- Department of Gastrointestinal Surgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: Solid pseudopapillary neoplasm (SPN) is a rare tumor with low malignant potential, which typically occurs in the pancreas. Extrapancreatic SPN is also extremely rare worldwide.
Case presentation: We report a case of a 70-year-old woman hospitalized with abdominal pain and bloating. The patient did not have any underlying diseases, such as diabetes, coronary heart disease, or hypertension. More than 30 years ago, the patient underwent surgery for “ectopic pregnancy”. The patient had no family history of hereditary disease, nor did any immediate family members have a history of cancer. Laboratory tests showed that her hemoglobin and albumin levels were low and she had a high level of cancer antigen 125 (CA125). Enhanced computed tomography (CT) showed a large tumor in the abdomen and pelvis. The patient subsequently underwent surgery, and it was found that the tumor was attached to the terminal ileum. Pathological findings suggested that the tumor was an extrapancreatic SPN, with an ectopic pancreas found in the tumor tissue. The patient did not receive chemotherapy or radiotherapy after surgery. After 13 months of follow-up, the patient was admitted again with abdominal pain. CT showed tumor recurrence with extensive systemic metastases. The patient and her family refused reoperation and biopsy, and the patient was discharged after the abdominal pain and anemia resolved.
Conclusion: We report a rare case of extrapancreatic SPN of ileal origin, which could be the first report worldwide. It had aggressive biological features, with recurrence and metastasis 13 months after surgery. For extrapancreatic SPN, the risk of recurrence should be assessed, and for tumors suspected of malignant behavior, a longer follow-up after discharge may be needed. Although SPN generally has a good prognosis after surgery, there is no consensus on whether postoperative chemotherapy and other treatments are needed for patients with high recurrence risk.
Introduction
Solid pseudopapillary neoplasm (SPN) of the pancreas is an uncommon pancreatic tumor, accounting for approximately 0.3%–2.7% of all pancreatic tumors (1). In 2010, the WHO classified SPN as a low-grade malignant pancreatic tumor, although 10%–15% of SPN exhibit aggressive behavior, in rare cases resulting in patient death (2). The main clinical manifestations of SPN are abdominal pain, abdominal distension, and other discomforts caused by the enlargement of the tumor mass pressing on the abdomen. Although it has been reported in people aged 2–85, it is most common in women aged 20–40, with a female-to-male ratio of approximately 10:1 (3). Pancreatic SPN is a nomenclature to describe its histological features. It does not originate from pancreatic tissue.
Extrapancreatic primary SPN is extremely rare, and only approximately 50 cases have been reported (4–43). This article aimed to improve clinicians' understanding of extrapancreatic SPN, reduce the rate of missed diagnosis and delayed treatment, and ultimately maximize patient benefit. Here, we report a rare case of extrapancreatic SPN of ileal origin, which could be the first report worldwide. All previously published studies involving extrapancreatic SPN were reviewed. Written informed consent was obtained from the patient for the surgical intervention and case publication.
Case presentation
A 70-year-old woman was admitted to the hospital with sudden abdominal pain and bloating. The patient did not have any underlying diseases, such as diabetes, coronary heart disease, or hypertension. More than 30 years ago, the patient underwent surgery for “ectopic pregnancy”. The patient had no family history of hereditary disease, nor did any immediate family members have a history of cancer. Physical examination revealed mid-abdominal tenderness and a palpable 6 cm × 6 cm mass, with a firm texture and unclear boundary. Abdominal enhanced computed tomography (CT) showed a large mixed density mass in the lower abdominal cavity-pelvis, approximately 162.5 mm × 105 mm × 182 mm (Figure 1A). The mass was closely related to the right appendage, the adjacent bowel and bladder were compressed and moved, and no obvious obstruction or dilation of the bowel was seen. A cystic low-density, nonenhancing shadow was seen in the left adnexal area, measuring approximately 27 mm × 21 mm. No obvious abnormality was found in the liver, gallbladder, spleen, kidney, or pancreas. Laboratory tests showed that hemoglobin was 80 g/L (reference: 115–150 g/L), albumin was 25.9 g/L (reference: 40.0–55.0 g/L), and other routine laboratory tests showed no obvious abnormality. Furthermore, cancer antigen 19–9 (CA19–9) and carcinoembryonic antigen (CEA) were within normal limits, but CA125 was elevated to 706.7 U/ml (reference: 0.00–35.00 U/ml).
