation as the most frequently encountered. Other complications were stroke (1.6%), pneumonia (16.5%), low cardiac output syndrome (2.7%), and acute kidney injury (3.2%). One of three patients who developed strokes had subarachnoid hemorrhage (SAH). Redo procedures were performed in three patients; two patients due to surgical bleeding and one patient had side port sutured to his superior vena cava.
There was no significant difference in incidence of in-hospital mortality between borderline and low PVRI groups (1.1% vs. 2.2%, OR 0.48 95% CI 0.04–5.48; p = 0.621). None of other variables (age, sex, defect size, TAPSE, small-sized LV, TVG, mPAP, PaO2, preoperative pulmonary vasodilator, Aox time and CPB time) showed any significant difference, thus multivariate analysis by logistic regression was not performed. Overall incidence of in-hospital mortality was 1.6% (3/183), none of them had pulmonary hypertension-related cause of death. One patient who belonged in borderline PVRI group died from excessive bleeding resulting in uncorrectable hemodynamic state despite optimal effort. Two patients from low PVRI group died from massive non-hemorrhagic stroke and sepsis with multi-organ dysfunction.
Table 4 detailed the incidence of overall morbidity and each of the components considered. Pulmonary hypertension crisis (PHC) occurred in seven patients and all of them belonged to borderline PVRI group. Five patients had post-AVT PVRI >8 WU.m2, after recalculation. The incidence of overall morbidity was higher in borderline PVRI group compared to low PVRI group before adjustment (32.6% vs. 13.2%, OR 3.28 (95% CI 1.5–6.72; p = 0.002)) and after adjustment (OR 2.63 (95% CI 1.02–6.77; p = 0.045)). Summarized in Table 5, preoperative TVG ≥64 mmHg, mPAP ≥36 mmHg, arterial PaO2 < 80 mmHg, and use of pulmonary vasodilator drug preoperatively were predictors for overall morbidity. However, after adjustment, only preoperative TVG ≥64 mmHg remained as independent predictors of overall morbidity (p = 0.034). Statistical analysis for overall morbidity summarized in Tables 5, 6.
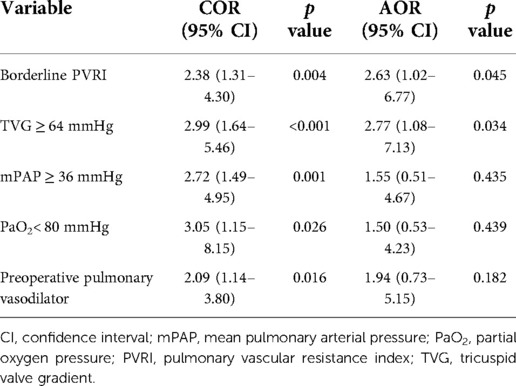
Table 6. Univariate and multivariate analysis of factors affecting overall morbidity after surgical ASD closure.
Prevalence of ASD-PAH ranged between 8%–10% (3). However, we reported a higher proportion of PAH in secundum ASD patients who underwent surgical closure (44%; 225/510 patients) in this study. This higher number might be due to our hospital is the national referral center for CHD in which most patients were referred with advanced stage of disease of whom required more specialized intervention. The demographic characteristics in our study were similar with previous studies which were mostly female patient and in third-to-fourth decade (1, 14). Clinical and diagnostic characteristics supported distinct hemodynamic properties between groups whom similar to other studies though no difference in size defect was found between groups. Conflicting findings in other studies exist regarding PH severity and defect size (16, 17). Determination of defect size in available studies was done using echocardiography where the accuracy of the results depends on the view taken, the form of ASD, as well as individual expertise. In our study, measurement of ASD defect size was confirmed at surgery.
Primary endpoint in this study showed no difference in in-hospital mortality between two groups with particularly low mortality rate (1.6%) which concordance with available statement regarding surgical ASD closure safety (1). The causes of death varied but very few had reported PH as the cause. Horvarth et al. (1992) reported two deaths from 166 patients underwent surgical ASD closure with one of them had pulmonary hypertension as the responsible cause of death (18). Although our study shared the same value of in-hospital mortality, we had completely different study subjects compared to other previous studies. All of our subjects had preoperative PH (confirmed by gold standard RHC) which were considered to have higher risk compared to patients without, while in other studies only partial of subjects were accompanied by PH known with different diagnostic approach. Currently, there is no previous study regarding ASD closure similar to ours. Whilst Bando et al. (4) already reported that preoperative PH was independent predictor of mortality after CHD correction [OR 2.2 95% CI 1.05–4.59; p = 0.036], three patients who died during the course of our study had no report of pulmonary hypertension crisis and none of the considered variables proven to be the independent cause of mortality.
