- 1National Local Joint Engineering Research Center for Precision Surgery and Regenerative Medicine, First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
- 2Department of Oncology, Xi'an No.3 Hospital, Xi'an, China
- 3Department of Hepatobiliary Surgery, First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
Background and Aims: Intrahepatic cholangiocarcinoma has an increasing global incidence and mortality rate. Hepatectomy is still the most effective curative treatment for patients with ICC, but the prognosis of patients with ICC is still poor even after curative resection. This study aimed to incorporate important factors obtained from SEER database to construct and validate a nomogram for predicting the cancer-specific survival of patients with ICC after hepatectomy.
Methods: We obtained patient data from SEER database. The nomogram was constructed base on six prognostic factors for predicting CSS rates in ICC patients. The nomogram was validated by C-index, ROC curve and calibration curves.
Results: A total of 919 patients with ICC after hepatectomy between 2000 and 2018 were included in this study. A nomogram based on six independent prognostic factors (Black race, AJCC T, AJCC N, AJCC M, chemotherapy and PLNR ≥ 0.15) was developed for the prediction of CSS at 3 and 5 years. The C-index of the nomogram and AJCC stage system were 0.709 and 0.657 in the training cohort respectively. The 3- and 5-year AUCs of nomogram were 0.744 and 0.75 in the training cohort. The calibration plots indicated that there was good agreement between the actual observations and predictions.
Conclusions: In conclusion, we constructed and validated a nomogram for predicting the 3- and 5-year CSS in ICC patients after hepatectomy. We have confirmed the precise calibration and acceptable discrimination power of our nomogram. The predictive power of this nomogram may be improved by considering other potential important factors and also by external validation.
Introduction
Intrahepatic cholangiocarcinoma (ICC), second only to hepatocellular carcinoma (HCC) as the most common liver cancer, has an increasing global incidence and mortality rate (1–3). ICC arises from the epithelial layer of the second-degree biliary tract, and has a high degree of malignancy (4, 5). Hepatectomy is still the most effective curative treatment for patients with ICC (6). Unfortunately, even after curative resection, the prognosis of patients with ICC remains poor with a five-year overall survival rate of only 20%–35% (7). Therefore, it is necessary to integrate multiple prognostic factors into an easy-to-use predictive system to better inform surgeons and patients with ICC.
Presently, the most commonly used classification system for patients with ICC is the American Joint Committee on Cancer (AJCC) TNM staging system (8). However, the AJCC system is not easy to apply and it neglects many significant prognostic factors such as race, age, and grade (9). A nomogram is a prognostic predictive tool that creates a user-friendly graph based on a statistical model (10). It can be used to calculate the probability of a clinical outcome by considering the prognostic weight of each factor. This tool is been widely used in clinical decision-making (11–13).
This study aimed to incorporate important factors obtained from the Surveillance, Epidemiology, and End Results (SEER) database to construct and validate a nomogram for predicting cancer-specific survival (CSS) in patients with ICC after hepatectomy. To the best of our knowledge, the nomogram of this study contains the most patients with ICC (919 patients) who have undergone hepatectomy. We compared our model with the AJCC staging system to determine whether it provides a more accurate prediction.
Materials and methods
Ethics statement
Our data were obtained from the SEER database with a signed data agreement (11187-Nov2021). The approval and informed consent of the institutional review committee were exempted since the SEER database is a public database and provides open access for anyone who has registered an account and signed the authorization.