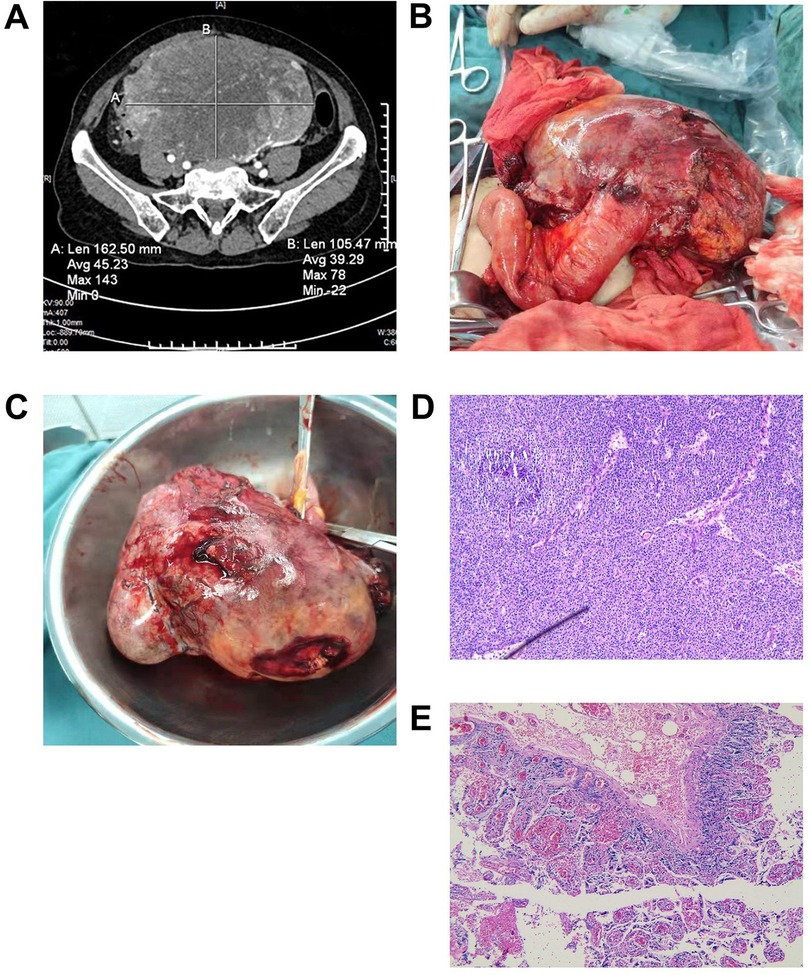
Figure 1. (A) An enhanced CT showed a huge mixed density mass in the lower abdominal cavity-pelvis. (B,C) Grossly, the tumor was attached to the terminal ileum, solid and cystic, with areas of necrosis and hemorrhage. (D) The tumor was histologically composed of cells arranged in the form of solid sheets and pseudopapillary areas (H & E, X 100). (E) The left adnexa showed the formation of white bodies in the left ovary (H & E, X 100). CT, computed tomography.
The patient had a definite diagnosis of a large tumor in the abdomen and pelvis. After communicating with the patient's family, we decided not to perform a preoperative biopsy and to surgically remove the tumor and perform a pathological biopsy. The patient underwent an exploratory laparotomy, and a large tumor in the abdomen and pelvis was revealed, approximately 20 cm × 20 cm in size. The tumor was attached to the terminal ileum, approximately 12 cm away from the ileocecal region, and could not be separated. We considered that the tumor probably originated in the small intestine (Figures 1B, C). The terminal mesentery was then segmented in a fan shape, and the intestinal tube was severed 3 cm distal to and 10 cm proximal to the tumor, followed by small bowel anastomosis. The pelvic cavity was explored again, and it was found that the right adnexa and uterus were atrophied. A cyst was observed in the left adnexa, approximately 3.0 cm × 3.0 cm in size, so the left adnexa was excised.
The postoperative recovery of the patient was uneventful, and she was discharged after half a month. The tumor was histologically composed of cells arranged in the form of solid sheets and pseudopapillary areas (Figure 1D). Ectopic pancreatic tissue was also observed histologically within the resected tumor. Immunohistochemistry (IHC) showed positive results for CD10, CD56, E-cadherin (weak positive), cytokeratin (CK), vimentin (Vim), progesterone receptor (PR), and succinate dehydrogenase complex subunit B (SDHB). In addition, the tumor cells showed nuclear staining for β-catenin, paranuclear dot-like staining for CD99, and focal staining for soluble protein-100 (S100) and synaptophysin (Syn). The proliferation index of Ki-67 was approximately 20%. The pathology of the left adnexa showed the formation of white bodies in the left ovary (Figure 1E). Combined with IHC, we confirmed that this was an extrapancreatic SPN of primary ileal origin.
