- 1Department of Orthopedic Surgery, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
- 2Department of Orthopedic Surgery, Hebei Jing-Xing Xian Hospital, Shijiazhuang, China
- 3Department of Hand Surgery, Cangzhou Hospital of Integrated Traditional and Western Medicine of Hebei Province, Cangzhou, China
Objective: This study aimed to investigate the association between new inflammatory indicators at admission and the occurrence of preoperative deep vein thrombosis (DVT) in patients with patella fractures.
Methods: A retrospective analysis of the medical records of patients aged 18 years or older who underwent surgical treatment for unilateral closed patella fractures at our hospital between August 2016 and August 2020. The incidence of preoperative DVT was detected by Duplex ultrasound (DUS). Partial blood routine and biochemical indexes were collected at admission, and the neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR) of inflammatory indexes were also calculated. ROC was used to analyze the cut-off value NLR, MLR, and PLR for predicting preoperative DVT, and univariate and multivariate analyses of the risk factors for preoperative DVT of patella fractures, and to verify whether other risk factors affecting the relationship between validation indexes and preoperative DVT.
Results: A total of 500 patients were included, of which 39 patients (7.8%) developed preoperative DVT. After univariate and multivariate analysis, preoperative time (in each day delay), male (vs. female), D-dimer > 0.6 mg/L, total cholesterol (TC) > 5.6 mmol/L, and PLR > 189.8 were the risk factors for preoperative DVT in patients with patella fracture. Inflammation index PLR combined with the other four risk factors significantly improved the predictive efficacy of preoperative DVT compared with PLR (P = 0.009).
Conclusion: Inflammatory index PLR is a risk factor for preoperative DVT in patients with patella fracture, and the efficacy of PLR in predicting DVT can be significantly improved when other risk factors (male, D-dimer > 0.6 mg/L, TC > 5.6 mmol/L, and PLR > 189.8 of preoperative time in each day delay) are combined. These data are useful for the clinical identification of patients at high risk of preoperative DVT in patella fractures.
Introduction
DVT is a common complication in patients with lower extremity fractures and carries a risk of fatal pulmonary embolism. DVT has been reported in patients with fractures at the time of admission (1). A patella fracture is a common fracture of the lower extremity with an incidence of 1% to 1.5% (2, 3). Although the incidence is not high, but once the occurrence of activities affecting the knee joint. In addition, the pain after fracture leads to the limitation of lower extremity movement, and the slow venous blood flow caused by immobilization of the affected extremity leads to the risk of DVT (4, 5). Previous studies have shown that the incidence of preoperative DVT in the patella fracture is 4.4% to 5.8% (6, 7).
Understanding the high-risk factors of DVT and early identification of high-risk patients are the key to preventing DVT. However, risk factors are inconsistent in different studies (7–9). Recent studies have found that inflammation is involved in the formation of DVT. Post-traumatic inflammatory response activates inflammatory cells and releases inflammatory factors in the body (10), which leads to systemic reaction and disorder of coagulation and anticoagulation mechanism (11). The neutrophil-to-lymphocyte ratio (NLR), monocyte/lymphocyte (MLR), and platelet to lymphocyte ratio (PLR) are brand new biomarkers of the inflammatory response, which can reflect the overall level of inflammation in the body (12–14). In DVT-related studies, NLR is a high-risk factor for death due to acute DVT in cancer patients (15). Peng et al. (16) found that MLR increased in patients with postoperative venous thromboembolism in patients with hip fractures. Moreover, Kuplay et al. (17) also found that PLR in cardiovascular patients increased significantly in proximal DVT. However, at present, it is not clear whether inflammation indicators NLR, MLR, and PLR are related to the occurrence of preoperative DVT after a patella fracture. And that pushed us to explore.
Our objective was to collect data from patients with surgically treated patella fractures in our hospital and analyze whether inflammatory indicators were risk factors for DVT. In addition, when inflammatory indicators are combined with other risk factors, whether the association between inflammatory indicators and DVT is affected.
Methods
Patients
This study retrospectively collected the data of patients with surgically treated patella fracture admitted to our hospital from August 2016 to August 2020. This study was approved by the Ethics Committee of the Hebei Cangzhou Hospital of Integrated Traditional Chinese Medicine and Western Medicine, focusing only on the examination results of patients. All patients enrolled signed informed consent forms. All data are obtained through our electronic medical records system. According to the empirical criteria, the sample size of logistic regression analysis should be 10 to 15 times the number of covariates. There were 36 covariates in this study, and the sample size should not be less than 36 × 10 = 360.
Inclusion and exclusion
Inclusion criteria were: patients' age to be 18 years old or older, unilateral patella fracture, and surgical treatment. Exclusion criteria: time >2 weeks from fracture, complicated fractures at other sites, open fractures, pathological fractures, severe autoimmune diseases, infectious diseases at the time of fracture, abnormal coagulation function, use of anticoagulants within 3 months of admission, incomplete medical records.