Study population
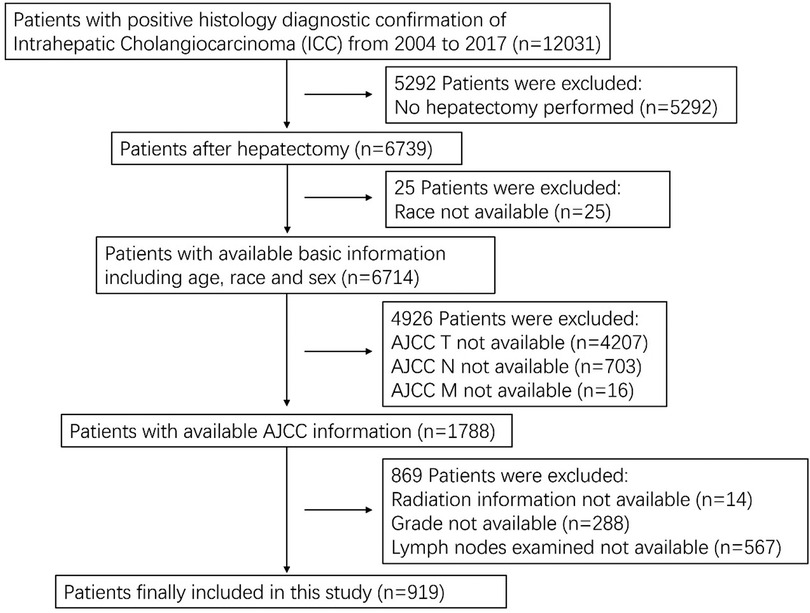
We obtained patient data from the SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000–2018) incidence database using SEER*Stat version 8.3.9. The data were obtained from the International Classification of Diseases for Oncology 3rd edition (ICD-O-3), primary site code C22.1 (intrahepatic bile duct), and histological/behavior code 8160.3 (cholangiocarcinoma). The exclusion criteria were as follows: (1) no hepatectomy performed; (2) incomplete basic information (age, race, and sex); (3) incomplete tumor information (AJCC T, N, M, grade, and lymph nodes examined). The staging system used was the 8th edition of the AJCC system which was calculated from the 6th or 7th edition TNM stages and included other characteristics such as tumor size.
A total of 12,031 patients with ICC were identified and 919 patients who underwent hepatectomy were included in this study. A flow chart for selecting the research samples is shown in Figure 1. The following additional data (variables) were used in the analysis: patient sex, age and race; AJCC staging for the extent of tumor (T), the extent of spread to lymph nodes (N), presence of metastasis (M), tumor grade, radiation (Y/N), chemotherapy (Y/N), number of examined regional nodes, number of positive regional nodes, months of survival, and vital status records. Positive lymph node ratio (PLNR) was calculated by dividing the number of lymph nodes examined by the number of positive lymph nodes.
Statistical analysis
The PLNR cutoff point was analyzed using an X-tile plot (14). A two-population model was implemented to segregate the patients into two groups according to PLNR (PLNR < 0.15 and PLNR ≥ 0.15).
For nomogram construction and validation, we randomly divided all patients with ICC after hepatectomy into training (n = 643) and validation (n = 276) cohorts at a ratio of 7:3 (15, 16). Multivariate cox proportional hazards regression analysis was performed to identify variables (P < 0.05) that significantly affected CSS in the training group. Using these identified prognostic factors, we constructed a nomogram for predicting the three- and five-year CSS rates in patients with ICC after hepatectomy.