The patient did not receive chemotherapy or radiotherapy after surgery. Thirteen months after surgery, the patient was admitted again with abdominal pain. Laboratory examination showed her hemoglobin was 88 g/L, and her CA125 was elevated to 47.4 However, CA19–9 and CEA were within normal limits. Enhanced CT of the chest and the whole abdomen showed that there was a mixed density mass in the right middle abdomen, approximately 59 mm × 57 mm × 92 mm (left and right × front and back × up and down), and the boundary was not clear (Figure 2A). A cystic and solid mass was seen next to the duodenum, approximately 35.2 mm × 22.7 mm, with an irregular shape and unclear demarcation with the duodenum. The solid part was significantly enhanced (Figure 2B). There were several nodular and clump-shaped hypodense shadows in the liver, and the larger shadows were located in the right lobe of the liver (Figure 2C). There were multiple solid nodules in both lungs, and the largest nodule was located in the dorsal segment of the left lower lobe (Figure 2D). The patient and her family refused reoperation and biopsy, and the patient was discharged after her abdominal pain and anemia resolved.

Figure 2. Enhanced CT of the chest and the whole abdomen showed tumor recurrence and extensive systemic metastases (A). There was a mixed density mass in the right middle abdomens, and the boundary was not clear (red arrowheads). (B) A cystic and solid mass was found next to the duodenum, irregular in shape, with unclear demarcation with the duodenum, and the solid part was significantly enhanced (red arrowheads). (C) There were several nodular and clump-shaped hypodense shadows in the liver, and the larger ones were located in the right lobe of the liver (red arrowheads). (D) There were multiple solid nodules in both lungs, and the largest one was located in the dorsal segment of the left lower lobe (red arrowheads). CT, computed tomography.
Discussion
We identified 50 cases with definite extrapancreatic SPN reported between 1990 and 2022 (including the case we reported). The characteristics of all cases are summarized in Table 1, based on the literature search (Supplementary Table S1 shows this in more detail). The most common sites of extrapancreatic SPN were the ovary (24.4%, 12/49), testis/paratestis (18.4%, 9/49), retroperitoneum (10.2%, 5/49), mesocolon (10.2%, 5/49), and omentum (10.2%, 5/49). Other rare sites included the stomach, liver, right adrenal, posterior mediastinum, mesentery, jejunum, and duodenum. Among the 50 patients, 16 were men, with a male-to-female ratio of 1:2.125. The average age of the patients was 39 years (range, 13–82 years), and tumors occurred mainly in people aged 20–40 (40%).
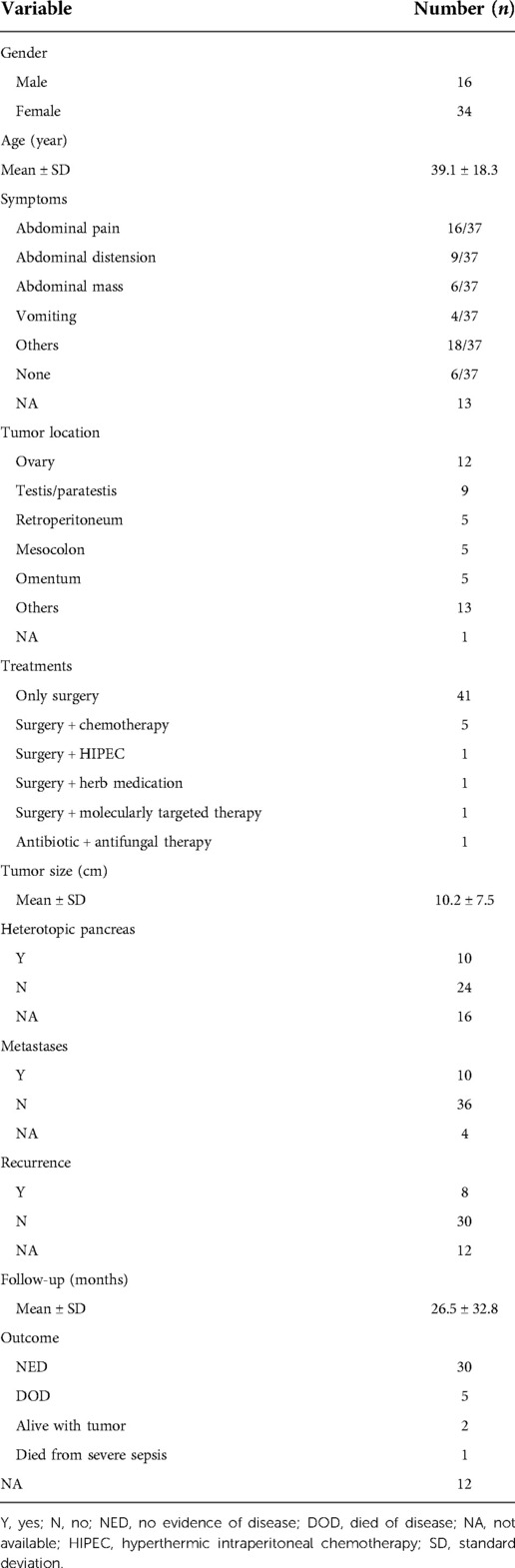
Table 1. Patient demographics and tumor characteristics of extrapancreatic solid pseudopapillary neoplasm.