Discussion about postoperative mortality cannot be separated with the most avoided complication of pulmonary hypertension, pulmonary hypertension crisis. Our study reported similar incidence rate of PHC (3.8%) with the available data (0.75%–4%) (5). High preoperative pulmonary vascular resistance (PVRI >6 WU.m2) and less vasoreactivity (decrease PVRI <20% after AVT) are risk factors for postoperative complication in patients with preoperative PH (19). Our study found all patients who experienced postoperative PHC were in the borderline PVRI group with five of them had final PVRI value >8 WU.m2 although they had good vasoreactivity.
The risk of mortality in patient experienced PHC is as high as 20% (9). In our study, all of the patients with PHC survived the disastrous event and were fully discharged from hospital in good condition. Furthermore, eleven patients who had preoperative PVRI >8 WU.m2 did not experienced mortality and only 6/11 patients had morbidity. These favorable findings might be due to optimal pre-and perioperative care such as administration of optimal preoperative pulmonary vasodilator, good adherence, and practice to recommendation regarding postoperative PH care (sedation, minimal manipulation, acidosis treatment, good hydration, administration of inotropic and inodilator as appropriate, oxygenation, and perioperative pulmonary vasodilator) in our patients (20). These advantages might not available in studies conducted before 1990 though they had lower operability PVRI limit such as in study by Bush et al. (PVRI ≤6 WU.m2) and Neutze et al. (PVRI <7 WU.m2), where perioperative care not as sophisticated as nowadays and pulmonary vasodilator were not widely used and available (21). Those studies however had CHD type and patient's characteristics that differed from our study. To be of note, there has not been a similar study for secundum ASD-PAH patient either locally or internationally.
Another enticing aspect in our study was more than half of patients (52.2%) in borderline PVRI group did not receive any perioperative pulmonary vasodilator and yet none of them had any event. The decisions to give perioperative therapy was not only based on preoperative hemodynamic but also on postoperative clinical and hemodynamic consideration. Widely known as the principal risk factor for incidence of postoperative PH, high PVRI value is not the solely predictor. Pulmonary vascular reactivity contribute to acute increase of postoperative pulmonary vascular pressure and there were no parameters that can truly predict this (22). Canter et al. found that genetic factor T1450N polymorphism of gene encoding carbamoyl-phosphate synthetase I (an important enzyme for endogenous nitric oxide production) play a role in the increase in pulmonary pressure in postoperative pediatric patients (23). Further research is needed regarding contributing factors.
Expected results for other postoperative morbidity components were reported from our study. Fouly et al. have stated that patients with preoperative PH had longer ICU stay and mechanical ventilation use (24). Patients whom developed postoperative pulmonary hypertension would inevitably had longer duration of mechanical ventilation as part of precautious ICU care and indirectly affect the ICU stay.
We reported borderline PVRI group and preoperative TVG ≥ 64 mmHg as independent predictor for overall morbidity. Different results reported by Horer et al. in which preoperative atrial fibrillation and greater defect size had longer ICU stay (14). However this study had different characteristic regarding PH, only some of them had preoperative PH with mean mPAP 18.6 ± 6.4 mmHg and PVR 2.26 ± 1.67 WU.
To our knowledge, our study was the first to assess the association of preoperative TVG with clinical outcome after surgical closure of ASD associated with PAH. Other studies mainly use systolic pulmonary arterial pressure (sPAP) both for defining PH and as predictor factor. Tricuspid valve gradient is the component for calculating estimated sPAP which represents the interaction between right ventricle and afterload (PVR) (25). Thus, an increase in PVR would be followed with an increase in TVG, which could be seen in patients with overall morbidity had similar proportion for borderline PVRI and TVG ≥64 mmHg groups.
Severe left ventricular dysfunction after ASD closure was a possible complication in adult patients. This condition would lead to low cardiac output syndrome (LCOS) and affect the outcome after closure. This undesired complication was postulated due to small LV size that can cause LV diastolic dysfunction (26). Our center found that LV end diastolic volume index ≤53.3 ml/m2 by magnetic resonance imaging (MRI) was predictor of LCOS after surgical ASD closure (27). Not all of our patients in this study were performed preoperative MRI, therefore we use echocardiography parameter to define small-sized LV. No difference in outcome was found regarding this variable.
Although the study was a retrospective study, this study managed to depict our achievements as national referral center for congenital heart disease. Existing barriers did not hinder our effort to provide optimal care in accordance with existing guidelines and evidence-based practice. Our study only remarked the short-term outcome whereas preoperative PAH could affect long-term outcome in relation to PAH reversibility after defect closure which raised another concern. Further study was needed to evaluate the PAH reversibility in ASD patients with preoperative borderline PVRI.
