
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg. , 06 January 2023
Sec. Vascular Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1027231
Objective: We aimed to investigate the effectiveness and safety of prophylactic sac embolization during endovascular aneurysm repair (EVAR) in patients suffering from abdominal aortic aneurysms.
Methods: We performed a systematic literature search of PubMed, Web of Science, EMbase, Cochrane Library, China National Knowledge Infrastructure (CNKI), VIP, Wanfang and China Biomedical Literature Database (CBM) to identify studies evaluating the outcomes of sac embolization vs. no embolization among patients who had received EVAR. The time limit of the search was from the establishing database to July 22, 2022. Outcome measures involved the type II endoleak rate, the other endoleak rate, the reintervention rate, mortality, and operation time. Fixed (no heterogeneity) or random effects models were constructed for each outcome. The outcomes are represented as the odds ratio (OR) with a 95% confidence interval (CI).
Results: Among the 2,622 studies screened, 13 studies involving 747 participants were included in the review. The incidence of early-term type II endoleak (OR = 0.2, 95% CI (0.13,0.31), P < 0.00001), mid-term type II endoleak (OR = 0.23, 95% CI (0.15,0.37), P < 0.00001), late-term type II endoleak (OR = 0.27, 95% CI (0.16,0.46), P < 0.00001) and reintervention (OR = 0.50, 95% CI (0.37,0.78), P = 0.002) within the sac embolization group were significantly lower than those in the non-embolization group. No significant differences were observed between the two groups were found for the other endoleak rates (OR = 0.67, 95% CI (0.34,1.32), P = 0.25), mortality (OR = 0.64, 95% CI (0.25,1.66), P = 0.36) and operation time operation (MD = 5.76, 95% CI (-8.30,19.83), P = 0.42).
Conclusions: EVAR combined with sac embolization effectively reduces the incidence of type II endoleak and the reintervention rate without enhancing the operation time. Therefore, more high-quality studies are still needed for validation due to the limited amount and quality of included literature.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022365648.
An abdominal aortic aneurysm (AAA) is a typical aneurysmal arterial disease. Where anatomy permits, endovascular aneurysm repair (EVAR) has become an alternative to open surgery for AAA (1). Some international clinical studies have depictedt that the short-term mortality after EVAR is significantly lower than open surgery (2–5). However, the problem of endoleaks after EVAR is quite significant.
Type II endoleak is the most common type, detected in 6% to 59% of patients after EVAR (6, 7). Risk factors related to type II endoleak include age, smoking, the maximum diameter of the aneurysm, the inferior mesenteric artery (IMA), and the number of patent lumbar arteries (LA) (8). Multiple studies have shown a significant association between higher rates of type II endoleaks and preoperatively patent sac branches (IMA and LA) (9, 10). IMA is the inflow vessel of type II endoleak and LA is the outflow vessel. Patent vessels provide a complex array of inflow and outflow vessels, maintaining the blood flow and pressure within the sac (11). In addition, persistent type II endoleaks with increase in sac size could lead to new type I, type III endoleaks, or EVAR-related complications. They have a potential risk of rupture and a mortality rate of up to 80% during rupture (12, 13). Currently, the treatment of type II endoleaks mainly involves transarterial embolization of IMA or LA, direct translumbar embolization of the aneurysm sac, laparoscopic ligation of IMA and/or LA, or conversion to open surgery. However, the recurrence and subsequent re-intervention are possible with increased follow-up monitoring. It also enhancest the financial burden of the patients (14–16). Therefore, effective management of type II endoleaks is quite challenging.
Intraoperative sac embolization during EVAR can reduce the incidence of type II endoleaks. However, there is no consensus on the efficacy of intraoperative sac embolization. We screened the available data and performed a meta-analysis to evaluate the effectiveness and safety of prophylactic sac embolization during EVAR in AAA patients.
A systematic literature search involving PubMed, Web of Science, EMbase, Cochrane Library, China Knowledge Network (CNKI), Vipshop, Wanfang and the Chinese Biomedical Literature Database (CBM) was performed to retrieve randomized controlled trials (RCT) and retrospective cohort studies of prophylactic sac embolization in EVAR due to AAA. The search time limit was from establishing the database to July 22, 2022. The Chinese search terms included: “embolization”, “endoleak”, “aortic aneurysm, abdominal”, “abdominal aortic aneurysm”. The English search terms included: “Embolotherapy”, “Embolotherapies”, “Therapeutic Embolization”, “Embolizations, Therapeutic”, “Therapeutic Embolizations”, “Embolization”, “Endoleak”, “Endoleaks”, “Perigraft Leak”, “Leak, Perigraft”, “Leaks, Perigraft”, “Perigraft Leaks”, “Abdominal Aortic Aneurysms”, “Aneurysms, Abdominal Aortic”, “Aortic Aneurysms, Abdominal”, “Abdominal Aortic Aneurysm”, “Aneurysm, Abdominal Aortic”, “Aneurysm, Abdominal Aortic”.
