
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 10 January 2023
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1026604
Aim: To investigate the predictive value of C-reactive protein (CRP) to serum albumin (ALB) ratio in the severity and prognosis of acute pancreatitis (AP), and compare the predictive value of the CRP/ALB ratio with the Ranson score, modified computed tomography severity index (MCTSI) score, and Bedside Index of Severity in Acute Pancreatitis (BISAP) score.
Methods: This cohort study retrospectively analyzed clinical data of AP patients from August 2018 to August 2020 in our hospital. Logistic regression analysis was utilized to determine the effects of CRP/ALB ratio, Ranson, MCTSI, and BISAP score on severe AP (SAP), pancreatic necrosis, organ failure, and death. The predictive values of CRP/ALB ratio, Ranson, MCTSI, and BISAP score were examined with the area under the curve (AUC) of the receiver operator characteristic (ROC) curve analysis. DeLong test was used to compare the AUCs between CRP/ALB ratio, Ranson, MCTSI, and BISAP score.
Results: Totally, 284 patients were included in this study, of which 35 AP patients (12.32%) developed SAP, 29 (10.21%) organ failure, 30 (10.56%) pancreatic necrosis and 11 (3.87%) died. The result revealed that CRP/ALB ratio on day 2 was associated with SAP [odds ratio (OR): 1.74, 95% confidence interval (CI): 1.32 to 2.29], death (OR: 1.73, 95%CI: 1.24 to 2.41), pancreatic necrosis (OR: 1.28, 95%CI: 1.08 to 1.50), and organ failure (OR: 1.43, 95%CI: 1.18 to 1.73) in AP patients. Similarly, CRP/ALB on day 3 was related to a higher risk of SAP (OR: 1.50, 95%CI: 1.24 to 1.81), death (OR: 1.8, 95%CI: 1.34 to 2.65), pancreatic necrosis (OR: 1.22, 95%CI: 1.04 to 1.42), and organ failure (OR: 1.21, 95%CI: 1.04 to 1.41). The predictive value of CRP/ALB ratio for pancreatic necrosis was lower than that of MCTSI, for organ failure was lower than that of Ranson and BISAP, and for death was higher than that of MCTSI.
Conclusion: The CRP/ALB ratio may be a novel but promising, easily measurable, reproducible, non-invasive prognostic score that can be used to predict SAP, death, pancreatic necrosis, and organ failure in AP patients, which can be a supplement of Ranson, MCTSI, and BISAP scores.
Acute pancreatitis (AP) is an inflammatory disease of the pancreas characterized by acute abdominal pain and elevated serum pancreatin, which leads to subsequent pancreatic autodigestion, edema, hemorrhage, necrosis, and even distal organ dysfunction (1, 2). The majority of AP cases are mild and with an acceptable prognosis, whereas approximately 20% of AP patients develop moderate-to-severe AP (MSAP) and even severe AP (SAP), characterized by rapid progression, poor prognosis, and a high mortality rate of 30% (3). The long hospital stays and a series of financial burdens brought by SAP make us have to keep alert to the severity and poor prognosis of AP (4, 5). Early recognition of disease severity, as well as early evaluation of prognostic factors, may be helpful for early therapeutic intervention so as to improve survival and prognosis.
At present, a variety of scores are used in the assessment of AP prognoses, such as Ranson, modified computed tomography severity index (MCTSI), and Bedside Index of Severity in Acute Pancreatitis (BISAP) scores (6–8). However, the calculation of those scores requires the use of numerous parameters and very complicated algorithms, which limits their use in clinical practice (8). Therefore, there is an urgent need for a simpler, faster, real-time tool to predict disease prognosis. In several studies, serum markers such as C-reactive protein (CRP) and albumin (ALB) have been reported to be associated with prognosis in AP (9–11). However, their predictive value is unsatisfactory when used alone (12). Accumulating studies reported that CRP/ALB ratio could predict the prognosis of diseases associated with inflammatory response (10, 13, 14). A study by Zhao et al. (12) demonstrated that the admission CRP/ALB ratio was significantly higher in the re-operation of AP patients under debridement. The CRP/ALB ratio may be associated with the prognosis of AP. Kaplan et al. (15) reported that the CRP/ALB ratio could predict the mortality of AP patients. However, to date, the relationship between the CRP/ALB ratio and severity of AP, such as SAP, organ failure, and pancreatic necrosis in patients with AP remains unclear. It is necessary to select a simple, non-invasive method to predict the severity of AP in order to help to improve survival.
Herein, we investigated the predictive value of the CRP/ALB ratio for the determination of severity and prognosis in patients who were with an AP diagnosis and compared the predictive value of the CRP/ALB ratio with the Ranson score, MCTSI score, and BISAP score.