The clinical symptoms of extrapancreatic SPN are often nonspecific, and some cases were even discovered incidentally during routine examinations. The symptoms are described in detail for 37 of the 50 patients. The clinical manifestations included abdominal pain in 16 cases (43.2%, 16/37), abdominal distension in 9 cases (24.3%, 9/37), abdominal mass in 6 cases (16.2%, 6/37), vomiting in 4 cases (10.8%, 4/37), and asymptomatic in 6 cases (16.2%, 6/37). A few patients had weight loss, nausea, fever, and fatigue. The patients' symptoms can also be different depending on the primary site, and they usually have more than one symptom. For example, menorrhagia, pelvic pain, and postmenopausal bleeding can occur in patients with SPN on the ovary. Laboratory tests of some patients may show decreased hemoglobin and increased white blood cells, but they are not specific. In addition, although serum markers (such as CEA, CA199, and CA125) can be increased, they are also not typical for diagnosing SPN (44). In our case, there was no change in CEA or CA-199. Although CA-125 was significantly elevated, the tumor originated in the ileum and was not an ovarian-related tumor.
In recent years, reports on SPN have gradually accumulated in various countries. However, there is still no clear conclusion about the origin of SPN. Some researchers found ectopic pancreatic tissue in the SPN tissue or at the tumor margin, so it is speculated that the SPN originated from this ectopic pancreatic tissue (16, 41–43). We found that in 50 cases of extrapancreatic SPN, only 10 (29.4%, 10/34) had ectopic pancreas, 24 (70.6%, 24/34) had no ectopic pancreas, and 16 did not mention it. Other researchers have found that during embryogenesis, the genital ridge is very close to the pancreatic primordium, so cells from the primordial gonad have the potential to migrate to the developing pancreas, thus leading to speculation that SPN may originate from germ ridge-related cells (45). This theory may also explain why extrapancreatic SPN tends to occur in the ovary and testis.
Imaging examinations play an important role in the initial diagnosis of SPN, among which the most commonly used imaging modalities are ultrasound, CT, and magnetic resonance imaging (MRI) (17, 46). The ultrasound features of SPN are mainly cystic and solid masses with heterogeneous internal echoes. Compared with ultrasound, CT can reveal the morphological structure of the entire tumor and the relationships between the surrounding tissues more clearly. It typically presents as a large mass of inhomogeneous density with solid and cystic components, with the solid component usually located at the margin of the mass and the cystic component in the center of the mass, often with an intact fibrous capsule. In addition, highly malignant SPN often exhibit local discontinuities of the capsule, unclear edges, and relatively large tumor volumes. On MRI, the solid component and cystic component have different signal responses. T1-weighted imaging mostly shows heterogeneously mixed signals, T2-weighted imaging is iso- or slightly hyperintense, and diffusion-weighted imaging is hyperintense. In addition, the presence of solid and cystic components on MRI with hemorrhage but no septum should be highly suspicious for SPN. Among the 50 cases of extrapancreatic SPN, CT was the most widely used diagnostic method. In our case, the tumor's location, invasion, and metastasis were also clarified by contrast-enhanced CT.
Pathological examination and IHC are the most reliable methods for diagnosing SPN. The average size of surgical resection specimens among the 50 cases was approximately 10.2 ± 7.5 cm (0.5–30 cm). The growth pattern of SPN is more diverse, and it can manifest as solid, pseudopapillary, and cystic structures in different proportions. Microscopically, one or more layers of tumor cells are arranged around the fibrovascular axis to form pseudopapillary protrusions, which are typical pathological features (9). IHC showed that almost all tumors were positive for β-catenin (nuclear staining), CD10, CD56, and vimentin. Most tumors were positive for CD99, α1-antitrypsin, NSE, P504s, and PR; some were positive for synaptophysin. However, they usually do not express chromogranin A or E-cadherin (3, 46). Based on these typical features, the diagnosis of SPN can be performed.