We included the studies depending on the following criteria: (1) Type of study: RCT or retrospective cohort study; (2) Subjects: AAA patients undergoing EVAR; (3) Intervention measures: the experimental group was treated with EVAR + intraoperative sac embolization, and the control group was treated with standard EVAR; (4) Outcome measures: incidence of type II endoleak, incidence of other endoleaks, reintervention rate, mortality, and operative time.
The exclusion criteria were:(1) Lack of control group; (2) Literature without primary outcome measures; (3) Duplicated studies; (4) Conference abstract; (5) Languages other than Chinese and English.
Two evaluators independently screened the literature, extracted the data, and examined them. Any inconsistencies were carefully discussed, and a third party was consulted to resolve the issue. Based on inclusion and exclusion criteria, the initial screening was done by reading the “title and abstract” of the article to exclude any irrelevant studies. Then the full text was assessed to recruit the final literature. After confirming the inclusion, the following data were extracted: basic information about the literature (first author, publication date, type of study), age, gender, sample size, follow-up time, embolic criteria, embolic material, monitoring mode, outcome indicators, and the critical elements of risk of bias assessment.
Statistical analysis was performed using RevMan 5.3 software. Continuous variables used the mean difference (MD) as the effect indicator, and dichotomous variables used the odds ratio (OR) with 95% confidence intervals (95% CI). They were calculated for each effect indicator and P-values, with P < 0.05 representing a statistically significant difference. Heterogeneity between studies was assessed using the I2 statistic with a cutoff value of 50%. If I2 ≤ 50%, there was no heterogeneity and a fixed effects model was used. On the other hand, if I2 > 50%, a random effects model was used, and further heterogeneity analysis was performed through source line descriptive analysis. Two evaluators assessed three RCTs for risk of bias using the risk of bias assessment tool recommended in the Cochrane Handbook 5.1.0, and nine cohort studies were evaluated using the NOS quality rating scale.
A total of 2,622 articles were obtained through the database search. After removing duplicate studies and screening based on the inclusion and exclusion criteria, according to the PRISMA 2020 statement (17), we included 13 studies (18–30) with 747 patients, of which 330 underwent sac embolization during EVAR. The literature screening flow diagram and the results are depicted in Figure 1.
The primary characteristics of the included studies are represented in Table 1, outcome measures of the included studies are demonstrated in Table 2, and the risk of bias assessment results of the RCT studies is shown in Table 3. The quality assessment of the cohort studies was performed with the Newcastle-Ottawa Scale (NOS), having with a total score of 9. The NOS scores of the included articles were ≥6, depicting high quality (Table 1).
Based on the follow-up time, the incidence of type II endoleaks was defined at 0–6 months, 7–18 months, and >18 months postoperatively as early, middle and late endoleaks. Seven studies reported the occurrence of early-term type II endoleak, and the meta-analysis indicated that the incidence of early-term type II endoleak was significantly lower within the embolization group than in the non-embolization group [OR = 0.2, 95% CI (0.13, 0.31), P < 0.00001] (Figure 2A). Eight studies reported the occurrence of mid-term type II endoleak, and the results revealed that the incidence of mid-term type II endoleak was significantly lower within the embolization group than in the non-embolization group [OR = 0.23, 95% CI (0.15, 0.37), P < 0.00001] (Figure 2B). Five articles reported the occurrence of late endoleaks, and meta-analysis showed that the incidence of late type II endoleak was significantly lower in the embolization group than in the non- embolization group [OR = 0.27, 95% CI (0.16, 0.46), P < 0.00001] (Figure 2C).