The nomogram was internally validated in the training cohort and externally validated in the validation cohort. To evaluate the discriminative ability of the nomogram, we used the concordance index (C-index) and receiver operating characteristic (ROC) curve to assess the area under the curve (AUC) (17, 18). A C-index or AUC of 0.5 indicates a discrimination ability that is no better than chance, whereas an AUC of 1.0 indicates a perfect discrimination ability (19). Calibration curves were constructed using a bootstrap approach, with 500 resamples, to compare the predicted CSS with the CSS observed in the study.
All statistical analyses were performed using SPSS (version 24.0; SPSS, Chicago, IL, USA) and R software (version 4.1.2; http://www.r-project.org/). A P value of < 0.05 was considered to indicate statistical significance.
Results
Patient characteristics
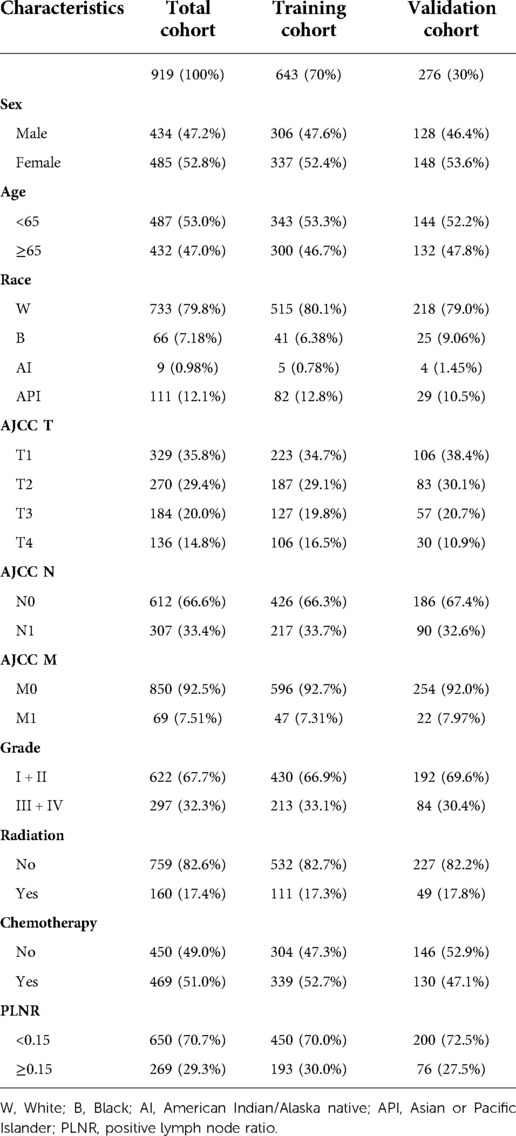
A total of 919 patients with ICC who underwent hepatectomy between the years 2000 and 2018 were included in this study. The training and validation cohorts consisted of 643 and 276 cases, respectively, which were selected using the random split-sample method (split ratio: 7:3). In the total cohort of patients with ICC after hepatectomy, the majority of patients were female (52.8%), under 65 years of age (59.4%) and white (66.5%). Furthermore, most of the patients had T1 (35.8%), N0 (66.6%), and M0 (92.5%), Patients with grade I and II tumor differentiation degree accounted for 67.7% of all cases. A large proportion of the patients did not receive radiation therapy (82.6%) but received chemotherapy (51.0%). Most patients with ICC after hepatectomy had a PLNR of < 0.15. The characteristics of patients with ICC after hepatectomy in the training and validation cohorts were similar to those in the total cohort (Table 1).
Screening for prognostic factors of CSS