In the literature we reviewed, a total of 49 patients with extrapancreatic SPN received surgery, and one patient received antibiotic and antifungal therapy due to severe infection. Among 50 cases of extrapancreatic SPN, five patients received chemotherapy, one received molecularly targeted therapy (the drug was imatinib), one received herbal medication, and one received HIPEC. However, the current number of cases is too small, and more clinical evidence is needed to evaluate the effect of adjuvant therapy on SPN. Ten patients with extrapancreatic SPN had tumor metastasis (21.7%, 10/46), 36 had no tumor metastasis (78.3%, 36/46), and 4 had no mention. Among the 50 patients with extrapancreatic SPN, 38 patients had follow-up information, and the follow-up time ranged from 3 to 144 months. There were 8 patients (21.1%, 8/38) with tumor recurrence, 30 (78.9%, 30/38) without recurrence, and 12 who did not mention recurrence. There were 38 patients with clear outcomes, and 32 patients (84.2%, 32/38) survived well, including 30 patients with no evidence of disease (NED) and 2 patients alive with tumors. There were six deaths (15.8%, 6/38), of which five died of SPN recurrence, and one died of severe sepsis.
Pancreatic SPN is a low-grade malignant tumor; only 15% will develop metastasis, and a long-term survival rate is observed in more than 95% of the cases. The metastasis rate of extrapancreatic SPN was 21.7%, and the survival rate of 38 patients with follow-up was 84.2%. Therefore, extrapancreatic SPN may have a favorable clinical course similar to that of SPN. Although the liver is the most common site of distant metastases, the mesentery, mentum, peritoneum, and lungs may also be involved. However, metastasis or invasion of adjacent organs is not a contraindication to surgery, and the patients could also have a longer survival time after reoperation (46). In addition to surgery, conventional adjuvant treatments can also be used for the treatment of SPN, such as hyperthermic intraperitoneal chemotherapy (HIPEC), radiofrequency ablation (RFA), transcatheter arterial embolization, radiotherapy, and chemotherapy (19).
Extrapancreatic SPN is a rare low-grade malignancy with a good overall prognosis. It mainly occurs in the ovary, testis/paratestis, retroperitoneum, mesocolon, and omentum. Abdominal pain, bloating, and a palpable mass are the most common symptoms. Laboratory tests and serum tumor markers are usually nonspecific. Immunohistochemical staining of biopsy or the surgical resection specimen is the main method for diagnosis and differential diagnosis. The most unique immunohistochemical marker is the abnormal nuclear staining of β-catenin. The best treatment for extrapancreatic SPN is still radical surgical resection. Even in the case of tumor metastasis, most patients can still be radically cured by surgical resection of the primary tumor and metastases because of the slow clinical progression after metastasis. For unresectable patients, there is limited evidence to support other treatments, such as chemotherapy.
Primary SPN occurring outside the pancreas are exceedingly rare. Our case is unique because this is the first reported extrapancreatic SPN of ileal origin. Due to its abnormal location, an accurate diagnosis was a challenge for pathologists. Another feature that makes this case unique is that the patient developed extensive lung and abdominal metastases 13 months after surgery. Some doctors have proposed risk criteria for recurrence after SPN, including diffuse tumor growth, capsular involvement, vascular or perineural invasion, lymph node metastasis or distant metastasis, and a Ki-67 index ≥4% is associated with SPN recurrence. Intraoperative tumor rupture may be the cause of peritoneal recurrence (21). Some researchers also found that the tumor size for cases with metastases was larger than that of nonmetastatic tumors (8.13 ± 1.03 cm for metastatic tumors and 5.20 ± 3.78 cm for nonmetastatic, range 7–9 cm, P < 0.012). Larger tumor size was significantly associated with the risk of metastasis and recurrence (P < 0.002) (46). The tumor resected from our patient was approximately 20 cm × 20 cm in size, and the postoperative pathological finding of the Ki-67 index was approximately 20%, so we speculated that these clinical features of the patient were one of the possible reasons for the recurrence. In addition, our patient only underwent surgery and did not receive other treatments, such as chemotherapy or radiotherapy. Although SPN generally has a good prognosis after surgery, there is no consensus on whether postoperative chemotherapy and other treatments are needed for patients with high recurrence risk. Whether this is related to postoperative recurrence also needs further research.
Conclusion
We report a rare case of extrapancreatic SPN of ileal origin, which could be the first report worldwide. It had aggressive biological features, with recurrence and metastasis 13 months after surgery. For extrapancreatic SPN, the risk of recurrence should be assessed, and for tumors suspected of malignant behavior, a longer follow-up after discharge may be necessary. Our case may extend our understanding of the biological behavior of extrapancreatic SPN and provide clinical experience with the diagnosis and treatment of extrapancreatic SPN to avoid a missed diagnosis and delayed treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical Committee of the Second Affiliated Hospital of Chongqing Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HL and ZX researched the literature and wrote the manuscript. YW and HG contributed to being involved in the treatment of the patient. YT and DW contributed to the manuscript review. JW and JZ wrote the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the “Kuanren Yingcai” team and individual special fund--excellent young talent (Jianbo Zhang) (grant no. KY2019Y008).