In this cohort study, we reviewed the data of AP patients in the Shanghai Tenth People's Hospital of Tongji University between August 2018 and August 2020. Totally, 284 patients were enrolled in the study. The inclusion criteria were: (1) all AP patients who were admitted to our hospital within 72 h of onset met the International Association of Pancreatology (IAP)/American Pancreatic Association (APA) guidelines (16); (2) patient was admitted to hospital within 72 h of onset, and abdominal enhanced CT examination was performed within 2–7 days of admission. The exclusion criteria were: (1) presence of malignant tumor, recent other infectious diseases, diseases of the blood system, rheumatic immune diseases, and organ dysfunction; (2) age <18 years; (3) acute onset of chronic pancreatitis (on the basis of patient history and CT imaging); (3) pregnant or lactation population; (4) combination with other digestive system diseases; (5) incomplete medical records. All procedures were implemented in accordance with the principles of the Declaration of Helsinki and the design of the work was reviewed and approved by our Ethics Committee (SHYS-IEC-5.0/22K161/P01). Once data is truly anonymized and individuals are no longer identifiable, patient consent was waived.
The clinical information of AP patients who met the inclusion criteria was collected from the medical record, including: (1) Information at the time of hospitalization: gender (male, female), age (years), etiology (gallstone, hyperlipidemia, alcohol, and others); (2) scoring system: Ranson score, MCTSI score, and BISAP score were evaluated for each patient, respectively; (3) laboratory indicators: white blood cell (WBC, 109/L), hemoglobin (Hb, g/L), neutrophil counts (109/L), lymphocyte counts (109/L), platelet counts (109/L), platelet to lymphocyte ratio (PLR), neutrophil to lymphocyte ratio (NLR), alanine transaminase (ALT, U/L), aspartate aminotransferase (AST, U/L), direct bilirubin (DB, umol/L), total bilirubin (TB, umol/L), gamma-glutamyl transpeptidase (GGT, U/L), alkaline phosphatase (AKP, U/L), amylase (AMY, U/L), lipase (LPS, U/L), blood urea nitrogen (BUN, mmol/L), serum creatinine (Scr, umol/L), calcium (Ca, mmol/L), and lactate dehydrogenase (LDH, U/L) after admission for all patients in this study; (4) patient outcomes: SAP, infectious pancreatic necrosis, organ failure, and death.
All AP patients who were admitted to our hospital within 72 h of onset met the guidelines for the Diagnosis and Treatment of Acute Pancreatitis revised in 2019 by the Pancreatic Surgical Science Section of the Chinese Medical Association Surgery Branch, which requires at least two conditions: (1) abdominal pain highly suggestive of AP; (2) elevations in serum amylase and/or lipase to more than 3 times the upper limit of normal; (3) the presence of characteristic radiological findings [ultrasonography or computerized tomography (CT)] of AP.
The definition of severity of AP was used as follows: (1) mild AP (MAP): no organ failure and no local or systemic complications; (2) MSAP: organ failure that resolves within 48 h (transient organ failure) and/or local or systemic complications without persistent organ failure; and (3) SAP: persistent single or multiple organ failure (>48 h). Following the modified Marshall scoring system, organ failure was defined as a score of 2 or more for one of three organ systems (respiratory, renal, and cardiovascular) (17). Death was defined as death during hospitalization.
According to the clinical outcome, all patients were divided into the death group (n = 11) and the survival group (n = 273). Patients were also divided into the SAP group (n = 35) and the non-SAP group (n = 249). According to the presence or absence of organ failure, the two groups were divided into the organ failure group (n = 29) and the without organ failure group (n = 255). Abdominal enhanced CT scan results confirmed the occurrence of pancreatic necrosis in 30 patients with pancreatitis in this study, and AP patients were divided into pancreatic necrosis group (n = 29) and non-pancreatic necrosis group (n = 255).
Normally distributed data described with mean ± SD, an unpaired student t-test was used for comparison between groups. Non-normally distributed data defined with median and interquartile range [M (Q1, Q3)] were compared between the two groups using the Mann–Whitney U test. Categorical variables expressed as numbers and percentages [n (%)] were compared between the two groups using the χ2 test or Fisher's exact test. Differential analysis was used to screen for possible confounding factors. Univariable logistic regression and multivariate logistic regression analysis were utilized to determine the effects of CRP/ALB ratio, Ranson score, MCTSI score, and BISAP score on SAP, infectious pancreatic necrosis, organ failure, and death. The predictive values of CRP/ALB ratio, Ranson score, MCTSI score, and BISAP score on SAP, infectious pancreatic necrosis, organ failure, and death were examined with the area under the curve (AUC) of the receiver operator characteristic (ROC) curve analysis. DeLong test was used to compare the AUCs between CRP/ALB ratio, Ranson score, MCTSI score, and BISAP score. Statistical power was tested for outcome with a smaller sample size. Pairwise comparisons were performed with a Bonferroni correction for multiple comparisons. The P value < 0.05 was considered to be statistically significant. SAS 9.4 software (SAS Institute, Cary, NC, United States) was performed to compute the statistical analysis.