The result of present study indicates that adult secundum ASD-PAH who had preoperative borderline PVRI did not have any different for in-hospital mortality post-surgical closure compared to low PVRI. However, the borderline PVRI group had higher incidence of overall morbidity. These findings supported the operability of patient with borderline PVRI, yet caution must be made for perioperative care to anticipate possible morbidity. Hence, readiness of the center to overcome post-operative pulmonary complication plays a key role in determining perioperative outcome.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethical Committee of Department of Cardiology and Vascular Medicine, Faculty of Medicine Universitas Indonesia, National Cardiovascular Center Harapan Kita. The patients/participants provided their written informed consent to participate in this study.
OL: Conceptualization; Data curation; Formal analysis; Investigation; Writing—original draft; Writing—review / editing. RI: Conceptualization; Data curation; Formal analysis; Investigation; Writing—original draft. RA: Conceptualization; Project administration; Visualization; Writing—review / editing. BR: Conceptualization; Project administration; Visualization; Writing—review / editing. LDL: Writing—review / editing. YK: Writing—review / editing. HSM: Writing—review / editing. RS: Writing—review / editing. All authors contributed to the article and approved the submitted version.
The authors thank the Pediatric Cardiology Staff: Radityo Prakoso, Olfi Leyla, Sisca Natalia Siagian, Aditya Sembiring; the Heart Surgery Staff: Dicky Fachri, Pribadi Busro; the Intensive Care Unit Staff Novik Wardhana; and Shindi Eugene Tiurma Tampubolon for their valuable contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ACHD, adult congenital heart disease; Aox, aortic cross clamp; ASD, atrial septal defect; AVT, acute vasoreactivity testing; CHD, congenital heart isease; CPB, cardiopulmonary bypass; CTEPH, chronic thromboembolic pulmonary hypertension; EDD, end diastolic diameter; ICU, intensive care unit; LCOS, low cardiac output syndrome; mPAP, mean pulmonary arterial pressure; MRI, magnetic resonance imaging; PAH, pulmonary arterial hypertension; PaO2, partial pressure of oxygen; PH, pulmonary hypertension; PHC, pulmonary hypertension crisis; PVD, pulmonary vascular disease; PVR, pulmonary vascular resistance; PVRI, pulmonary vascular resistance index; Qp/Qs, pulmonary blood flow/systemic blood flow; RHC, right heart catheterization; sPAP, systolic pulmonary arterial pressure; TAPSE, tricuspid annular plane systolic excursion; TEE, trans-esophageal echocardiography; TTE, transthoracic echocardiography; TVG, tricuspid valve gradient
1. Sachdeva R. Moss and Adams’ heart disease in infants, childrend, and adolescents. eight edit. (Allen HD, Driscoll DJ, Shaddy RE, Feltes TF, eds.). Lippincot Williams and Wilkins; 2013.
2. Rahajoe AU. Management of patients with congenitally malformed hearts in Indonesia. Cardiol Young. (2007) 17(6):584–8. doi: 10.1017/S1047951107001588
3. Schwerzmann M, Pfammatter JP. Approaching atrial septal defects in pulmonary hypertension. Expert Rev Cardiovasc Ther. (2015) 13(6):693–701. doi: 10.1586/14779072.2015.1047763
4. Bando K, Turrentine MW, Sharp TG, Sekine Y, Aufiero TX, Sun K, et al. Pulmonary hypertension after operations for congenital heart disease: analysis of risk factors and management. J Thorac Cardiovasc Surg. (1996) 112(6):1600–9. doi: 10.1016/S0022-5223(96)70019-3
5. Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. Pediatric Pulmonary Hypertension Guidelines from the American Heart Association and American Thoracic Society. Vol 132; 2015.
6. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. (2016) 37(1):67–119. doi: 10.1093/eurheartj/ehv317
7. Yao A. “Treat-and-repair” strategy for atrial septal defect and associated pulmonary arterial hypertension. Circ J. (2015) 80(1):69–71. doi: 10.1253/circj.CJ-15-1235
8. Campbell M. Natural history of atrial septal defect. Br Hear Journal Cardiol Clin. (1970) 32:820–6. doi: 10.1136/hrt.32.6.820
9. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003
10. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. (2019) 53(1):1801913. doi: 10.1183/13993003.01913-2018
11. Lafarge CG, Miettinen OS. The estimation of oxygen consumption. Cardiovasc Res. (1970) 4(1):23–30. doi: 10.1093/cvr/4.1.23
12. Talwar S, Kumar M, Sreenivas V, Choudhary S, Sahu M, Airan B. Factors determining outcomes in grown up patients operated for congenital heart diseases. Ann Pediatr Cardiol. (2016) 9(3):222–8. doi: 10.4103/0974-2069.189113
13. Kogon B, Grudziak J, Sahu A, Jokhadar M, McConnell M, Book W, et al. Surgery in adults with congenital heart disease: risk factors for morbidity and mortality. Ann Thorac Surg. (2013) 95(4):1377–82. doi: 10.1016/j.athoracsur.2012.11.076
14. Hörer J, Eicken A, Müller S, Schreiber C, Cleuziou J, Prodan Z, et al. Risk factors for prolonged intensive care treatment following atrial septal defect closure in adults. Int J Cardiol. (2008) 125(1):57–61. doi: 10.1016/j.ijcard.2007.02.022
15. LaPar DJ, Gillen JR, Crosby IK, Sawyer RG, Lau CL, Kron IL, et al. Predictots of operative mortality in cardiac surgical patients with prolonged intensive care unit duration. J Am Coll Surg. (2013) 216(6):1116–23. doi: 10.1016/j.jamcollsurg.2013.02.028
16. Cossío-Aranda J, Zamora KDV, Nanda NC, Uzendu A, Keirns C, Verdejo-Paris J, et al. Echocardiographic correlates of severe pulmonary hypertension in adult patients with ostium secundum atrial septal defect. Echocardiography. (2016) 33(12):1891–6. doi: 10.1111/echo.13358
17. Gabriels C, De Meester P, Pasquet A, De Backer J, Paelinck BP, Morissens M, et al. A different view on predictors of pulmonary hypertension in secundum atrial septal defect. Int J Cardiol. (2014) 176(3):833–40. doi: 10.1016/j.ijcard.2014.08.009
18. Horvath KA, Burke RP, Collins JJ, Cohn LH. Surgical treatment of adult atrial septal defect: early and long-term results. J Am Coll Cardiol. (1992) 20(5):1156–9. doi: 10.1016/0735-1097(92)90372-T
19. Hill NS, Roberts KR, Preston IR. Postoperative pulmonary hypertension: etiology and treatment of a dangerous complication. Respir Care. (2009) 54(7):958–68. doi: 10.4187/002013209793800439
20. Kaestner M, Schranz D, Warnecke G, Apitz C, Hansmann G, Miera O. Pulmonary hypertension in the intensive care unit. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European paediatric pulmonary vascular disease network, endorsed by ISHLT and DGPK. Heart. (2016) 102:ii57–66. doi: 10.1136/heartjnl-2015-307774
21. Giglia TM, Humpl T. Preoperative pulmonary hemodynamics and assessment of operability: is there a pulmonary vascular resistance that precludes cardiac operation? Pediatr Crit Care Med. (2010) 11(SUPPL. 2):57–69. doi: 10.1097/PCC.0b013e3181d10cce
22. Adatia I, Beghetti M. Early postoperative care of patients with pulmonary hypertension associated with congenital cardiac disease. Cardiol Young. (2009) 19(4):315–9. doi: 10.1017/S1047951109990175
23. Canter JA, Summar ML, Smith HB, Rice GD, Hall LD, Ritchie MD, et al. Genetic variation in the mitochondrial enzyme carbamyl-phosphate synthetase I predisposes children to increased pulmonary artery pressure following surgical repair of congenital heart defects: a validated genetic association study. Mitochondrion. (2007) 7(3):204–10. doi: 10.1016/j.mito.2006.11.001
24. Fouly MAE-H, Mohammad WA. Effect of pulmonary arterial hypertension on results of surgical closure of VSD. Al-Azhar Assiut Med J. (2015) 13(3):15–26.
25. Roberts JD, Forfia PR. Diagnosis and assessment of pulmonary vascular disease by Doppler echocardiography. Pulm Circ. (2011) 1(2):160–81. doi: 10.4103/2045-8932.83446
Keywords: atrial septal defect (ASD), pulmonary hypertension (PAH), pulmonary vascular resistance index (PVRI), surgical closure of ASD, mortality, morbidity, cardiac catherization
Citation: Lilyasari O, Istisakinah R, Ariani R, Rahmat B, Liastuti LD, Kurniawati Y, Muliawan HS and Sukmawan R (2022) Operability of atrial septal defect with borderline pulmonary vascular resistance index: A study in developing country. Front. Surg. 9:1031451. doi: 10.3389/fsurg.2022.1031451
Received: 30 August 2022; Accepted: 23 September 2022;
Published: 20 October 2022.
Edited by:
Sebastian Michel, LMU Munich University Hospital, GermanyReviewed by:
Paul Philipp Heinisch, Congenital and Pediatric Heart Surgery, German Heart, Germany© 2022 Lilyasari, Istisakinah, Ariani, Rahmat, Liastuti, Kurniawati, Muliawan and Sukmawan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oktavia Lilyasari T2t0YXZpYV9saWx5YXNhcmlAeWFob28uY29t
Specialty Section: This article was submitted to Heart Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.