Acknowledgements
We would like to thank Dr Huaying Xiong for the revisions of language and grammar.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1020044/full#supplementary-material.
References
1. Chen H, Huang Y, Yang N, Yan W, Yang R, Zhang S, et al. Solid-pseudopapillary neoplasm of the pancreas: a 63-case analysis of clinicopathologic and immunohistochemical features and risk factors of malignancy. Cancer Manag Res. (2021) 13:3335–43. doi: 10.2147/CMAR.S304981
2. Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG clinical guideline: diagnosis and management of pancreatic cysts. Am J Gastroenterol. (2018) 113:464–79. doi: 10.1038/ajg.2018.14
3. Michalova K, Michal M Jr, Kazakov DV, Sedivcova M, Hes O, Hadravsky L, et al. Primary signet ring stromal tumor of the testis: a study of 13 cases indicating their phenotypic and genotypic analogy to pancreatic solid pseudopapillary neoplasm. Hum Pathol. (2017) 67:85–93. doi: 10.1016/j.humpath.2017.07.010
4. Junzu G, Yanbin S, Suxia W, Janjun D. A case of extrapancreatic solid pseudopapillary tumor in the retroperitoneum. Jpn J Radiol. (2012) 30:598–601. doi: 10.1007/s11604-012-0084-5
5. Lemoine A, Asmandar S, Boutroux H, Tounian P, Ducou Le Pointe H, Coulomb A, et al. Extrapancreatic primary solid pseudopapillary tumor in the gastric antrum: case report. Pediatr Blood Cancer. (2020) 67:e28415. doi: 10.1002/pbc.28415
6. Klöppel G, Maurer R, Hofmann E, Lüthold K, Oscarson J, Forsby N, et al. Solid-cystic (papillary-cystic) tumours within and outside the pancreas in men: report of two patients. Virchows Arch A Pathol Anat Histopathol. (1991) 418:179–83. doi: 10.1007/BF01600295
7. Slidell MB, Schmidt EF, Jha RC, Rossi CT, Becker TE, Guzzetta PC. Solid pseudopapillary tumor in a pancreatic rest of the jejunum. J Pediatr Surg. (2009) 44:E25–7. doi: 10.1016/j.jpedsurg.2009.01.074
8. Hibi T, Ojima H, Sakamoto Y, Kosuge T, Shimada K, Sano T, et al. A solid pseudopapillary tumor arising from the greater omentum followed by multiple metastases with increasing malignant potential. J Gastroenterol. (2006) 41:276–81. doi: 10.1007/s00535-005-1753-2
9. Yoshikawa A, Ryu Y, Takata H, Asaumi Y, Sakatoku M, Terahata S. An extrapancreatic solid-pseudopapillary neoplasm in the greater omentum. BJR Case Rep. (2017) 3:20170008. doi: 10.1259/bjrcr.20170008
10. Syriac S, Kesterson J, Izevbaye I, de Mesy Bentley KL, Lele S, Mhawech-Fauceglia P. Clinically aggressive primary solid pseudopapillary tumor of the ovary in a 45-year-old woman. Ann Diagn Pathol. (2012) 16:498–503. doi: 10.1016/j.anndiagpath.2011.04.007
11. Deshpande A, Munshi M. Cytology of papillary solid-cystic neoplasm of the pancreas presenting as an extrapancreatic mass: a case report. Acta Cytol. (2005) 49:81–6. doi: 10.1159/000326101
12. Kim YI, Kim ST, Lee GK, Choi BI. Papillary cystic tumor of the liver. A case report with ultrastructural observation. Cancer. (1990) 65:2740–6. doi: 10.1002/1097-0142(19900615)65:12%3C2740::aid-cncr2820651223%3E3.0.co;2-0
13. Tornóczky T, Kálmán E, Jáksó P, Méhes G, Pajor L, Kajtár GG, et al. Solid and papillary epithelial neoplasm arising in heterotopic pancreatic tissue of the mesocolon. J Clin Pathol. (2001) 54:241–5. doi: 10.1136/jcp.54.3.241
14. Tariq N, Qureshi A, Dian A. Extra pancreatic solid pseudopapillary tumour in a young male. J Pak Med Assoc. (2016) 66:1337–8.27686317
15. Tez M, Ozalp N, Zülfikaroğlu B, Koç M. A solid pseudopapillary tumour arising from mesocolon without ectopic pancreas. HPB Surg. (2010) 2010:206186. 2010: doi: 10.1155/2010/206186