We identified six independent prognostic factors in the training cohort based on univariate and multivariate cox proportional hazard regression analyses. Black race [hazard ratio (HR) = 1.885, P < 0.01], AJCC T3/T4 (HR = 2.352/2.819, P < 0.001), AJCC N1 (HR = 1.787, P < 0.01), AJCC M1 (HR = 1.685, P < 0.01), chemotherapy (HR = 0.612, P < 0.001) and PLNR ≥ 0.15 (HR = 1.738, P < 0.01) were significantly associated with CSS in patients with ICC after hepatectomy (Figure 2 and Table 2).

Figure 2. Forest plot of multivariate cox regression analysis for CSS in patients with ICC after hepatectomy. W, White; B, Black; AI, American Indian/Alaska native; API, Asian or Pacific Islander; PLNR, positive lymph node ratio.
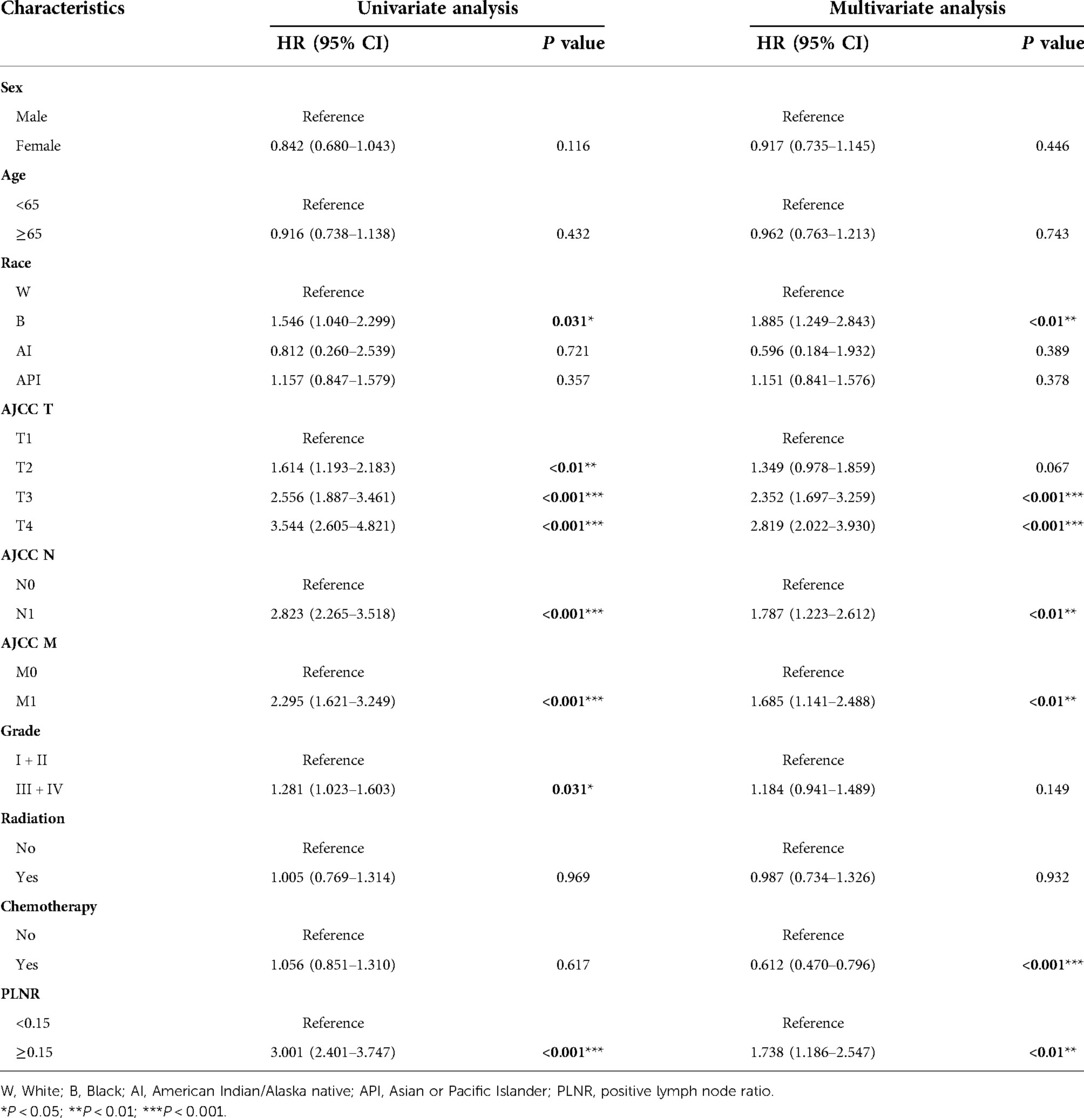
Table 2. Univariate and multivariate cox regression analysis based on all variables for ICC patient cancer-specific survival (training cohort).
Nomogram construction
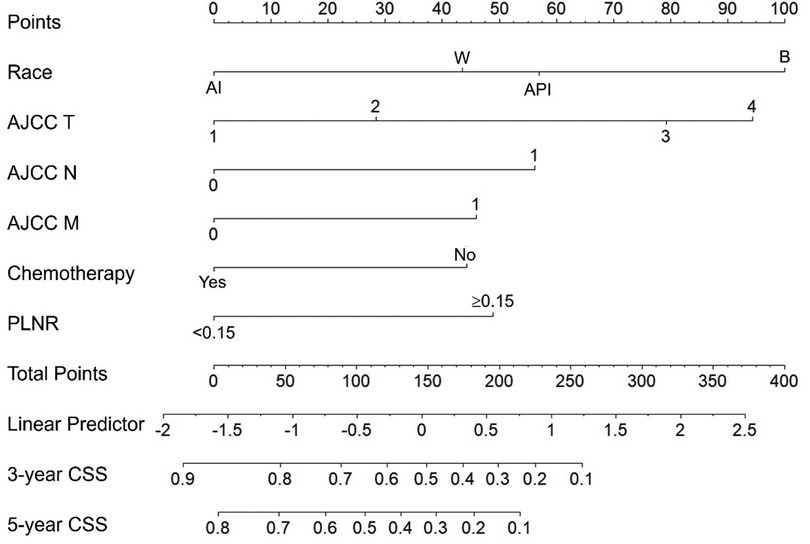
As shown in Figure 3, our nomogram was constructed based on independent prognostic factors in the training cohort to predict 3- and 5- years CSS of patients with ICC after hepatectomy. The nomogram demonstrated that patient race contributed the most to the prognosis, followed by AJCC T, AJCC N, PLNR, AJCC M and chemotherapy. When using the nomogram, every patient with ICC obtained scores based on the level of each independent prognostic factor. The total score was calculated by adding the scores for each factor. The 3- and 5- years CSS could be estimated by the total points.
Nomogram validation
The C-index of the nomogram in this study (training cohort = 0.709, validation cohort = 0.683) was higher than that of the AJCC staging system (training cohort = 0.657, validation cohort = 0.657). And the AUCs of the nomogram were also higher than the AJCC staging system in both training (3-year AUC: 0.744 vs. 0.715, 5-year AUC: 0.75 vs.0.702, Figures 4A,B) and validation (3-year AUC: 0.74 vs. 0.685, 5-year AUC: 0.776 vs. 0.735, Figures 4,D) cohorts. The C-index and AUCs demonstrated the acceptable discrimination performance of our nomogram. Furthermore, the calibration plots for the CSS at 3- and 5-years showed a good agreement between prediction by nomogram and actual observation (Figure 5).