Data collection
The data collected mainly included the following aspects: demographic data, comorbidities, injuries, blood routine and biochemical test results, and routine DUS test results of DVT.
The demographic data include sex, age, body mass index (BMI), current smoking, alcohol consumption, American Society of Anesthesiologists (ASA). The comorbidities included hypertension, diabetes, chronic heart disease, cerebrovascular disease, and allergies to any medications, all of which were self-reported by patients. Injury-related data included the following: time from injury to hospital admission, mechanism of injury (low or high energy), time from hospital admission to operation, and total length of stay. Low energy injury refers to a fall at a high level while the patient is standing. High energy injury refers to injury caused by traffic accident or position injury higher than their own height.
Blood collection and test results information
Venous blood was collected from the elbow veins on an empty stomach for the first time after admission.Vein blood samples were collected in vacuum tubes containing sodium citrate anticoagulant. Blood samples were sent to the laboratory of our hospital for blood analysis within 6 h after collection. Laboratory tests included measurements of levels of total protein (TP), albumin (ALB), alkaline phosphatase (ALP), total bilirubin (TBIL), γ-glutamyl transferase (GGT), hypersensitive C-reactive protein (HCRP), total bile acid (TBA), creatine kinase (CK), lactic dehydrogenases (LDH), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL-C) level, low-density lipoprotein (LDL-C) level, very low-density lipoprotein (VLDL) level, glucose (GLU), uric acid (UA), white blood cell (WBC) count, neutrophile (NEUT) count, lymphocyte (LYM) count, monocytes (MON) count, red blood cell (RBC) count, hemoglobin (HGB) level, platelet (PLT) count, D-dimer level. Neutrophile-to-lymphocyte rate (NLR), monocytes-to-lymphocyte rate (MLR), platelet-to-lymphocyte rate (PLR) were calculated according to blood routine indexes.
Diagnosis and prevention of DVT
The patient could not perform off-bed weightbearing exercises because of pain in the injured extremity, but lay in bed until surgery. After admission, all patients were given Low Molecular Weight Heparin Injection (LMWHI) subcutaneous (abdominal subcutaneous) for anticoagulation (4250 I.U., once daily). In addition to drug prophylaxis, the patient underwent physical prophylaxis for DVT with ankle pump exercise in bed (1,000 times, once daily). Drug anticoagulation was discontinued on the day of operation. Patients were screened for DVT by DUS on the first day after admission and every 3 days thereafter, up to the day of operation. If any symptoms of thrombosis (e.g., lower extremity distention, pain, and acute varicose superficial veins) occur in the preoperative time, DUS should be performed immediately to confirm DVT. The occurrence of DVT in patients was detected by the same group of senior ultrasound doctors in our hospital. The “Guidelines for the Diagnosis and Treatment of Deep Vein Thrombosis (2016 3rd Edition)” issued by the Chinese Medical Association was used to diagnose and treat DVTs (18). The veins examined include: femoral common vein, superficial and deep femoral vein, popliteal vein, posterior and anterior tibial vein and peroneal vein. Patients diagnosed with DVT are consulted by vascular surgeons to determine the treatment of thrombosis. The timing of the patient's operation is determined by a senior orthopedic surgeon. The observation period was from the time of fracture to the time of operation, and the time of DVT the first detected by DUS was recorded. Patients were divided into DVT group and non-DVT group according to whether preoperative DVT occurred.
Statistical analysis
SPSS 25.0 (IBM, Armonk, New York) and MedCalc 20.0 (MedCalc Software, Ostend, Belgium) was used for statistical analysis. For biomarker NLR, PLR, MLR, and the plasma D-dimer level, we constructed receiver operating characteristic (ROC) to determine the optimal cutoff value for each variable. On basis of the cutoff values determined, each variable was divided in to two groups. Categorical variables were analyzed by chi-square or Fisher's exact test. Student T test or Mann-Whitney test was performed for continuous variables. Univariate analysis of differences between DVT group and non-DVT group. Multivariate logistic regression model and stepwise backward elimination method were used to investigate the independent risk factors that predicted the occurrence of DVT. The univariate analysis results of P < 0.10 were included in the model, and the correlation strength was expressed by odds ratio (OR) and 95% confidence interval (95% CI). Hosmer–Lemeshow test was employed to evaluate the fitting degree of the final model, and a P > 0.05 represented the acceptable result. The ability of inflammatory indicators combined with other independent risk factors to predict preoperative DVT was evaluated by ROC curve again. The combination of indicators and individual inflammatory indicators were compared to predict whether there was statistical difference in preoperative DVT. The significance level was set as P < 0.05.