16. Khaniya S, Shakya VC, Koirala R. Solid pseudopapillary tumor in an ectopic pancreas: an unusual presentation. J Surg Case Rep. (2017) 3:rjx050. doi: 10.1093/jscr/rjx050
17. Gurzu S, Bara T, Sincu M, Gabos S, Vlad DM, Bara T Jr, et al. Solid pseudopapillary neoplasm of pancreas: two case reports. Medicine (Baltimore). (2019) 98:e16455. doi: 10.1097/MD.0000000000016455
18. Cheuk W, Beavon I, Chui DT, Chan JK. Extrapancreatic solid pseudopapillary neoplasm: report of a case of primary ovarian origin and review of the literature. Int J Gynecol Pathol. (2011) 30:539–43. doi: 10.1097/PGP.0b013e31821724fb
19. Choi HW, Park HJ, Hong SA, Park SB, Lee ES, Ahn HS, et al. Radiologic findings in extrapancreatic solid pseudopapillary tumor with aggressive behavior: a case report. J Korean Med Sci. (2017) 32:2079–84. doi: 10.3346/jkms.2017.32.12.2079
20. Zhu H, Xia D, Wang B, Meng H. Extrapancreatic solid pseudopapillary neoplasm: report of a case of primary retroperitoneal origin and review of the literature. Oncol Lett. (2013) 5:1501–4. doi: 10.3892/ol.2013.1242
21. Wu H, Huang YF, Liu XH, Xu MH. Extrapancreatic solid pseudopapillary neoplasm followed by multiple metastases: case report. World J Gastrointest Oncol. (2017) 9:497–501. doi: 10.4251/wjgo.v9.i12.497
22. Kushner BS, Chatterjee D, Hammill C. Rare aggressive solid pseudopapillary neoplasm of the ovary with metastatic disease following surgical resection. BMJ Case Rep. (2020) 13:e238136. doi: 10.1136/bcr-2020-238136
23. Chakrabarti S, Ghosh S, Sarkar R. Solid pseudopapillary tumour of extrapancreatic origin presenting as mesenteric cystic mass: a diagnostic dilemma. J Clin Diagn Res. (2016) 10:ED01-2. doi: 10.7860/JCDR/2016/19355.8312
24. Singh K, Patel N, Patil P, Paquette C, Mathews CA, Lawrence WD. Primary ovarian solid pseudopapillary neoplasm with CTNNB1 c.98C > G (p.S33C) point mutation. Int J Gynecol Pathol. (2018) 37:110–6. doi: 10.1097/PGP.0000000000000396
25. Thai E, Dalla Valle R, Silini EM. Primary solid papillary tumor of the liver. Pathol Res Pract. (2012) 208:250–3. doi: 10.1016/j.prp.2012.01.005
26. Kominami A, Fujino M, Murakami H, Ito M. β-catenin mutation in ovarian solid pseudopapillary neoplasm. Pathol Int. (2014) 64:460–4. doi: 10.1111/pin.12194
27. Michal M, Bulimbasic S, Coric M, Sedivcova M, Kazakov DV, Michal M, et al. Pancreatic analogue solid pseudopapillary neoplasm arising in the paratesticular location. The first case report. Hum Pathol. (2016) 56(56):52–6. doi: 10.1016/j.humpath.2016.06.007
28. Komforti MK, Edelman M, Fan C, Liang SX. Solid pseudopapillary neoplasm presenting as a primary ovarian mass in an eighteen-year-old female: report of a case and review of the literature. Virchows Arch. (2018) 472:285–91. doi: 10.1007/s00428-017-2231-y
29. Stoll LM, Parvataneni R, Johnson MW, Gui D, Dorigo O, Sullivan P. Solid pseudopapillary neoplasm, pancreas type, presenting as a primary ovarian neoplasm. Hum Pathol. (2012) 43:1339–43. doi: 10.1016/j.humpath.2011.12.018
30. Walter T, Hommell-Fontaine J, Hervieu V, Adham M, Poncet G, Dumortier J, et al. Primary malignant solid pseudopapillary tumors of the gastroduodenal area. Clin Res Hepatol Gastroenterol. (2011) 35:227–33. doi: 10.1016/j.clinre.2011.01.004
31. Guo X, Li N, Ren K, Wu L, Ma LI, Wu S, et al. Extrapancreatic solid pseudopapillary tumors: a clinicopathological analysis of two cases. Mol Clin Oncol. (2016) 4:845–50. doi: 10.3892/mco.2016.802
32. He S, Yang X, Zhou P, Cheng Y, Sun Q. Solid pseudopapillary tumor: an invasive case report of primary ovarian origin and review of the literature. Int J Clin Exp Pathol. (2015) 8:8645–9.26339451