The study population consisted of 284 patients, 123 females (43.31%) and 161 (56.69%) males. The median age of the patients was 59.50 (IQR 39.00–70.00) years. The median hospitalization length of the patients was 9 (IQR 8–13) days. In terms of etiology, 154 (54.23%) of the patients were due to gallstone, 69 (24.30%) were due to hyperlipidemia, 13 (4.58%) due to alcohol, and 61 (21.48%) due to other etiologies (autoimmune diseases, metabolic diseases, trauma or postoperative, drugs, viral infection, etc., or the specific etiologies of pancreatitis cannot be determined). The baseline characteristics of patients are summarized in Table 1.
Thirty-five patients had SAP. There were statistically significant differences in hyperlipidemia, WBC, neutrophil counts, PLR, NLR, AMY, BUN, Scr, Ca, and LDH between patients with and without SAP, which may be the factors affecting SAP. Differences in population characteristics between patients with and without SAP are shown in Table 1.
Finally, 11 patients died. Ranson, MCTSI, BISAP, WBC, neutrophil counts, PLR, NLR, AMY, BUN, Scr, Ca, LDH, CRP, ALB, CRP/ALB on day 2, and CRP, ALB, CRP/ALB on day 3 may be associated with the death of AP. Differences in population characteristics between death and survival are depicted in Table 2.
Pancreatic necrosis occurred in 29 patients. The AP patients with pancreatic necrosis were younger than patients without pancreatic necrosis. Hyperlipidemia may be the factor affecting pancreatic necrosis in AP patients. Higher levels of Ranson, MCTSI, BISAP, WBC, neutrophil counts, NLR, BUN, Ca, LDH, CRP, CRP/ALB were observed in AP patients with pancreatic necrosis. The differences between patients with and without pancreatic necrosis are shown in Table 3.
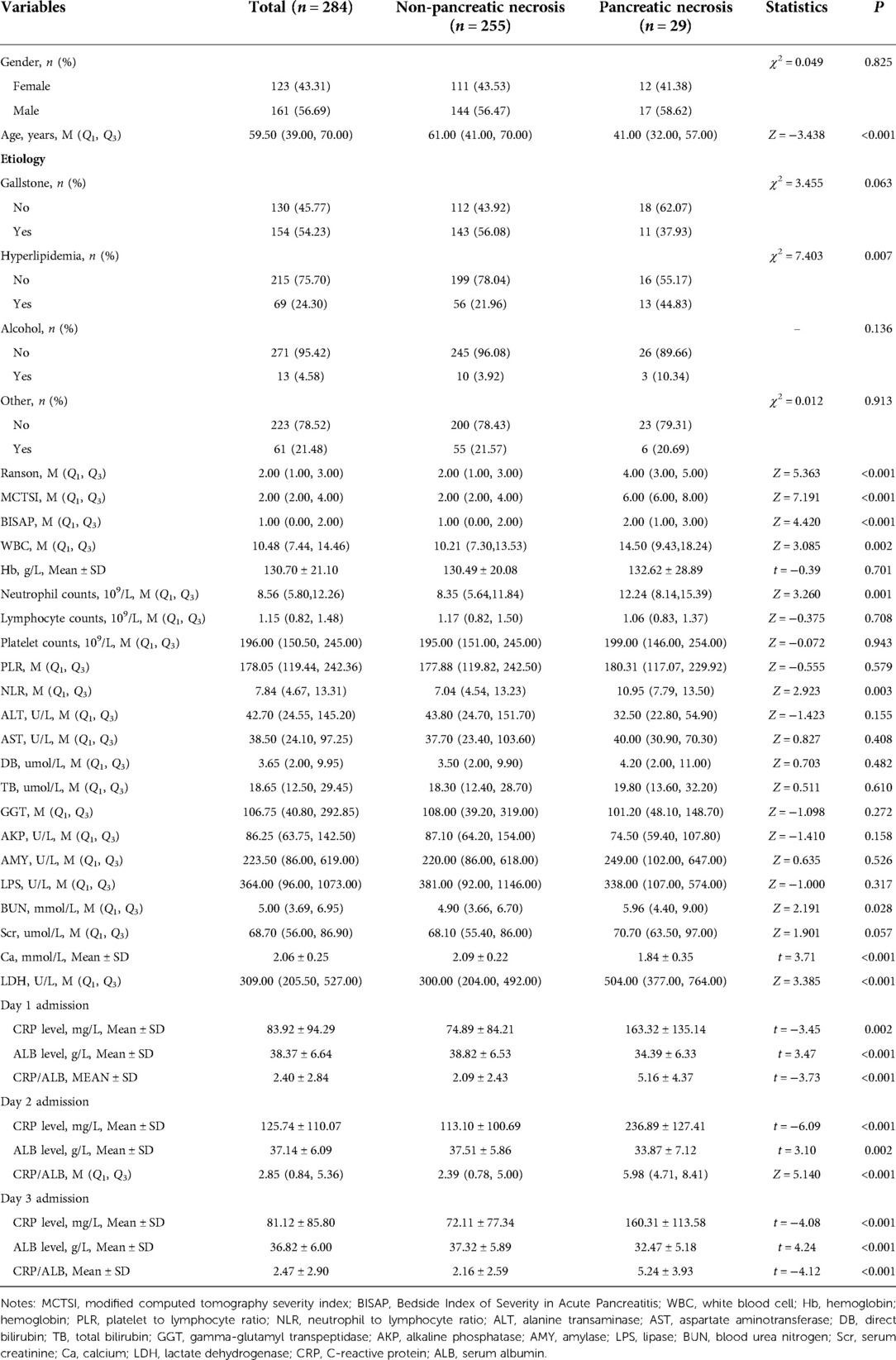
Table 3. Differences of population characteristics between patients with and without pancreatic necrosis.