Results
In the window period, a total of 612 cases of patella fracture patients. According to the exclusion criteria, fracture over 2 weeks; complicated fractures at other sites, open fractures, a total of 54 cases, pathological fracture, severe autoimmune diseases and infectious diseases at the time of fracture, use of anticoagulants within 3 months of admission, a total of 32 cases, 26 cases of incomplete medical records. A total of 500 patients were included.
They were divided into two groups based on the presence or absence of preoperative DVT: 461 patients in non-DVT group and 39 patients in DVT group, respectively. In the DVT group, there were 32 (82.1%) males and 7 (17.9%) females, with an average age of 57.0 ± 15.3 years (range, 28–84 years). In the non-DVT group, there were 273 (59.2%) males and 188 (40.8%) females, with an average age of 52.1 ± 14.9 years (range, 18–86 years). There were 275 (55%) left patella fractures and 225 (45%) right patella fractures. The mean days from fracture to admission was 0.2 ± 0.4 days (range, 0.02 to 5 days), the mean days from fracture to operation was 4.1 ± 2.1 days (range, 0–14 days), and mean total hospital stay was 11.3 ± 3.8 days (rang, 5–26 days).
DVT patient information
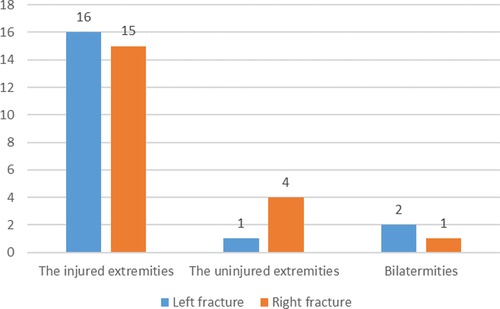
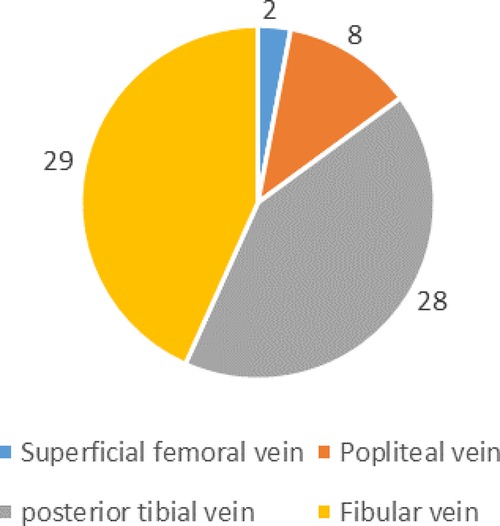
Preoperative DVT was detected in 39 patients 7.8% (39/500). None of the patients had pulmonary embolism. 79.5% (31/39) occurred in the injured lower extremity and 20.5% (8/39) in the uninjuried lower extremity, as shown in Figure 1. Among all the thrombus, DVT was found in only 4 deep veins, namely superficial femoral vein, popliteal vein, posterior tibial meridians and fibular vein. Their specific distribution is shown in Figure 2. There were 67 clots in 39 patients, with an average of 1.7 clots each patient, and 17 patients had only 1 clots. The mean time from fracture to DVT detection was 3.5 ± 2.2 days (range 0.1–11.1 days).
ROC analysis of inflammatory indicators for the diagnosis of DVT optimal cutoff value
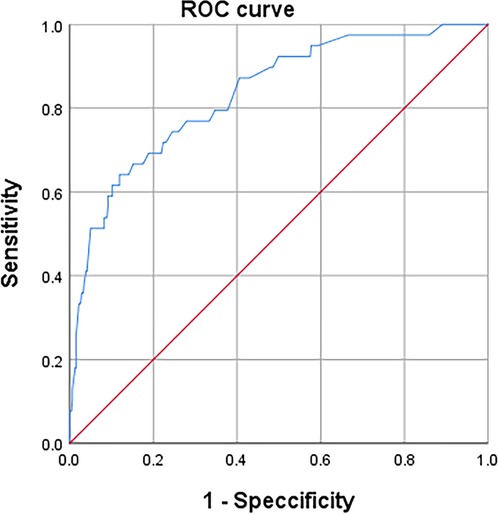
According to the ROC curve (Figure 3), the optimal cut off values for predicting DVT of continuous variables NLR, MLR, PLR and D-dimer are selected as follows: NLR, 4.6, AUC, 0.632; MLR, 0.7, AUC, 0.625; PLR, 189.8, AUC, 0.733; D-dimer, 0.6, AUC, 0.701 (as follow Table 1). These inflammatory biomarkers were converted into dichotomous variables based on thresholds.