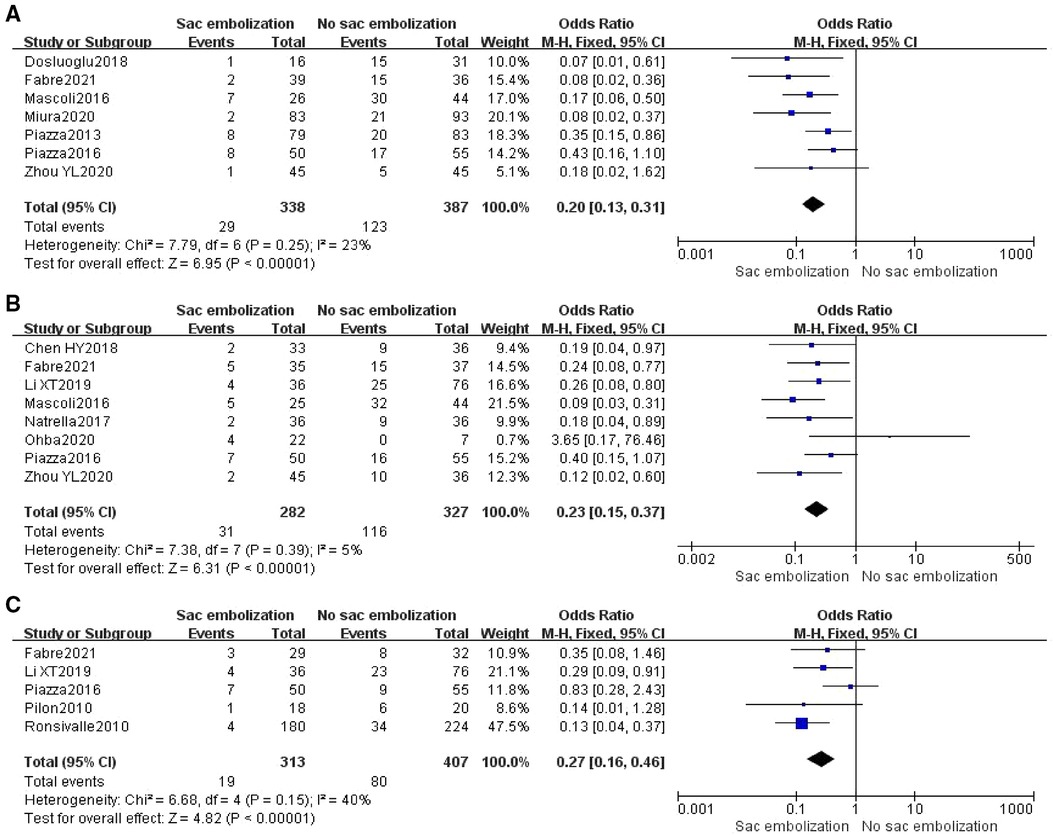
Figure 2. Incidence of early-term (A), mid-term (B), and late-term (C) type II endoleak in the embolization group compare with the non- embolization group.
Based on the inclusion criteria, subgroup analysis revealed that sac embolization could significantly decrease the rate of type II endoleak during the final follow-up among the regular and high-risk groups (P < 0.05) (Figure 3).
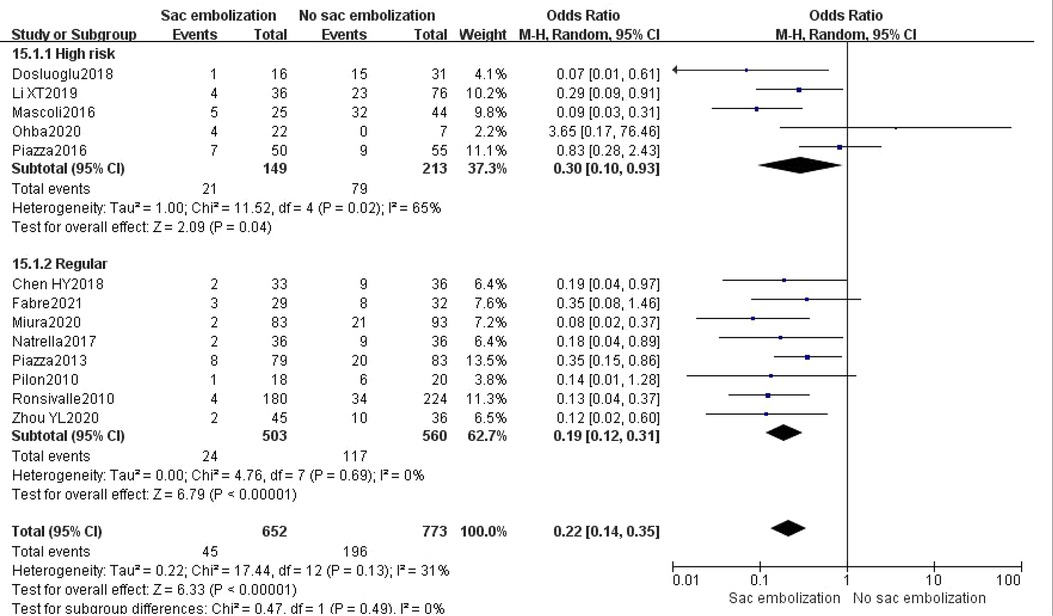
Figure 3. Incidence of type II endoleak at the last follow-up in the high risk group compare with the regular group.