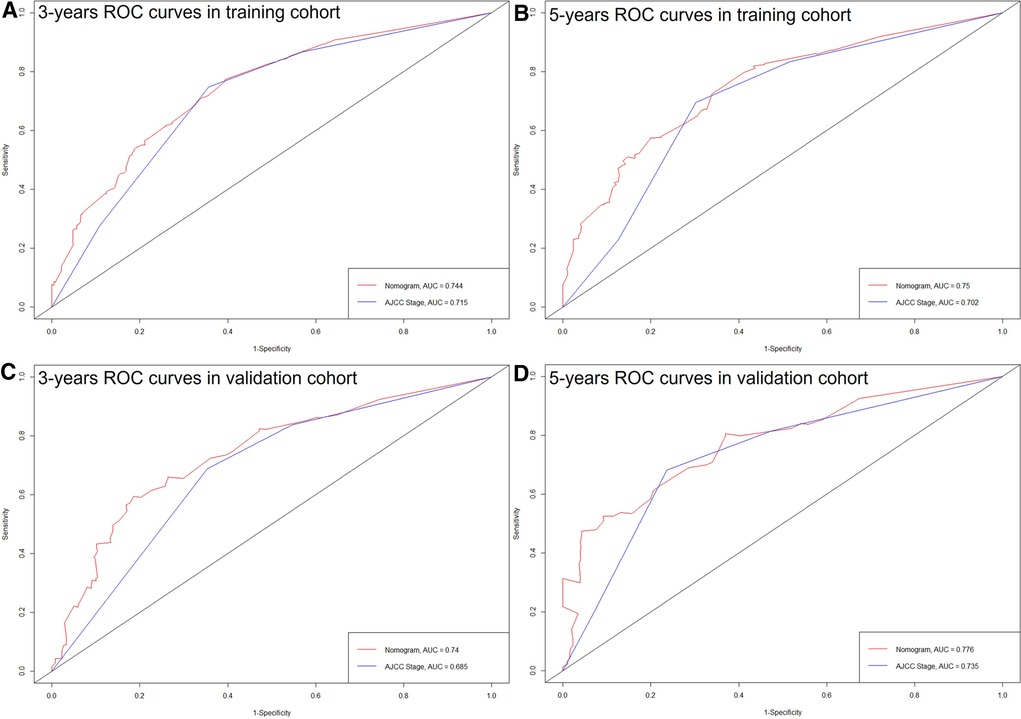
Figure 4. ROC curves of the nomogram and the AJCC staging system. (A) 3 years in training cohort; (B) 5 years in training cohort; (C) 3 years in validation cohort; (D) 5 years in validation cohort.
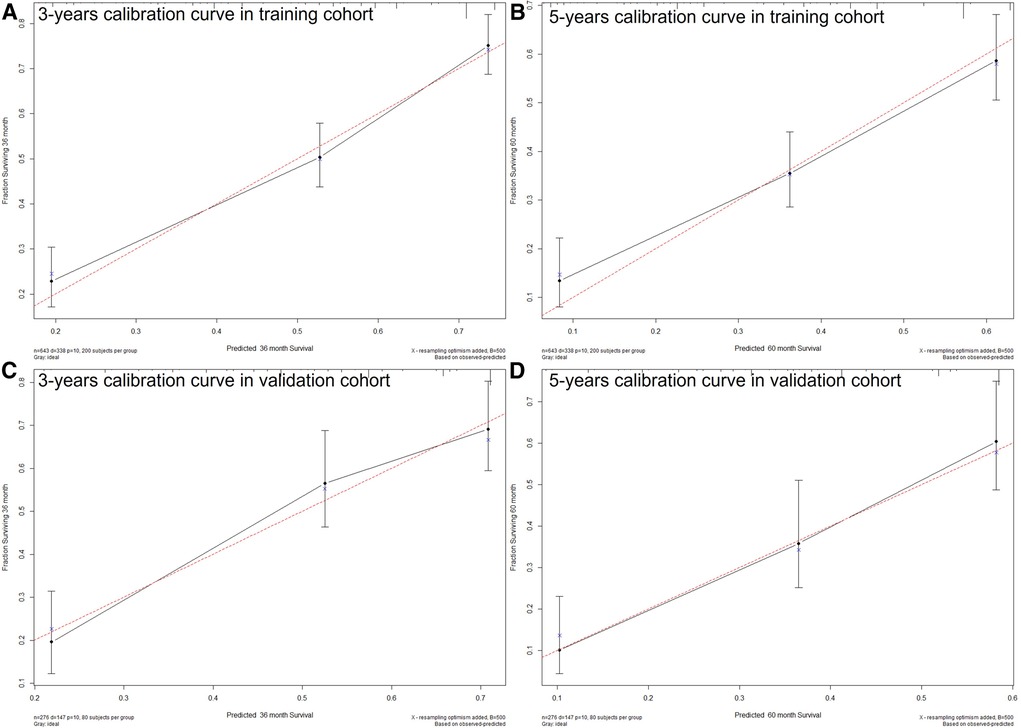
Figure 5. The calibration plots show excellent agreement between observed outcomes and predicted survival probabilities. (A) 3 years in training cohort; (B) 5 years in training cohort; (C) 3 years in validation cohort; (D) 5 years in validation cohort.