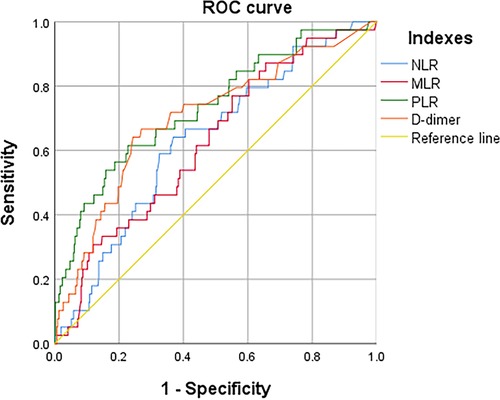
Figure 3. ROC to determine the optimal cutoff value for NLR, LMR, PLR, and D-dimer, when in continuous variable.
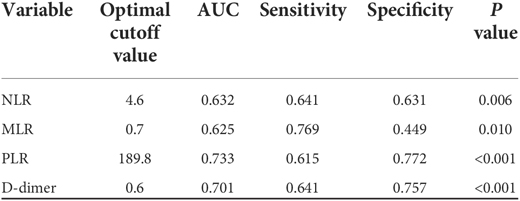
Table 1. The ROC and AUC to determine the optimal cutoff value for each index in continuous variable.
Univariate analysis of DVT group and non-DVT group
As shown in Table 2, we found statistically that gender, time from fracture to operation, TP < 60 g/L, LDH > 250 U/L, TC > 5.2 mmol/L, PLT > 300*109/L, NLR > 4.6, MLR > 0.7, PLR > 189.8, and D-dimer > 0.6 mg/L was significantly different between DVT group and non-DVT group (P < 0.05).
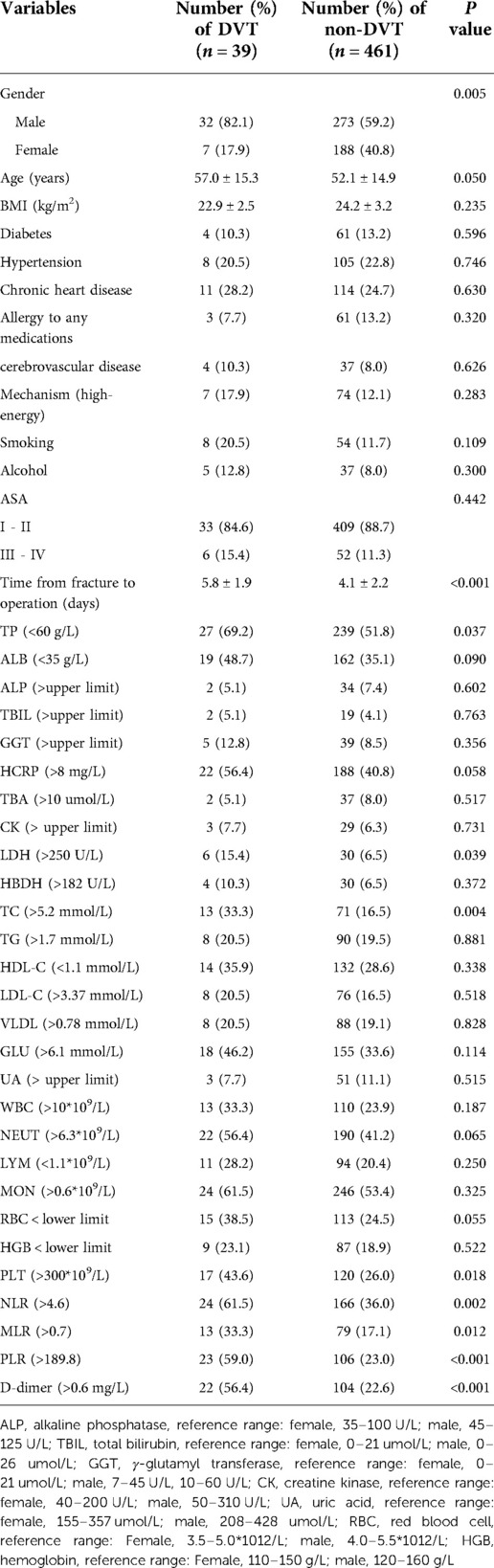
Table 2. Univariate analyses of risk factors associated with preoperative DVT after patella fracture.
Multivariate analysis predicted the independent risk factors for preoperative DVT and compared the differences after combination.