According to the embolic material, subgroup analysis depicted that using coils and fibrin glue + coils could significantly reduce the type II endoleak rate during the final follow-up (P < 0.01). However there was no statistically significant difference in using fibrin glue alone groups [OR = 0.44, 95% CI (0.01, 17.01), P = 0.66] (Figure 4).
Other endoleaks included type I, type III or type IV. Six studies described the occurrence of other endoleaks. The results depicted no statistically significant difference during incidence of other endoleaks between the embolization and the non- embolization groups [OR = 0.67, 95% CI (0.34, 1.32), P = 0.25] (Figure 5).
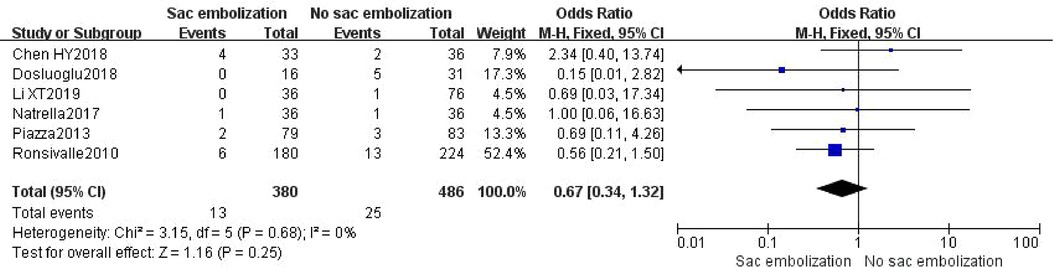
Figure 5. Incidence of other endoleaks in the embolization group compare with the non- embolization group.
Ten studies reported the occurrence of mortality. The meta-analysis described no statistically significant difference in mortality among the embolization and non-embolization groups in the random effects model [OR = 0.64, 95% CI (0.25, 1.66), P = 0.36] (Figure 6).
Nine studies reported re-intervention, and meta-analysis showed that the re-intervention rate was lower in the embolization group compared to the non-embolization group, indicating a statistically significant difference [OR = 0.50, 95% CI (0.37, 0.78), P = 0.002]. Subgroup analysis revealed that the re-intervention rate associated with type II endoleak was significantly lower in the embolization group than in the non-embolization group [OR = 0.22, 95% CI (0.10, 0.48), P < 0.01]. However, there was no statistically significant difference in the re-intervention rate associated with other factors in the two groups [OR = 0.84, 95% CI (0.49, 1.44), P = 0.52] (Figure 7).
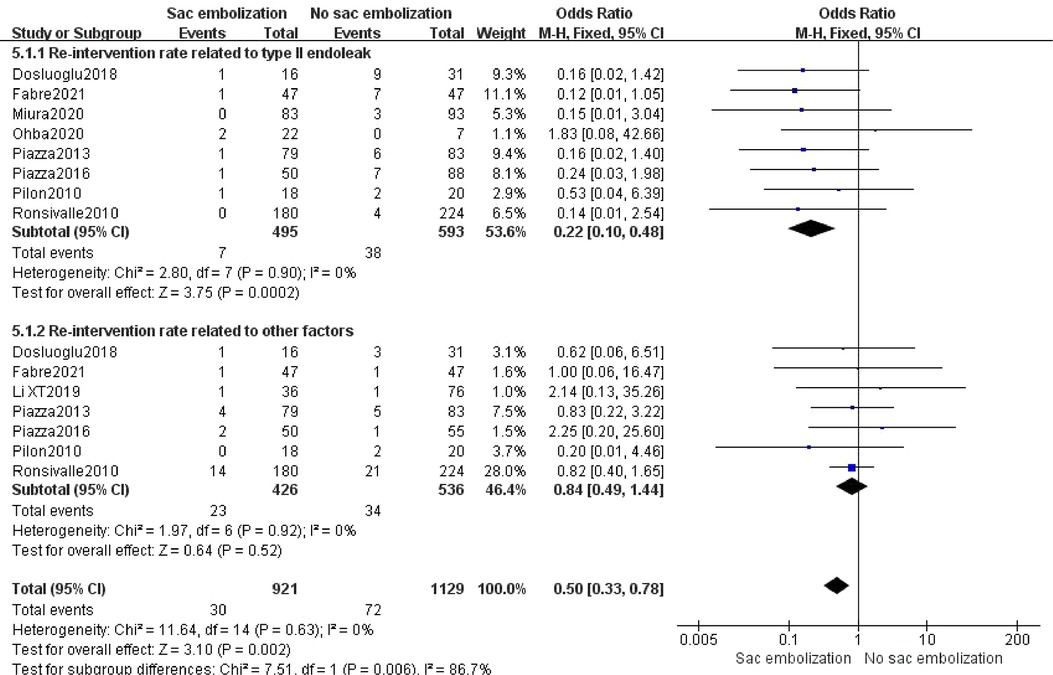
Figure 7. The rate of re-intervention in the embolization group compare with the non- embolization group.