Table 4 shows the difference in population characteristics between organ failure and without organ failure. There were statistically significant differences in WBC, NLR, AST, DB, BUN, Scr, Ca and LDH between AP patients with organ failure, and those without organ failure.
CRP/ALB ratio on day 1 was related to SAP [odds ratio (OR): 1.32, 95% confidence interval (CI): 1.12 to 1.55, P < 0.001), pancreatic necrosis (OR: 1.20, 95% CI: 1.04 to 1.38, P = 0.010), however, CRP/ALB ratio on day 1 was not related to death (OR: 1.22, 95% CI: 0.98 to 1.53, P = 0.078), and organ failure (OR: 1.09, 95% CI: 0.95 to 1.26, P = 0.220). CRP/ALB ratio on day 2 was associated with SAP (OR: 1.74, 95% CI: 1.32 to 2.29, P < 0.001), death (OR: 1.73, 95%CI: 1.24 to 2.41, P = 0.001), pancreatic necrosis (OR: 1.28, 95%CI: 1.08 to 1.50, P = 0.003), and organ failure (OR: 1.43, 95%CI: 1.18 to 1.73, P < 0.001) in AP patients. Similarly, CRP/ALB on day 3 was related to higher risk of SAP (OR: 1.50, 95%CI: 1.24 to 1.81, P < 0.001), death (OR: 1.8, 95%CI: 1.34 to 2.65, P < 0.001), pancreatic necrosis (OR: 1.22, 95%CI: 1.04 to 1.42, P = 0.014), and organ failure (OR: 1.21, 95%CI: 1.04 to 1.41, P = 0.015). Ranson, MCTSI, and BISAP scores could also predict the risk of SAP, death, pancreatic necrosis, and organ failure in AP patients. Evaluations of the prognosis values of CRP /ALB, Ranson, MCTSI, and BISAP in AP patients are presented in Table 5.
The predictive values of CRP/ALB ratio were compared with Ranson, MCTSI, and BISAP for predicting SAP, death, pancreatic necrosis, and organ failure among AP patients. The calculated AUC of the CRP/ALB ratio for SAP was 0.753 (95% CI: 0.655 to 0.852) on day 1, lowering than the AUC of Ranson (0.932, 95% CI: 0.896 to 0.967), MCTSI (0.899, 95% CI: 0.838 to 0.960), BISAP (0.914, 95% CI: 0.871 to 0.956), day 2 (0.895, 95% CI: 0.835 to 0.954), and day 3 (0.895, 95% CI: 0.839 to 0.950). The predictive value of CRP/ALB ratio on day 1 (AUC: 0.715, 95% CI: 0.603 to 0.828), day 2 (AUC: 0.791, 95% CI: 0.699 to 0.883), and day 3 (AUC: 0.783, 95% CI: 0.697 to 0.869) for pancreatic necrosis was lower than that of MCTSI (AUC: 0.892, 95% CI: 0.831 to 0.953). The predictive value of CRP/ALB ratio for organ failure on day 1 (AUC: 0.698, 95% CI: 0.675 to 0.891), day 2 (AUC: 0.783, 95% CI: 0.675 to 0.891), and day 3 (AUC: 0.793, 95% CI: 0.699 to 0.887) was lower than that of Ranson (AUC: 0.953, 95% CI: 0.928 to 0.979) and BISAP (AUC: 0.941, 95% CI: 0.905 to 0.976). The calculated AUC of the CRP/ALB ratio for death on day 3 was 0.943 (95% CI: 0.891 to 0.995), higher than the calculated AUC of the MCTSI (0.871, 95% CI: 0.763 to 0.978). Comparison outcomes of predictive values between CRP/ALB ratio and Ranson, MCTSI, and BISAP scores are shown in Table 6. Statistical power result indicated that the small sample size of CRP/ALB ratio on day 3 for death has not lowered statistical power. The predictive values of CRP/ALB ratio and Ranson, MCTSI, and BISAP are shown in Figure 1.