Discussion
Although the AJCC staging system is widely used for predicting prognosis in patients with ICC, it has inherent defects because it neglects many additional risk factors other than the TNM factors. Moreover, the AJCC staging system has not yet been specifically developed for postoperative prognostic prediction (8). It is widely known that a model has relatively good discrimination if its C-index and AUC exceed 0.7 (20). Consequently, we observed that the C-index of the AJCC staging system was 0.657 for survival prediction in both the training and validation cohorts.
Nomograms have been shown to be more accurate and user-friendly than the conventional staging system in many cancers (21, 22), and liver resection is the only established curative method for ICC patients. Therefore, we constructed a more comprehensive model based on a combination of various risk factors to better predict the prognosis of patients with ICC after hepatectomy. The nomogram in this study performed well in predicting cancer-specific survival, and its prediction was supported by the C-index (0.744) and calibration curve.
Other researchers have made efforts in the past. In 2013, Wang et al. established a prognosis nomogram for ICC after partial hepatectomy and focused on the influence of laboratory examination results such as AFP, CEA, and CA-199 in patients with ICC after hepatectomy (8). It was a single-center study of 367 patients. Our study has the advantage of being a population based study with a larger sample size (919 patients). Similarly, Yuan et al. developed a prognostic nomogram for patients with ICC in 2021 (23). The biggest difference was that they included all patients with ICC no matter whether those patients underwent hepatectomy or not. As we know, hepatectomy is still the most effective curative treatment for patients with ICC. Therefore, the patients with ICC who did not undergo surgery were expected to have worse survival outcomes. Furthermore, our study found that race was an important prognostic factor, which was not included in the Yuan et al. study.
The effects of racial factors on patients with ICC have rarely been studied. Firas et al. (24) found that African Americans had a lower incidence of ICC than Whites. Linlin et al. (25) reported that race did not influence the prognosis of patients with ICC. In our study, black patients had a worse prognosis than white (HR = 1.885, P < 0.01). However, the reason for this is unclear. Lee et al. (26) reported that black patients with cholangiocarcinoma had a lower surgical rate than white patients did (odd's ratio: 0.73; P < 0.001). This could be due to socioeconomic reasons, such as uninsured status and Medicaid insurance. However, there were no racial or socioeconomic differences in the multimodal therapy once the patients accessed surgical care. In our study, all patients underwent surgery, although the number of black patients after surgery was few (66/919). Therefore, this result needs to be further verified in future studies. The possible influence of genetics behind this disparity is also worth exploring.
Furthermore, the benefits of chemotherapy for surgically resected ICC remain poorly defined. A randomized study indicated that chemotherapy improved the survival of patients with ICC (6.5 months vs. 2.5 months) but it was not significant (P = 0.1) (27). John et al. (28) proposed that chemotherapy for resected ICC should be strongly considered for tumors harboring high-risk features, their conclusion was based on the National Cancer Database. Primrose et al. (29) discovered that capecitabine can improve overall survival in patients with resected biliary tract cancer when used as adjuvant chemotherapy following surgery and could be considered a standard of care. In our study, chemotherapy significantly improved the prognosis of patients with ICC who have undergone resection (HR = 0.612, P < 0.001), which verified the benefit of chemotherapy in patients with ICC after hepatectomy.
It is well known that positive lymph node (PLN) is an important negative prognostic indicator for cancer patients. However, owing to the different numbers of examined lymph nodes, the number of PLN could vary among patients with a similar prognosis (30, 31). Therefore, we chose PLNR as the research object in our study, which combined the number of PLN and the number of examined lymph nodes. We identified that PLNR ≥ 0.15 is a significant poor prognostic factor in patients with ICC who have undergone resection (HR = 1.738, P < 0.01).