Six studies reported the operation time, and the random effect model analysis showed no statistically significant difference in operation time between the embolization and the non-embolization groups [MD = 5.76, 95% CI (−8.30, 19.83), P = 0.42] (Figure 8).
Funnel plots were created using RevMan 5.3 software depending on the incidence of early, middle, and late-term type II endoleaks. It showed substantial symmetry on both sides, establishing a low publication bias for inclusion (Figure 9).
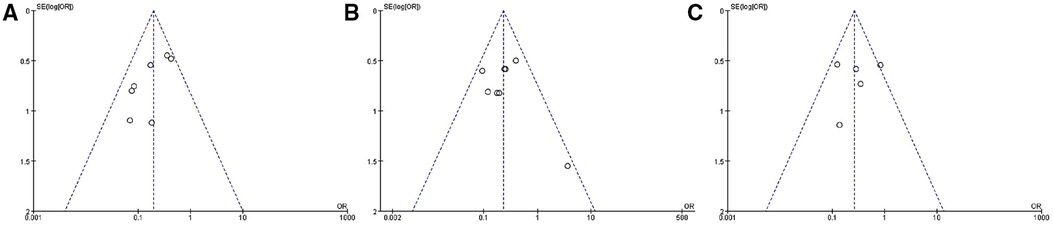
Figure 9. Publication bias (according to incidence of early-term (A), mid-term (B), and late-term (C) type II endoleak).
EVAR has the advantages of less trauma, lower anesthetic requirements, and faster postoperative recovery compared to traditional open surgery. However, managing the postoperative EVAR sac is extremely important, and the reduction in aneurysm diameter during follow-up is an important marker for successful EVAR (31). Type II endoleak is the most common complication after EVAR. Some type II endoleaks may close or increase the diameter of the aneurysm sac and re-intervention (19). Sac embolization in EVAR is performed by developing new access on the opposite side of the delivery route of the main stent, thereby leaving the 4F contrast catheter within the AAA cavity. After successfully undergoing the standard EVAR, the coils or liquid embolization agent was delivered from the pre-positioned 4F catheter within the sac.
This study showed that EVAR combined with sac embolization could significantly reduce the incidence of type II endoleak in the early, middle, and late-term postoperative periods (P < 0.01). There was no significant effect on the incidence of other endoleaks (type I, III, and IV endoleaks) (P = 0.25). EVAR combined with sac embolization could also significantly decrease the re-intervention rate (P = 0.0007). Regarding safety, there was no significant difference in mortality and operation time between the sac embolization and the non- embolization groups.
In previous studies, different centers have reported different efficacy of sac embolization due to various follow-up times. The early-term effectiveness of EVAR combined with sac embolization has been widely recognized (18, 19, 25, 30) and confirmed by our studies. Pilon (26)and Ronsivalle (27) reported significant long-term efficacy. In an RCT study in 2021, Fabre et al. (19) reported that sac embolization prevented type II endoleaks at 1 month, 6 months, and 12 months. However, no statistically significant difference was observed in the incidence of type II endoleaks between the two groups at 24 months (P = 0.19). Piazza et al. (25) also described no significant difference in the rate of type II endoleaks among the two groups at 24 months after surgery (P = 0.57). Our study confirmed the efficacy of EVAR combined with sac embolization, significantly reducing the incidence of type II endoleaks during the middle and late term.
Thirty-eight cases of other endoleaks were reported in six studies, including 13 from the embolization group (11 cases of type I endoleaks and two from of type III endoleaks), and 25 from the non-embolization group (21 cases of type I endoleaks, one of type III endoleak and three of type IV endoleaks), with no statistically significant difference between the two groups (P = 0.25). A separate analysis for the type I endoleak incidence in the two groups also showed no statistically significant difference [OR = 0.67, 95% CI (0.33, 1.38), P = 0.28].