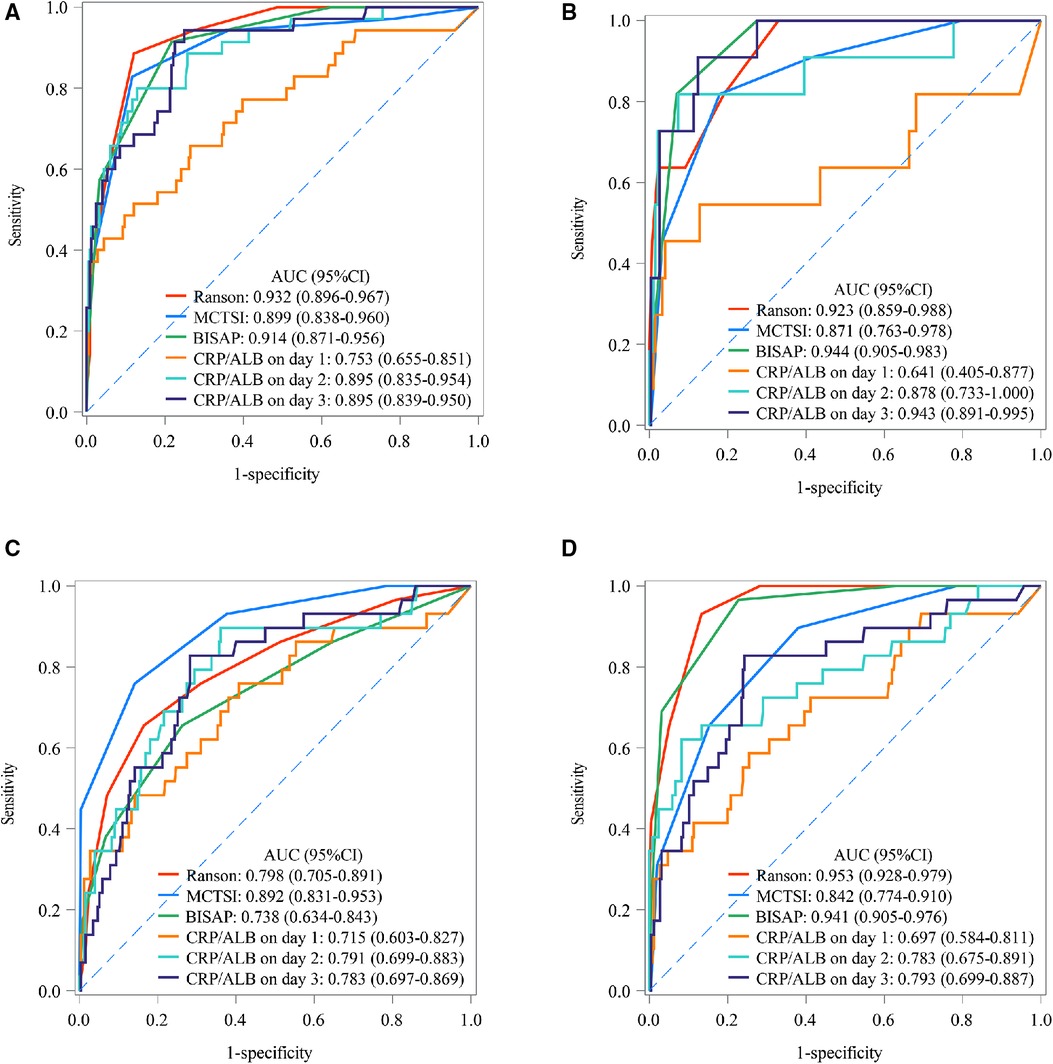
Figure 1. The AUC of CRP/ALB ratio, ranson, MCTSI, and BISAP in SAP, death, pancreatic necrosis, and organ failure; (A) SAP; (B) death; (C) pancreatic necrosis; (D) organ failure. MCTSI, modified computed tomography severity index; BISAP, bedside index of severity in acute pancreatitis; AUC, area under the curve; CRP, C-reactive protein; ALB, serum albumin; SAP, severe acute pancreatitis.
AP is a relatively common condition worldwide and is characterized by acute and severe upper abdominal pain (18). Accurate and timely identification of the severity and prognostic factors of AP patients is critical. Currently, Ranson Score, BISAP, and MCTSI were frequently used for early identification of the severity of AP (19). Each score has specific applications, but all have limitations. A simple, repeatable, and non-invasive laboratory procedure, is needed to predict the severity and prognostic factors of AP patients. In this study, we used the CRP/ALB ratio to predict SAP, death, pancreatic necrosis, and organ failure among AP patients. CRP/ALB ratio on day 1 was related to SAP and pancreatic necrosis, nevertheless, CRP/ALB ratio on day 1 was not related to death and organ failure. CRP/ALB ratio on day 2 and day 3 was associated with SAP, death, pancreatic necrosis, and organ failure in AP patients. The findings demonstrated the predictive value of the CRP/ALB ratio for the determination of the severity and prognosis of AP. However, CRP/ALB ratio may not superior to the Ranson, MCTSI, and BISAP scores. The predictive value of the CRP/ALB ratio may be helpful to the assessment of SAP and prognosis in AP patients.