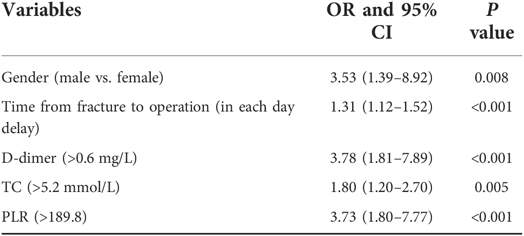
Multivariate logistic regression model analysis found five independent risk factors for preoperative DVT, which were male(vs. female) (P = 0.008), time from fracture to operation (in each day delay) (P < 0.001), D-dimer > 0.6 mg/L (P < 0.001), TC > 5.2 mmol/L (P = 0.005), and PLR > 189.8 (P < 0.001), as shown in Table 3. The H-L test of demonstrated The good fitness of The final model (X2 = 5.680, P = 0.683; Nagelkerke R2 = 0.289). After PLR was combined with other 4 factors, it was found that AUC, sensitivity and specificity for predicting preoperative DVT were 0.833, 0.641 and 0.883 (Figure 4). Better than PLR alone in predicting DVT efficacy (AUC, 0.833 vs. 0.733, P = 0.009).
Discussion
Patients with patella fracture were selected for a retrospective analysis of the occurrence of preoperative DVT and related risk factors, and the risk factors were linearized to predict the occurrence of DVT again. In this study, we found that the incidence of preoperative DVT was 7.8%, and identified male, time from fracture to operation (in each day delay), D-dimer > 0.6 mg/L (P < 0.001), TC > 5.2 mmol/L (P = 0.005), and PLR > 189.8 were risks factor for preoperative DVT in patients with patella fracture. The AUC of preoperative DVT after patella fracture predicted by ROC analysis after PLR combined with other 4 risk factors was 0.833, the sensitivity was 0.883, and the specificity was 0.641.
Data from this study showed that the incidence of preoperative DVT was 7.8% in patients with patella fractures. Previous studies have shown that the incidence of preoperative DVT is between 4.1% to 52.5% in patients with perioperative knee fracture, including fractures of the distal femur, patella, and proximal tibia (6, 7, 19–21). The incidence of DVT in the perioperative period of preoperative patella fracture was 4.1%–5.8% (6, 7), and the results of this study were basically consistent with them. In terms of location, 79.5% of DVT occurred in the affected lower extremities, and 85% of DVT occurred in the posterior tibial vein and fibular vein, which was consistent with Yang et al. (6) and Luo et al. (22).
In this study, male was a risk factor for the occurrence of preoperative DVT in patients, and the risk of male was 2.53 times higher than that of female. In other studies, the risk of DVT in males is 1.29–2.35 times higher than that in females (6, 18, 23). However, females in childbearing age are higher than males, while males are higher than females in non-childbearing age (24, 25). In our sample, 66.7% (26/39) of the DVT group were over 50 years old, which was consistent with the epidemiological statistical results. Some reports indicate that the difference may be related to hormone levels and genetics in women (24, 26, 27). The results of this study also showed that for each additional day before surgery, the risk of developing preoperative DVT increased by 31%. It's also an easy ending to understand. The patient's bed rest after fracture slows down the blood velocity of the lower extremities. On the other hand, the hypercoagulability of blood after trauma contributes to the occurrence of DVT, which also conforms to Virchow's Law (28). Similar results have been confirmed in spinal fracture (29), hip fracture (30), patella fracture (6) and foot fracture (8). With the increase of preoperative time, the risk of DVT in patients is 5% to 29%.
D-dimer is a recognized diagnostic index for the occurrence of DVT, which reflects the coagulation and fibrinolysis state of the body (31–33). Our results also show this characteristic of D-dimer. There is evidence that the D-dimer threshold for DVT diagnosis in patients with spinal and tibial fractures is 1.08 mg/L and 1.76 mg/L, respectively (21, 34). In this study, the threshold was 0.6 mg/L by ROC analysis. In terms of risk, we found that patients with D-dimer > 0.6 mg/L were 3.78 times more likely to develop DVT than patients with D-dimer ≤ 0.6 mg/L. It is well known that high plasma TC levels increase the incidence of cardiovascular events and death (35). We found that TC > 5.2 mmol/L increased the incidence of preoperative DVT, and multivariate analysis showed that the risk of DVT increased by 0.8 times. Leiba et al. (36) found that patients with high cholesterol significantly increased in patients with DVT, which was consistent with our research results.
Although NLR and MLR univariate analyses showed differences between the DVT and non-DVT groups. However, multivariate analysis did not identify an independent risk factor for preoperative DVT. We found that the AUC of PLR predicting DVT at admission was 0.733, and the sensitivity and specificity were 0.615 and 0.772, respectively. Patients with PLR > 189.8 had a 2.73-fold increased risk of preoperative DVT. Systemic inflammatory response and immunosuppression can be triggered immediately after trauma (37). Platelets, as cellular effector factors of inflammation and thrombosis, can also be activated after trauma (38). It may stimulate this inflammatory response in vivo and cause the increase of platelets (39). There is evidence that platelets play an important role in DVT (40). Multiple studies have shown that high PLR is associated with DVT (41–43), and there is also evidence that high PLR is associated with acute DVT (15). Other studies have shown that high PLR increases the risk of venous thrombosis by nearly 3 times (43). The results of this study are consistent with those reported.