Several nomograms have been constructed to predict the prognosis of patients with ICC after hepatectomy (8, 32, 33), but our study had a larger sample size and the data were population based. Our study has some limitations. First, this large-sample study was based on the SEER database, which may have inherent bias. Second, our study included 6,739 patients with ICC after hepatectomy initially. However, only 919 patients were finally included after rigor screening, which may weaken the external validity of our nomogram. Third, our nomogram was internally validated. It would be better to validate it externally using other populations.
Conclusion
In this study, we constructed and validated a nomogram for predicting the three- and five-year CSS in patients with ICC after hepatectomy. Six independent prognostic factors were identified, which are as follows: Black race, AJCC T, AJCC N, AJCC M, chemotherapy, and PLNR ≥ 0.15. The precise calibration and acceptable discrimination power of the nomogram were verified. The nomogram could be improved by further external validation and including additional potential factors that were not available in the SEER database.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
LK: conceptualization. Huang GB and Song WL: data curation. Zhang YC and Ren BY: formal analysis. Lv Y: supervision. Liu K: writing—original draft. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Key R&D Project of Shaanxi Province (No. 2020GXLH-Z-001) and the Scientific Development Funding of the First Affiliated Hospital of Xi'an Jiaotong University (No. 2022QN-17).
Acknowledgments
We thank the staff at the SEER database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. (2004) 40(3):472–7. doi: 10.1016/j.jhep.2003.11.030
2. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. (2014) 383(9935):2168–79. doi: 10.1016/S0140-6736(13)61903-0
3. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, et al. The global burden of cancer 2013. JAMA Oncol. (2015) 1(4):505–27. doi: 10.1001/jamaoncol.2015.0735
4. Aljiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. (2009) 208(1):134–47. doi: 10.1016/j.jamcollsurg.2008.09.007
5. Najran P, Lamarca A, Mullan D, McNamara MG, Westwood T, Hubner RA, et al. Update on treatment options for advanced bile duct tumours: radioembolisation for advanced cholangiocarcinoma. Curr Oncol Rep. (2017) 19(7):50. doi: 10.1007/s11912-017-0603-8
6. Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology. (2009) 136(4):1134–44. doi: 10.1053/j.gastro.2009.02.038
7. Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. (2014) 149(6):565–74. doi: 10.1001/jamasurg.2013.5137
8. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. (2013) 31(9):1188–95. doi: 10.1200/JCO.2012.41.5984
9. Liu K, Huang G, Chang P, Zhang W, Li T, Dai Z, et al. Construction and validation of a nomogram for predicting cancer-specific survival in hepatocellular carcinoma patients. Sci Rep. (2020) 10(1):21376. doi: 10.1038/s41598-020-78545-2
10. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. (2008) 26(8):1364–70. doi: 10.1200/JCO.2007.12.9791
11. Li W, Xiao Y, Xu X, Zhang Y. A novel nomogram and risk classification system predicting the cancer-specific mortality of patients with initially diagnosed metastatic cutaneous melanoma. Ann Surg Oncol. (2021) 28(7):3490–500. doi: 10.1245/s10434-020-09341-5
12. Zhang H, Li X, Zhang Y, Huang C, Wang Y, Yang P, et al. Diagnostic nomogram based on intralesional and perilesional radiomics features and clinical factors of clinically significant prostate cancer. J Magn Reson Imaging. (2021) 53(5):1550–8. doi: 10.1002/jmri.27486
13. Zuo Z, Zhang G, Song P, Yang J, Li S, Zhong Z, et al. Survival nomogram for stage IB non-small-cell lung cancer patients, based on the SEER database and an external validation cohort. Ann Surg Oncol. (2021) 28(7):3941–50. doi: 10.1245/s10434-020-09362-0
14. Zhou L, Zhao Y, Zheng Y, Wang M, Tian T, Lin S, et al. The prognostic value of the number of negative lymph nodes combined with positive lymph nodes in esophageal cancer patients: a propensity-matched analysis. Ann Surg Oncol. (2020) 27(6):2042–50. doi: 10.1245/s10434-019-08083-3
15. Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Eur Urol. (2015) 67(6):1142–51. doi: 10.1016/j.eururo.2014.11.025
16. Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the milan criteria. JAMA Surg. (2016) 151(4):356–63. doi: 10.1001/jamasurg.2015.4257
17. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. (1982) 143(1):29–36. doi: 10.1148/radiology.143.1.7063747
18. Wolbers M, Koller MT, Witteman JC, Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. (2009) 20(4):555–61. doi: 10.1097/EDE.0b013e3181a39056
19. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. (2015) 16(4):e173–80. doi: 10.1016/S1470-2045(14)71116-7
20. Steyerberg EW, Roobol MJ, Kattan MW, van der Kwast TH, de Koning HJ, Schröder FH. Prediction of indolent prostate cancer: validation and updating of a prognostic nomogram. J Urol. (2007) 177(1):107–12; discussion 12. doi: 10.1016/j.juro.2006.08.068
21. Hu CY, Pan ZY, Yang J, Chu XH, Zhang J, Tao XJ, et al. Nomograms for predicting long-term overall survival and cancer-specific survival in lip squamous cell carcinoma: a population-based study. Cancer Med. (2019) 8(8):4032–42. doi: 10.1002/cam4.2260
22. Pan Z, You H, Bu Q, Feng X, Zhao F, Li Y, et al. Development and validation of a nomogram for predicting cancer-specific survival in patients with Wilms’ tumor. J Cancer. (2019) 10(21):5299–305. doi: 10.7150/jca.32741
23. Yuan C, Hu Z, Wang K, Zou S. Development and validation a nomogram for predicting overall survival in patients with intrahepatic cholangiocarcinoma. Front Surg. (2021) 8:659422. doi: 10.3389/fsurg.2021.659422
24. Baidoun F, Sarmini MT, Merjaneh Z, Moustafa MA. Controversial risk factors for cholangiocarcinoma. Eur J Gastroenterol Hepatol. (2022) 34(3):338–44. doi: 10.1097/MEG.0000000000002313
25. Yin L, Zhao S, Zhu H, Ji G, Zhang X. Primary tumor resection improves survival in patients with multifocal intrahepatic cholangiocarcinoma based on a population study. Sci Rep. (2021) 11(1):12166. doi: 10.1038/s41598-021-91823-x
26. Lee RM, Liu Y, Gamboa AC, Zaidi MY, Kooby DA, Shah MM, et al. Race, ethnicity, and socioeconomic factors in cholangiocarcinoma: what is driving disparities in receipt of treatment? J Surg Oncol. (2019) 120(4):611–23. doi: 10.1002/jso.25632
27. Glimelius B, Hoffman K, Sjödén PO, Jacobsson G, Sellström H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. (1996) 7(6):593–600. doi: 10.1093/oxfordjournals.annonc.a010676
28. Miura JT, Johnston FM, Tsai S, George B, Thomas J, Eastwood D, et al. Chemotherapy for surgically resected intrahepatic cholangiocarcinoma. Ann Surg Oncol. (2015) 22(11):3716–23. doi: 10.1245/s10434-015-4501-8
29. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. (2019) 20(5):663–73. doi: 10.1016/S1470-2045(18)30915-X
30. Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol. (2003) 169(3):943–5. doi: 10.1097/01.ju.0000032474.22093.06
31. Qiu C, Dong W, Su B, Liu Q, Du J. The prognostic value of ratio-based lymph node staging in resected non-small-cell lung cancer. J Thorac Oncol. (2013) 8(4):429–35. doi: 10.1097/JTO.0b013e3182829c16
32. Cai Y, Zhang B, Li J, Li H, Liu H, Xie K, et al. A novel nomogram based on hepatic and coagulation function for evaluating outcomes of intrahepatic cholangiocarcinoma after curative hepatectomy: a multi-center study of 653 patients. Front Oncol. (2021) 11:711061. doi: 10.3389/fonc.2021.711061
Keywords: nomogram, cancer-specific survival, intrahepatic cholangiocarcinoma, hepatectomy, prognosis
Citation: Huang G, Song W, Zhang Y, Ren B, Lv Y and Liu K (2023) Prognostic nomogram for cancer-specific survival in patients with intrahepatic cholangiocarcinoma after hepatectomy: A population study of 919 patients. Front. Surg. 9:1025521. doi: 10.3389/fsurg.2022.1025521
Received: 23 August 2022; Accepted: 21 November 2022;
Published: 6 January 2023.
Edited by:
Giuseppe Zimmitti, Fondazione Poliambulanza Istituto Ospedaliero, ItalyReviewed by:
Marco Ramera, Fondazione Poliambulanza Istituto Ospedaliero, ItalyJasper Sijberden, Amsterdam University Medical Center, Netherlands
© 2023 Huang, Song, Zhang, Ren, Lv and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kang Liu a2FuZ2xpdUB4anR1LmVkdS5jbg==
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Gaobo Huang1,2
Gaobo Huang1,2 Yanchao Zhang
Yanchao Zhang Yi Lv
Yi Lv Kang Liu
Kang Liu