The impact of type II endoleaks on survival is remains unclear (18). Seike et al. (32) retrospectively analyzed the clinical data of 17,099 patients and show a correlation between type II endoleaks patients and late adverse events, including re-intervention, death, rupture, and aneurysm sac enlargement. Batt et al. (33) reported that survival with or without type II endoleak (T2E) had no statistical difference (P = 0.49). Sidloff et al. (34) found that a late type II endoleak was associated with survival (P = 0.008) but early type II endoleak was not (P = 0.06). Our study found that intraoperative embolization significantly reduced the incidence of postoperative type II endoleaks. Moreover, there was no significant difference in postoperative mortality between the two groups (P = 0.36), demanding confirmation through various RCTs.
In a study of 3,595 patients, van Marrewijk et al. (35) observed that 55% of patients with type II endoleaks required re-intervention, significantly higher than the 15% re-intervention rate among patients without type II endoleak. In our analysis, embolization effectively decreased the re-intervention rate (P = 0.002), and subgroup analysis revealed that embolization significantly reduced the re-intervention rate associated with type II endoleaks (P < 0.01). For re-intervention due to other reasons, there was no significant difference between the embolization and the non-embolization groups (P = 0.52). An issue with the embolization procedure is the potential for prolonging of operative time and radiation exposure, in our analysis, sac embolization did not extend the operative time (P = 0.42).
The study was conducted by following the methods and requirements of meta-analysis. However, there are certain limitations: (1) the sample size of some of the RCTs or retrospective cohort studies included in this study was small and required expansion; (2) lack of standardized in the treatment of study methods, such as lack of blinding and propensity matching, which could be biased; (3) different centers included their studies based on different prevention criteria, embolic materials and re-intervention criteria, which could affect the analysis analysis.
In conclusion, compared with standard EVAR, EVAR combined with intraoperative sac embolization significantly decreases the incidence of postoperative type II endoleak and re-intervention rate but does not enhance the operation time. This study is limited by the quantity and quality of the included literature. Therefore, more high-quality studies are needed to validate the results.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
YZ and KL completed data extraction, processing and article writing; QN provide ideas; LF, DZ and CS provide statistical support. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ichihashi S, Takahara M, Fujimura N, Nagatomi S, Iwakoshi S, Bolstad F, et al. Multicentre randomised controlled trial to evaluate the efficacy of pre-emptive inferior mesenteric artery embolisation during endovascular aortic aneurysm repair on aneurysm sac change: protocol of clarify IMA study. BMJ open. (2020) 10(2):e031758. doi: 10.1136/bmjopen-2019-031758
2. Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet (London, England). (2016) 388(10058):2366–74. doi: 10.1016/S0140-6736(16)31135-7
3. Brown LC, Powell JT, Thompson SG, Epstein DM, Sculpher MJ, Greenhalgh RM. The UK EndoVascular aneurysm repair (EVAR) trials: randomised trials of EVAR versus standard therapy. Health Technol Assess (Winchester, England). (2012) 16(9):1–218. doi: 10.3310/hta16090
4. Patel R, Powell JT, Sweeting MJ, Epstein DM, Barrett JK, Greenhalgh RM. The UK EndoVascular aneurysm repair (EVAR) randomised controlled trials: long-term follow-up and cost-effectiveness analysis. Health Technol Assess (Winchester, England). (2018) 22(5):1–132. doi: 10.3310/hta22050
5. de Guerre L, Dansey K, Li C, Lu J, Patel PB, van Herwaarden JA, et al. Late outcomes after endovascular and open repair of large abdominal aortic aneurysms. J Vasc Surg. (2021) 74(4):1152–60. doi: 10.1016/j.jvs.2021.02.024