33. Fukunaga M. Pseudopapillary solid cystic tumor arising from an extrapancreatic site. Arch Pathol Lab Med. (2001) 125:1368–71. doi: 10.5858/2001-125-1368-PSCTAF
34. Deshpande V, Oliva E, Young RH. Solid pseudopapillary neoplasm of the ovary: a report of 3 primary ovarian tumors resembling those of the pancreas. Am J Surg Pathol. (2010) 34:1514–20. doi: 10.1097/PAS.0b013e3181f133e9
35. Miyazaki Y, Miyajima A, Maeda T, Yuge K, Hasegawa M, Kosaka T, et al. Extrapancreatic solid pseudopapillary tumor: case report and review of the literature. Int J Clin Oncol. (2012) 17:165–8. doi: 10.1007/s10147-011-0261-z
36. Lin DL, Li H, Jiang TJ, Wu J, Zhao H, Hu SS, et al. Extrapancreatic solid pseudopapillary neoplasm: report of a unique case of primary posterior mediastinum origin and review of the literature. Transl Cancer Res. (2020) 9:3024–9. doi: 10.21037/tcr.2020.02.58
37. Michalova K, Michal M, Sedivcova M, Kazakov DV, Bacchi C, Antic T, et al. Solid pseudopapillary neoplasm (SPN) of the testis: comprehensive mutational analysis of 6 testicular and 8 pancreatic SPN. Ann Diagn Pathol. (2018) 35:42–7. doi: 10.1016/j.anndiagpath.2018.04.003
38. Mengoli MC, Bonetti LR, Intersimone D, Fedeli F, Rossi G. Solid pseudopapillary tumor: a new tumor entity in the testis? Hum Pathol. (2017) 62:242–3. doi: 10.1016/j.humpath.2016.08.011
39. Basu A, Jha A. Solid and cystic tumor arising from an extrapancreatic site–a case report. Nepal Med Coll J. (2003) 5:107–8.15024781
40. Gahlot GP, Mridha AR, Sable M, Sharma MC, Pramanik R, Kumar L. Solid pseudopapillary neoplasm of the ovary with metastases to the omentum and regional lymph nodes. Indian J Pathol Microbiol. (2016) 59:348–50. doi: 10.4103/0377-4929.188107
41. Ishikawa O, Ishiguro S, Ohhigashi H, Sasaki Y, Yasuda T, Imaoka S, et al. Solid and papillary neoplasm arising from an ectopic pancreas in the mesocolon. Am J Gastroenterol. (1990) 85:597–601.2337064
42. Elorza Orúe JL, Ruiz Díaz I, Tubía Landaberea J, San Vicente Leza M. Solid and papillary tumor on ectopic pancreas in transversal mesocolon. Rev Esp Enferm Dig. (1991) 79:429–31.
43. Kövári E, Járay B, Pulay I. Papillary cystic neoplasms in the ectopic pancreas. Orv Hetil. (1996) 137:923–5.
44. Song H, Dong M, Zhou J, Sheng W, Zhong B, Gao W. Solid pseudopapillary neoplasm of the pancreas: clinicopathologic feature, risk factors of malignancy, and survival analysis of 53 cases from a single center. Biomed Res Int. (2017) 2017: 5465261. doi: 10.1155/2017/5465261
45. Kosmahl M, Seada LS, Janig U, Harms D, Kloppel G. Solid-pseudopapillary tumor of the pancreas: its origin revisited. Virchows Arch. (2000) 436:473–80. doi: 10.1007/s004280050475
Keywords: diagnosis, extrapancreatic solid pseudopapillary neoplasm, ileum, origin, treatment
Citation: Liu H, Xu Z, Wang Y, Gu H, Tang Y, Wu D, Wang J and Zhang J (2022) Case report: A case report and literature review of extrapancreatic solid pseudopapillary neoplasm. Front. Surg. 9:1020044. doi: 10.3389/fsurg.2022.1020044
Received: 15 August 2022; Accepted: 3 October 2022;
Published: 4 November 2022.
Edited by:
Sami Akbulut, İnönü University, TurkeyReviewed by:
Oscar Paredes, Instituto Nacional de Enfermedades Neoplásicas (INEN), PeruFirdaus Hayati, University of Malaysia Sabah, Malaysia
© 2022 Liu, Xu, Wang, Gu, Tang, Wu, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jijian Wang d2FuZ2pqMTk2M0AxNjMuY29t Jianbo Zhang emhhbmdqaWFuYm9AaG9zcGl0YWwuY3FtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Hang Liu
Hang Liu Zhiquan Xu†
Zhiquan Xu† Yaxu Wang
Yaxu Wang