Yao et al. (42) found that patients with postoperative DVT had higher preoperative PLR, but lower sensitivity and specificity in predicting DVT. We found similar results. Independent PLR showed low efficacy and low sensitivity and specificity in predicting preoperative DVT (AUC, 0.733, sensitivity, 0.615, specificity, 0.772). We linearized the combination of inflammatory indicator PLR with other 4 independent risk factors at admission, and found that the AUC for predicting DVT was 0.834, and the sensitivity and specificity were 0.641 and 0.839, respectively, which further proved that PLR after combination was highly correlated with the occurrence of preoperative DVT. The predictive efficacy and specificity of DVT were improved (P = 0.009). However, the sensitivity is not high, only 0.641. We analyzed that PLR may be affected by other factors, but this needs further confirmation.
There are some limitations of this study: First, we did not collect anticoagulant and blood drawing time after admission and before fasting venous blood drawing. Second, the amount of intravenous fluids in different patients before surgery may not be completely consistent. Third, none of the patients moved out of bed before surgery. These causes may influence independent factor results or the incidence of DVT.
Conclusions
By collecting data from patients with patella fractures, it was found that the incidence of preoperative DVT for patella fractures was 7.8%. Multivariate analysis showed that male, preoperative time (in each day delay), TC > 5.2 mmol/L, D-dimer > 0.6 mg/L, and PLR > 189.8 were independent risk factors for preoperative DVT of patella fracture. PLR combined with other risk factors improved the ability to predict preoperative DVT. Better than PLR alone (P = 0.009). PLR is expected to be a predictor of DVT, especially when other risk factors are combined.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
(I) Conception and design: SD, JL; (II) Administrative support: JZ; (III) Provision of study materials or patients: JZ; (IV) Collection and assembly of data: DW; (V) Data analysis and interpretation: HW; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zuo J, Hu Y. Admission deep venous thrombosis of lower extremity after intertrochanteric fracture in the elderly: a retrospective cohort study. J Orthop Surg Res. (2020) 1(15):549. doi: 10.1186/s13018-020-02092-9
2. Melvin JS, Mehta S. Patella fractures in adults. J Am Acad Orthop Surg. (2011) 4(19):198–207. doi: 10.5435/00124635-201104000-00004
3. Wild M, Windolf J, Flohé S. Fractures of the patella. Unfallchirurg. (2010) 5(113):401–11. quiz 412. doi: 10.1007/s00113-010-1768-x
4. Zee AA, van Lieshout K, van der Heide M, Janssen L, Janzing HM. Low molecular weight heparin for prevention of venous thromboembolism in patients with lower-limb immobilization. Cochrane Database Syst Rev. (2017) 8(8):Cd006681. doi: 10.1002/14651858.CD006681.pub4
5. Matthews JH, Terrill AJ, Barwick AL, Butterworth PA. Venous thromboembolism in podiatric foot and ankle surgery. Foot Ankle Spec. (2018) 5(11):444–50. doi: 10.1177/1938640017750256
6. Yang W, Wang H, Wei Q, Ding K, Jia Y, Li C, et al. Preoperative incidence and risk factors of deep vein thrombosis in patients with an isolated patella fracture. BMC Musculoskelet Disord. (2022) 1(23):204. doi: 10.1186/s12891-022-05163-6
7. Tan Z, Hu H, Deng X, Zhu J, Zhu Y, Ye D, et al. Incidence and risk factors for deep venous thrombosis of lower extremity after surgical treatment of isolated patella fractures. J Orthop Surg Res. (2021) 1(16):90. doi: 10.1186/s13018-021-02240-9
8. Meng H, Zhu Y, Zhang J, Li J, Zhao K, Zhang Y, et al. Incidence and risk factor for preoperative deep vein thrombosis (DVT) in isolated calcaneal fracture, a prospective cohort study. Foot Ankle Surg. (2021) 5(27):510–4. doi: 10.1016/j.fas.2020.06.007
9. Zhang W, Huai Y, Wang W, Xue K, Chen L, Chen C, et al. A retrospective cohort study on the risk factors of deep vein thrombosis (DVT) for patients with traumatic fracture at Honghui Hospital. BMJ Open. (2019) 3(9):e024247. doi: 10.1136/bmjopen-2018-024247