CRP is a valuable marker of inflammation in the acute phase that is produced during infection, ischemia, and trauma and is synthesized by liver cells, smooth muscle cells, macrophages, endothelial cells, lymphocytes, and adipocytes in response to the regulation of pro-inflammatory cytokines, especially interleukin-6 (10, 20). Because of its short half-life and high sensitivity, CRP is often used for the detection and evaluation of inflammation (21). Serum ALB levels reflect patients' nutritional status; a low serum ALB level indicates a state of malnutrition (22). In previous studies, ALB has been shown to be inversely associated with inflammation severity, disease prognoses, and mortality in AP (23). The CRP/ALB ratio, a combined index of the ALB and CRP levels, is known to be related more consistently to prognosis than a single marker, accurately reflecting the degree of inflammation or nutritional deficiency (24–26). Many studies have investigated the prognostic value of the CRP/ALB ratio in a variety of diseases (14, 27, 28). The present study showed that day 2 and day 3 CRP/ALB ratio could predict the SAP and prognostic outcome of AP. A study by Wang et al. (29) reported that high levels of CRP and low ALB levels were associated with in-hospital mortality in patients with SAP. Zhao et al. found that the admission CRP/ALB ratio was significantly higher in the re-operation of AP patients under debridement (12). A study by Kaplan et al. indicated that the CRP/ALB ratio could predict the mortality of AP patients with a sensitivity of 92.1% and specificity of 58.0% (15). Nevertheless, in this study, the day 1 CRP/ALB ratio has not been confirmed to be useful to predict mortality and organ failure in AP patients. We speculate the possible reason for this result is the lower CRP/ALB ratio on the first day. When infection and inflammation occur, the level of serum CRP rises rapidly within a few hours, and reaches the peak in 24–48 h, however, serum ALB might be declined a few days after the initiation of pancreatic inflammation (30). Thus, combined CRP/ALB ratio could be increased along with progression of pancreatic inflammation due to aforementioned scenario. The cut-off value of the CRP/ALB ratio on the prognosis of AP needs further study. Moreover, the day 3 CRP/ALB ratio showed a better predictive performance in predicting mortality of AP patients compared with the day 2 CRP/ALB ratio. Similarly, Sun et al. found that CRP/ALB at 72 h may be one of the best indicators for the assessment of clinical therapy and prognosis of patients with sepsis (31). Monitoring of ambulatory CRP/ALB level changes in patients with AP may be necessary. All in all, CRP/ALB ratio may be an easy-to-measure, reproducible, non-invasive tool to predict the SAP and the prognosis of patients with AP.
Our study could not demonstrate that the CRP/ALB ratio is superior to the Ranson, MCTSI, and BISAP scores. The Ranson score is one of the earliest scores to assess the severity of AP that consists of 11 indicators that are assessed at admission and 48 h after admission (32). However, some of these indicators are not routinely collected during the early stages of AP, making early prediction difficult. The BISAP score that was introduced in 2008, consists of five indicators to predict AP mortality within 24 h of admission, which is simple to use, however, has low SAP predictive sensitivity (33, 34). In 2004, Mortele et al. formulated the MCTSI including a simplified evaluation of peripancreatic inflammation and the extent of pancreatic parenchymal necrosis and incorporated the extrapancreatic complications (vascular, gastrointestinal, and extrapancreatic parenchymal complications as well as the presence of pleural effusion and/or ascites) in the assessment (35). These indicators are closely related to the prognostic indicators of AP patients. Nevertheless, the calculation of the MCTSI score still requires the use of numerous parameters. A clinical course of AP greatly varies between patients and this makes the accurate classification and prediction of disease severity very important for both clinical decision-making and research recruitment (36). Our study demonstrated the predictive value of CRP/ALB in predicting SAP. The easily calculated CRP/ALB ratio may allow the estimation of the risk of SAP and adverse prognosis outcomes of AP, providing additional information that may facilitate the estimation of a patient's overall condition.
This study provides a simpler and more feasible tool for assessing the severity and prognosis of patients with AP. CRP/ALB value can help identify high-risk patients in advance and remind medical staff to strengthen ward care for high-risk patients. The CRP/ALB value can also distinguish MAP from SAP, and the results of this study can provide a reference for hospitals and health decision makers to develop more effective treatment strategies for AP gradient. However, the current study had some limitations. Firstly, it was a single-center retrospective study with a small number of cases included, which may limit the prognostic value of the CRP/ALB ratio. Secondly, we did not find an association between CRP/ALB ratio on day 1 and death and organ failure in AP patients. Thirdly, the lack of external verification in this study may limit the generalization of our results. Finally, from our findings, the CRP/ALB ratio may not be better than Ranson, MCTSI, and BISAP scores. The predictive value of the CRP/ALB ratio on SAP and the prognosis of AP needs to be further clarified.
CRP/ALB ratio could predict SAP, death, pancreatic necrosis, and organ failure for AP, which may be a supplementary tool for the evaluations of SAP and prognosis for AP patients.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The studies involving human participants were reviewed and approved by Shanghai Tenth People's Hospital of Tongji University. The patients/participants provided their written informed consent to participate in this study.
YZ, WX, YL, YZ and YZ designed the study. YZ, WX and YL wrote the manuscript. WC collected, analyzed and interpreted the data. YZ and YZ critically reviewed, edited and approved the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Klochkov A, Kudaravalli P, Lim Y, Sun Y. Alcoholic pancreatitis. In: Statpearls. Treasure Island, FL: StatPearls Publishing(2022).