6. Ikoma A, Nakai M, Sato M, Sato H, Minamiguchi H, Sonomura T, et al. Systolic sac pressure Index for the prediction of persistent type II endoleak for 12 months after endovascular abdominal aortic aneurysm repair. Cardiovasc Intervent Radiol. (2016) 39(4):522–9. doi: 10.1007/s00270-015-1191-3
7. Nuckles B, Nadal L, Berger A, Salzler G, Elmore JR, Ryer EJ. Outcomes of type II endoleak treatment using ethylene vinyl alcohol copolymer (onyx(TM)). Vasc Endovascular Surg. (2021) 55(1):50–7. doi: 10.1177/1538574420964644
8. Guo Q, Du X, Zhao J, Ma Y, Huang B, Yuan D, et al. Prevalence and risk factors of type II endoleaks after endovascular aneurysm repair: a meta-analysis. PloS one. (2017) 12(2):e0170600. doi: 10.1371/journal.pone.0170600
9. Baum RA, Carpenter JP, Cope C, Golden MA, Velazquez OC, Neschis DG, et al. Aneurysm sac pressure measurements after endovascular repair of abdominal aortic aneurysms. J Vasc Surg. (2001) 33(1):32–41. doi: 10.1067/mva.2001.111807
10. Velazquez OC, Baum RA, Carpenter JP, Golden MA, Cohn M, Pyeron A, et al. Relationship between preoperative patency of the inferior mesenteric artery and subsequent occurrence of type II endoleak in patients undergoing endovascular repair of abdominal aortic aneurysms. J Vasc Surg. (2000) 32(4):777–88. doi: 10.1067/mva.2000.108632
11. Chew DK, Dong S, Schroeder AC, Hsu HW, Franko J. The role of the inferior mesenteric artery in predicting secondary intervention for type II endoleak following endovascular aneurysm repair. J Vasc Surg. (2019) 70(5):1463–8. doi: 10.1016/j.jvs.2019.01.090
12. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. (2018) 67(1):2–77. doi: 10.1016/j.jvs.2017.10.044
13. Guirguis-Blake JM, Beil TL, Senger CA, Coppola EL. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US preventive services task force. Jama. (2019) 322(22):2219–38. doi: 10.1001/jama.2019.17021
14. Bryce Y, Schiro B, Cooper K, Ganguli S, Khayat M, Lam CK, et al. Type II endoleaks: diagnosis and treatment algorithm. Cardiovasc Diagn Ther. (2018) 8(Suppl 1):S131–s137. doi: 10.21037/cdt.2017.08.06
15. Lalys F, Durrmann V, Duménil A, Göksu C, Cardon A, Clochard E, et al. Systematic review and meta-analysis of preoperative risk factors of type II endoleaks after endovascular aneurysm repair. Ann Vasc Surg. (2017) 41:284–93. doi: 10.1016/j.avsg.2016.08.021
16. Qiu CT, li YJ, li RG, Liu Y, Lu HQ, Li Y, et al. Progress in prevention and treatment of type II endoleaks after endovascular AneurysmRepair. MedicalREcapitulat. (2022) 28(13):2619–24. doi: 10.3969/j.issn.1006-2084.2022.13.021
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Research ed). (2021) 372:n71. doi: 10.1136/bmj.n71
18. Dosluoglu HH, Rivero M, Khan SZ, Cherr GS, Harris LM, Dryjski ML. Pre-emptive nonselective perigraft aortic sac embolization with coils to prevent type II endoleak after endovascular aneurysm repair. J Vasc Surg. (2019) 69(6):1736–46. doi: 10.1016/j.jvs.2018.10.054
19. Fabre D, Mougin J, Mitilian D, Cochennec F, Garcia Alonso C, Becquemin JP, et al. Prospective, randomised two centre trial of endovascular repair of abdominal aortic aneurysm with or without sac embolisation. Eur J Vasc Endovasc Surg. (2021) 61(2):201–9. doi: 10.1016/j.ejvs.2020.11.028
20. Mascoli C, Freyrie A, Gargiulo M, Gallitto E, Pini R, Faggioli G, et al. Selective intra-procedural AAA sac embolization during EVAR reduces the rate of type II endoleak. Eur J Vasc Endovasc Surg. (2016) 51(5):632–9. doi: 10.1016/j.ejvs.2015.12.009
21. Miura S, Kurimoto Y, Maruyama R, Masuda T, Yanase Y, Iba Y, et al. Endovascular aortic aneurysm repair without type 2 endoleak using concomitant N-butyl-2-cyanoacrylate injection into the abdominal aortic aneurysm sac. Ann Vasc Surg. (2020) 66:110–9. doi: 10.1016/j.avsg.2019.12.006
22. Natrella M, Rapellino A, Navarretta F, Iob G, Cristoferi M, Castagnola M, et al. Embo-EVAR: a technique to prevent type II endoleak? A single-center experience. Ann Vasc Surg. (2017) 44:119–27. doi: 10.1016/j.avsg.2017.01.028