10. Bortolotti P, Faure E, Kipnis E. Inflammasomes in tissue damages and immune disorders after trauma. Front Immunol. (2018) 9:1900. doi: 10.3389/fimmu.2018.01900
11. Borgel D, Bianchini E, Lasne D, Pascreau T, Saller F. Inflammation in deep vein thrombosis: a therapeutic target? Hematology. (2019) 1(24):742–50. doi: 10.1080/16078454.2019.1687144
12. Budzianowski J, Pieszko K, Burchardt P, Rzeźniczak J, Hiczkiewicz J. The role of hematological indices in patients with acute coronary syndrome. Dis Markers. (2017) 2017:3041565. doi: 10.1155/2017/3041565
13. Ertem AG, Yayla C, Acar B, Kirbas O, Unal S, Uzel Sener M, et al. Relation between lymphocyte to monocyte ratio and short-term mortality in patients with acute pulmonary embolism. Clin Respir J. (2018) 2(12):580–6. doi: 10.1111/crj.12565
14. Qin B, Ma N, Tang Q, Wei T, Yang M, Fu H, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. (2016) 3(26):372–6. doi: 10.3109/14397595.2015.1091136
15. Ming L, Jiang Z, Ma J, Wang Q, Wu F, Ping J. Platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, and platelet indices in patients with acute deep vein thrombosis. Vasa. (2018) 2(47):143–7. doi: 10.1024/0301-1526/a000683
16. Peng J, Wang H, Zhang L, Lin Z. Construction and efficiency analysis of prediction model for venous thromboembolism risk in the elderly after hip fracture. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2021) 2(46):142–8. doi: 10.11817/j.issn.1672-7347.2021.190722
17. Kuplay H, Erdoğan SB, Bastopcu M, Arslanhan G, Baykan DB, Orhan G. The neutrophil-lymphocyte ratio and the platelet-lymphocyte ratio correlate with thrombus burden in deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. (2020) 3(8):360–4. doi: 10.1016/j.jvsv.2019.05.007
18. Zhu Y, Chen W, Li J, Zhao K, Zhang J, Meng H, et al. Incidence and locations of preoperative deep venous thrombosis (DVT) of lower extremity following tibial plateau fractures: a prospective cohort study. J Orthop Surg Res. (2021) 1(16):113. doi: 10.1186/s13018-021-02259-y
19. Zhang J, Zhao K, Li J, Meng H, Zhu Y, Zhang Y. Age over 65 years and high levels of C-reactive protein are associated with the risk of preoperative deep vein thrombosis following closed distal femur fractures: a prospective cohort study. J Orthop Surg Res. (2020) 1(15):559. doi: 10.1186/s13018-020-02089-4
20. Liu D, Zhu Y, Chen W, Li J, Zhao K, Zhang J, et al. Relationship between the inflammation/immune indexes and deep venous thrombosis (DVT) incidence rate following tibial plateau fractures. J Orthop Surg Res. (2020) 1(15):241. doi: 10.1186/s13018-020-01765-9
21. Li J, Zhu Y, Chen W, Zhao K, Zhang J, Meng H, et al. Incidence and locations of deep venous thrombosis of the lower extremity following surgeries of tibial plateau fractures: a prospective cohort study. J Orthop Surg Res. (2020) 1(15):605. doi: 10.1186/s13018-020-02136-0
22. Luo Z, Chen W, Li Y, Wang X, Zhang W, Zhu Y, et al. Preoperative incidence and locations of deep venous thrombosis (DVT) of lower extremity following ankle fractures. Sci Rep. (2020) 1(10):10266. doi: 10.1038/s41598-020-67365-z
23. Zixuan L, Chen W, Li Y, Wang X, Zhang W, Zhu Y, et al. Incidence of deep venous thrombosis (DVT) of the lower extremity in patients undergoing surgeries for ankle fractures. J Orthop Surg Res. (2020) 1(15):294. doi: 10.1186/s13018-020-01809-0
24. Roach RE, Lijfering WM, Rosendaal FR, Cannegieter SC, le Cessie S. Sex difference in risk of second but not of first venous thrombosis: paradox explained. Circulation. (2014) 1(129):51–6. doi: 10.1161/circulationaha.113.004768
25. Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. (2007) 4(5):692–9. doi: 10.1111/j.1538-7836.2007.02450.x
26. van Hylckama Vlieg A, Helmerhorst FM, Vandenbroucke JP, Doggen CJ, Rosendaal FR. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case-control study. Br Med J. (2009) 339:b2921. doi: 10.1136/bmj.b2921
27. Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. Br Med J. (2008) 7655(336):1227–31. doi: 10.1136/bmj.39555.441944.BE
28. Wolberg AS, Rosendaal FR, Weitz JI, Jaffer IH, Agnelli G, Baglin T, et al. Venous thrombosis. Nat Rev Dis Primers. (2015) 1:15006. doi: 10.1038/nrdp.2015.6