2. Tang Y, Sun M, Liu Z. Phytochemicals with protective effects against acute pancreatitis: a review of recent literature. Pharm Biol. (2022) 60(1):479–90. doi: 10.1080/13880209.2022.2039723
3. Leppäniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, et al. Executive summary: WSES guidelines for the management of severe acute pancreatitis. J Trauma Acute Care Surg. (2020) 88(6):888–90. doi: 10.1097/ta.0000000000002691
4. Karim T, Jain A, Kumar V, Kumar RB, Kumar L, Patel M. Clinical and severity profile of acute pancreatitis in a hospital for low socioeconomic Strata. Indian J Endocrinol Metab. (2020) 24(5):416–21. doi: 10.4103/ijem.IJEM_447_20
5. Yang DJ, Li M, Yue C, Hu WM, Lu HM. Development and validation of a prediction model for deep vein thrombosis in older non-mild acute pancreatitis patients. World J Gastrointest Surg. (2021) 13(10):1258–66. doi: 10.4240/wjgs.v13.i10.1258
6. Chatterjee R, Parab N, Sajjan B, Nagar VS. Comparison of acute physiology and chronic health evaluation II, modified computed tomography severity Index, and bedside Index for severity in acute pancreatitis score in predicting the severity of acute pancreatitis. Indian J Crit Care Med. (2020) 24(2):99–103. doi: 10.5005/jp-journals-10071-23343
7. Teng TZJ, Tan JKT, Baey S, Gunasekaran SK, Junnarkar SP, Low JK, et al. Sequential organ failure assessment score is superior to other prognostic indices in acute pancreatitis. World J Crit Care Med. (2021) 10(6):355–68. doi: 10.5492/wjccm.v10.i6.355
8. Tahir H, Rahman S, Habib Z, Khan Y, Shehzad S. Comparison of the accuracy of modified CT severity Index score and neutrophil-to-lymphocyte ratio in assessing the severity of acute pancreatitis. Cureus. (2021) 13(8):e17020. doi: 10.7759/cureus.17020
9. Lu L, Feng Y, Liu YH, Tan HY, Dai GH, Liu SQ, et al. The systemic immune-inflammation Index may be a novel and strong marker for the accurate early prediction of acute kidney injury in severe acute pancreatitis patients. J Invest Surg. (2022) 35(5):962–6. doi: 10.1080/08941939.2021.1970864
10. Park J, Lim SJ, Choi HJ, Hong SH, Park CS, Choi JH, et al. Predictive utility of the C-reactive protein to albumin ratio in early allograft dysfunction in living donor liver transplantation: a retrospective observational cohort study. PloS One. (2019) 14(12):e0226369. doi: 10.1371/journal.pone.0226369
11. Hong W, Lin S, Zippi M, Geng W, Stock S, Basharat Z, et al. Serum albumin is independently associated with persistent organ failure in acute pancreatitis. Can J Gastroenterol Hepatol. (2017) 2017:5297143. doi: 10.1155/2017/5297143
12. Zhao Z, Yu Y, Xie R, Yang K, Xu D, Li L, et al. Prognostic value of the creatinine-albumin ratio in acute pancreatitis debridement. BMC Surg. (2020) 20(1):322. doi: 10.1186/s12893-020-00991-6
13. Liu Z, Jin K, Guo M, Long J, Liu L, Liu C, et al. Prognostic value of the CRP/alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol. (2017) 24(2):561–8. doi: 10.1245/s10434-016-5579-3
14. Kim MH, Ahn JY, Song JE, Choi H, Ann HW, Kim JK, et al. The C-reactive protein/albumin ratio as an independent predictor of mortality in patients with severe sepsis or septic shock treated with early goal-directed therapy. PloS One. (2015) 10(7):e0132109. doi: 10.1371/journal.pone.0132109
15. Kaplan M, Ates I, Akpinar MY, Yuksel M, Kuzu UB, Kacar S, et al. Predictive value of C-reactive protein/albumin ratio in acute pancreatitis. Hepatobiliary Pancreat Dis Int. (2017) 16(4):424–30. doi: 10.1016/s1499-3872(17)60007-9
16. Working Group IAP/APA Acute Pancreatitis Guideline. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. (2013) 13(4 Suppl 2):e1–15. doi: 10.1016/j.pan.2013.07.063
17. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. (2013) 62(1):102–11. doi: 10.1136/gutjnl-2012-302779
18. Wang L, Zeng YB, Chen JY, Luo Q, Wang R, Zhang R, et al. A simple new scoring system for predicting the mortality of severe acute pancreatitis: a retrospective clinical study. Medicine (Baltimore). (2020) 99(23):e20646. doi: 10.1097/md.0000000000020646
19. Silva-Vaz P, Abrantes AM, Morgado-Nunes S, Castelo-Branco M, Gouveia A, Botelho MF, et al. Evaluation of prognostic factors of severity in acute biliary pancreatitis. Int J Mol Sci. (2020) 21(12):4300. doi: 10.3390/ijms21124300
20. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. (2018) 9:754. doi: 10.3389/fimmu.2018.00754
21. Lee KJ, Kim HM, Choi JS, Kim YJ, Kim YS, Cho JH. Comparison of predictive systems in severe acute pancreatitis according to the revised Atlanta classification. Pancreas. (2016) 45(1):46–50. doi: 10.1097/mpa.0000000000000433
22. Saito H, Kono Y, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, et al. Postoperative Serum albumin is a potential prognostic factor for older patients with gastric cancer. Yonago Acta Med. (2018) 61(1):72–8. doi: 10.33160/yam.2018.03.010
23. Li S, Zhang Y, Li M, Xie C, Wu H. Serum albumin, a good indicator of persistent organ failure in acute pancreatitis. BMC Gastroenterol. (2017) 17(1):59. doi: 10.1186/s12876-017-0615-8
24. Oh TK, Ji E, Na HS, Min B, Jeon YT, Do SH, et al. C-Reactive protein to albumin ratio predicts 30-day and 1-year mortality in postoperative patients after admission to the intensive care unit. J Clin Med. (2018) 7(3):39. doi: 10.3390/jcm7030039
25. Zhang J, Zhang C, Li Q, Zhang J, Gu X, Zhao W, et al. C-Reactive protein/albumin ratio is an independent prognostic predictor of survival in advanced cancer patients receiving palliative care. J Palliat Med. (2019) 22(12):1536–45. doi: 10.1089/jpm.2019.0102
26. Wang W, Ren D, Wang CS, Li T, Yao HC, Ma SJ. Prognostic efficacy of high-sensitivity C-reactive protein to albumin ratio in patients with acute coronary syndrome. Biomark Med. (2019) 13(10):811–20. doi: 10.2217/bmm-2018-0346
27. Xu H, Hu L, Wei X, Niu J, Gao Y, He J, et al. The predictive value of preoperative high-sensitive C-reactive protein/albumin ratio in systemic inflammatory response syndrome after percutaneous nephrolithotomy. J Endourol. (2019) 33(1):1–8. doi: 10.1089/end.2018.0632
28. Zhou T, Zhao Y, Zhao S, Yang Y, Huang Y, Hou X, et al. Comparison of the prognostic value of systemic inflammation response markers in small cell lung cancer patients. J Cancer. (2019) 10(7):1685–92. doi: 10.7150/jca.29319
29. Wang X, Cui Z, Li H, Saleen AF, Zhang D, Miao B, et al. Nosocomial mortality and early prediction of patients with severe acute pancreatitis. J Gastroenterol Hepatol. (2010) 25(8):1386–93. doi: 10.1111/j.1440-1746.2010.06376.x
30. Seo HS. The role and clinical significance of high-sensitivity C-reactive protein in cardiovascular disease. Korean Circ J. (2012) 42(3):151–3. doi: 10.4070/kcj.2012.42.3.151
31. Sun R, Sun X, Yang H, Liu Q. Retrospective analysis of serum C-reactive protein/albumin ratio for the prognosis of the adult patients with sepsis. Zhonghua Wei Zhong Bing Ji Iiu Yi Xue. (2016) 28(5):413–7. PMID: 29920034
32. Qiu L, Sun RQ, Jia RR, Ma XY, Cheng L, Tang MC, et al. Comparison of existing clinical scoring systems in predicting severity and prognoses of hyperlipidemic acute pancreatitis in Chinese patients: a retrospective study. Medicine (Baltimore). (2015) 94(23):e957. doi: 10.1097/md.0000000000000957
33. Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. (2008) 57(12):1698–703. doi: 10.1136/gut.2008.152702
34. Yang YX, Li L. Evaluating the ability of the bedside Index for severity of acute pancreatitis score to predict severe acute pancreatitis: a meta-analysis. Medical principles and practice: international journal of the Kuwait university. Health Science Centre. (2016) 25(2):137–42. doi: 10.1159/000441003
35. Mortele KJ, Wiesner W, Intriere L, Shankar S, Zou KH, Kalantari BN, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol. (2004) 183(5):1261–5. doi: 10.2214/ajr.183.5.1831261
Keywords: c-reactive protein/albumin ratio, Ranson score, MCTSI score, BISAP score, prognosis, acute pancreatitis
Citation: Zhao Y, Xia W, Lu Y, Chen W, Zhao Y and Zhuang Y (2023) Predictive value of the C-reactive protein/albumin ratio in severity and prognosis of acute pancreatitis. Front. Surg. 9:1026604. doi: 10.3389/fsurg.2022.1026604
Received: 24 August 2022; Accepted: 22 November 2022;
Published: 10 January 2023.
Edited by:
Riccardo Casadei, University of Bologna, ItalyReviewed by:
Artur Rebelo, University Hospital in Halle, Germany© 2023 Zhao, Xia, Lu, Chen, Zhao and Zhuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zhao emhhb3lhbjA4MDRAb3V0bG9vay5jb20= Yugang Zhuang emh1YW5neXVnYW5nQG91dGxvb2suY29t
†These authors share first authorship
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.