23. Ohba S, Shimohira M, Hashizume T, Muto M, Ohta K, Sawada Y, et al. Feasibility and safety of sac embolization using N-butyl cyanoacrylate in emergency endovascular aneurysm repair for ruptured abdominal aortic aneurysms or isolated iliac artery aneurysms. J Endovasc Ther. (2020) 27(5):828–35. doi: 10.1177/1526602820923954
24. Piazza M, Frigatti P, Scrivere P, Bonvini S, Noventa F, Ricotta JJ, et al. Role of aneurysm sac embolization during endovascular aneurysm repair in the prevention of type II endoleak-related complications. J Vasc Surg. (2013) 57(4):934–41. doi: 10.1016/j.jvs.2012.10.078
25. Piazza M, Squizzato F, Zavatta M, Menegolo M, Ricotta JJ, Lepidi S, et al. Outcomes of endovascular aneurysm repair with contemporary volume-dependent sac embolization in patients at risk for type II endoleak. J Vasc Surg. (2016) 63(1):32–8. doi: 10.1016/j.jvs.2015.08.049
26. Pilon F, Tosato F, Danieli D, Campanile F, Zaramella M, Milite D. Intrasac fibrin glue injection after platinum coils placement: the efficacy of a simple intraoperative procedure in preventing type II endoleak after endovascular aneurysm repair. Interact Cardiovasc Thorac Surg. (2010) 11(1):78–82. doi: 10.1510/icvts.2009.231167
27. Ronsivalle S, Faresin F, Franz F, Rettore C, Zanchetta M, Olivieri A. Aneurysm sac “thrombization” and stabilization in EVAR: a technique to reduce the risk of type II endoleak. J Endovasc Ther. (2010) 17(4):517–24. doi: 10.1583/09-3004.1
28. Chen HY, Dai YQ, Zhuang H, Zhan TH, Chen C, Guo PF, et al. Efficacy of intraprocedural perigraft arterial sac embolization during endovascular aortic aneurysm repair for prevention of type II endoleak. Chinese J Interv Imaging and Ther. (2018) 15(11):645–9. doi: 10.13929/j.1672-8475.201803031
29. Li XT, Huang YY, Chen RQ, Guo MH, Guo PF. Intraoperative sac coiling embolization in type II endoleak high risk population after endovascular aortic repair. Chin J Gen Surg. (2019) 09:745–9. doi: 10.3760/cma.j.issn.1007-631X.2019.09.001
30. Zhou YR, Pan YM, Zheng Z, Zha ZB. Observation of the feasibility and effectiveness of para-graft tumor lumen embolization for the prevention of type II internal fistula during abdominal aortic aneurysm repair. J Clin and Exp Med. (2020) 19(14):1525–8. doi: 10.3969/j.issn.1671-4695.2020.14.020
31. Fujimura N, Matsubara K, Takahara M, Harada H, Asami A, Shibutani S, et al. Early sac shrinkage is a good surrogate marker of durable success after endovascular aneurysm repair in Japanese patients. J Vasc Surg. (2018) 67(5):1410–8. doi: 10.1016/j.jvs.2017.08.076
32. Seike Y, Matsuda H, Shimizu H, Ishimaru S, Hoshina K, Michihata N, et al. Nationwide analysis of persistent type II endoleak and late outcomes of endovascular abdominal aortic aneurysm repair in Japan: a propensity-matched analysis. Circulation. (2022) 145(14):1056–66. doi: 10.1161/CIRCULATIONAHA.121.056581
33. El Batti S, Cochennec F, Roudot-Thoraval F, Becquemin JP. Type II endoleaks after endovascular repair of abdominal aortic aneurysm are not always a benign condition. J Vasc Surg. (2013) 57(5):1291–7. doi: 10.1016/j.jvs.2012.10.118
34. Sidloff DA, Gokani V, Stather PW, Choke E, Bown MJ, Sayers RD. Type II endoleak: conservative management is a safe strategy. Eur J of Vasc and Endovas Surg. (2014) 48(4):391–9. doi: 10.1016/j.ejvs.2014.06.035
Keywords: abdominal aortic aneurysm, endovascular aneurysm repair, embolization, type II endoleak, SAC
Citation: Chen Q, Zhang Y, Lei K, Fu L, Zhang D, Sun W, Shi C and Niu Q (2023) Efficacy and safety of prophylactic intraoperative sac embolization in EVAR for abdominal aortic aneurysm: A meta-analysis. Front. Surg. 9:1027231. doi: 10.3389/fsurg.2022.1027231
Received: 24 August 2022; Accepted: 24 October 2022;
Published: 6 January 2023.
Edited by:
Wei Guo, Chinese PLA General Hospital, ChinaReviewed by:
Saket Singh, Yale Medicine, United States© 2023 Zhang, Lei, Fu, Zhang, Shi, Sun, Chen and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qibing Niu MzEyMTk5MTIzQHFxLmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Vascular Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.