29. Wang H, Lv B, Zhang Z, Wang S, Ding W. Prevalence and predictors for preoperative deep vein thrombosis in patients with thoracolumbar fractures caused by high-energy injuries. World Neurosurg. (2020) 141:e431–6. doi: 10.1016/j.wneu.2020.05.162
30. Fu YH, Liu P, Xu X, Wang PF, Shang K, Ke C, et al. Deep vein thrombosis in the lower extremities after femoral neck fracture: a retrospective observational study. J Orthop Surg (Hong Kong). (2020) 1(28):2309499019901172. doi: 10.1177/2309499019901172
31. Tran HA, Gibbs H, Merriman E, Curnow JL, Young L, Bennett A, et al. New guidelines from the thrombosis and haemostasis society of Australia and New Zealand for the diagnosis and management of venous thromboembolism. Med J Aust. (2019) 5(210):227–35. doi: 10.5694/mja2.50004
32. Imai N, Miyasaka D, Shimada H, Suda K, Ito T, Endo N. Usefulness of a novel method for the screening of deep vein thrombosis by using a combined D-dimer- and age-based index before total hip arthroplasty. PLoS One. (2017) 2(12):e0172849. doi: 10.1371/journal.pone.0172849
33. Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. (2003) 13(349):1227–35. doi: 10.1056/NEJMoa023153
34. Ma J, Du P, Qin J, Zhou Y, Liang N, Hu J, et al. Incidence and risk factors predicting deep venous thrombosis of lower extremity following spinal fractures. Sci Rep. (2021) 1(11):2441. doi: 10.1038/s41598-021-82147-x
35. Leiherer A, Ulmer H, Muendlein A, Saely CH, Vonbank A, Fraunberger P, et al. Value of total cholesterol readings earlier versus later in life to predict cardiovascular risk. EBioMedicine. (2021) 67:103371. doi: 10.1016/j.ebiom.2021.103371
36. Leiba M, Seligsohn U, Sidi Y, Harats D, Sela BA, Griffin JH, et al. Thrombophilic factors are not the leading cause of thrombosis in Behçet's Disease. Ann Rheum Dis. (2004) 11(63):1445–9. doi: 10.1136/ard.2003.014241
37. Lord JM, Midwinter MJ, Chen YF, Belli A, Brohi K, Kovacs EJ, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. (2014) 9952(384):1455–65. doi: 10.1016/s0140-6736(14)60687-5
38. Morris RS, Schaffer BS, Lundy JB, Pidcoke HF, Chung KK, Darlington DN, et al. Immunopathological response to severe injury: platelet activation and the Th-17 immune response. Blood Coagul Fibrinolysis. (2018) 1(29):48–54. doi: 10.1097/mbc.0000000000000665
39. Tekin YK. Are neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios associated with mortality in pediatric trauma patients? A retrospective study. Rambam Maimonides Med J. (2019) 4(10):e0022. doi: 10.5041/rmmj.10376
40. Montoro-García S, Schindewolf M, Stanford S, Larsen OH, Thiele T. The role of platelets in venous thromboembolism. Semin Thromb Hemost. (2016) 3(42):242–51. doi: 10.1055/s-0035-1570079
41. Xue J, Ma D, Jiang J, Liu Y. Diagnostic and prognostic value of immune/inflammation biomarkers for venous thromboembolism: is it reliable for clinical practice? J Inflamm Res. (2021) 14:5059–77. doi: 10.2147/jir.S327014
42. Yao C, Zhang Z, Yao Y, Xu X, Jiang Q, Shi D. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio for acute deep vein thrombosis after total joint arthroplasty: a retrospective study. J Orthop Surg Res. (2018) 1(13):40. doi: 10.1186/s13018-018-0745-x
Keywords: risk factor, inflammatory indicators, deep vein thrombosis, patella fracture, platelet-to-lymphocyte ratio (PLR)
Citation: Diao S, Li J, Zhao J, Wang D, Wang H, Xu X and Zhou J (2022) Risk factors and new inflammatory indicators of deep vein thrombosis after adult patella fractures. Front. Surg. 9:1028542. doi: 10.3389/fsurg.2022.1028542
Received: 26 August 2022; Accepted: 3 October 2022;
Published: 2 November 2022.
Edited by:
Paphon Sa-ngasoongsong, Mahidol University, ThailandReviewed by:
Wei Chen, The Third Hospital of Hebei Medical University, ChinaJianlin Xiao, Jilin University, China
© 2022 Diao, Li, Zhao, Wang, Wang, Xu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junlin Zhou anVubGluemhvdV9hcnRpY2xlQG91dGxvb2suY29t
‡ORCID Junlin Zhou orcid.org/0000-0001-8646-8779
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Shuo Diao1,†
Shuo Diao1,† Junlin Zhou
Junlin Zhou