
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 05 January 2023
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1019454
Aim: Little attention has been paid in the prognosis of colorectal signet ring cell carcinoma (SRCC). This study aims to explore the predictive capacity of log odds of positive lymph nodes (LODDS), lymph node ratio (LNR), and pN stage in the prognosis of patients with colorectal SRCC.
Methods: A retrospective cohort study was designed, and data were extracted from the Surveillance, Epidemiology and End Results (SEER) database. Data on demographic characteristics, clinicopathological features, and treatment were extracted. Outcomes were overall survival (OS) and cancer-specific survival (CSS). Association of LODDS, LNR, and pN stage with OS and CSS were explored using Cox proportional hazard model and Cox competing risk model, respectively, with results showing as hazard ratio and 95% confidence interval (CI). Predictive performance of LODDS, LNR, and pN stage in OS and CSS was assessed by calculating C-index.
Results: A total of 2,198 patients were included in this study. LODDS, LNR, and pN stage were associated with the OS and CSS of colorectal SRCC patients (all P < 0.05). LODDS showed a good performance in the OS (C-index: 0.704, 95% CI: 0.690–0.718), which was superior to LNR (C-index: 0.657, 95% CI: 0.643–0.671) and pN stage (C-index: 0.643, 95% CI: 0.629–0.657). The C-index of LODDS, LNR, and pN stage for CSS was 0.733 (95% CI: 0.719–0.747), 0.713 (95% CI: 0.697–0.729), and 0.667 (95% CI: 0.651–0.683), respectively.
Conclusions: LODDS displayed a better predictive capacity in the OS and CSS than LNR and pN stage, indicating that LODDS may be effective to predict the prognosis of colorectal SRCC in the clinic.
Signet ring cell carcinoma (SRCC) is a rare and special subtype of colorectal cancer (CRC), accounting for approximately 1% of all CRC cases (1). SRCC, originating from undifferentiated stem cells of colorectal mucosa, is characterized by rapid development, poor differentiation, diffuse infiltration, and high metastatic rate (2, 3). Evidence has indicated that SRCC is an independent risk factor for the poor prognosis of CRC patients (4). Compared to patients with colorectal nonvariant adenocarcinoma, SRCC patients have a lower 5-year overall survival (OS) and cancer-specific survival (CSS) (1). Although there are many clinical studies on the prognosis of CRC, little attention has been paid to that of SRCC. Accurately estimating the prognosis of SRCC cases may help implement individualized treatment and select the optimal treatment strategy to increase the survival rate.
Lymph node metastasis (LNM) is an important risk factor for the poor prognosis and strongly affects therapeutic decisions in CRC (5). The 5-year OS ranges from 70% to 90% in CRC patients with negative lymph nodes (NLNs), while it ranges from 30% to 60% in patients with positive lymph nodes (PLNs) (6). The American Joint Committee on Cancer (AJCC) pathological nodal classification (pN) stratifies nodal involvement according to the number of involved lymph nodes and is regarded as the most important prognostic factor for CRC patients (6). The accuracy of pN stage is influenced by the number of examined lymph nodes (must be ≥12), and 48%–63% of cases have inadequate lymph node examination (7). This may lead to underestimated stages and improper treatment. To address the limitations of the pN stage system, lymph node ratio (LNR) is proposed, which is defined as the ratio of the number of PLNs to the total number of retrieved lymph nodes (TLNs) (8). LNR is less influenced by TLNs and has been reported as a prognostic factor in CRC (8, 9). However, for patients without LNM (pN0 patients), LNR cannot predict the prognosis better than pN stage system. Also, the number of NLNs significantly affects prognosis (10, 11). Both LNR and pN stage are not to consider the effect of NLNs.
Log odds of PLNs (LODDS), defined as the log of the ratio of the number of PLNs to the number of NLNs, has been introduced as a prognostic marker in rectal cancer (9). Xu et al. has reported that LODDS showed a good predictive capacity in the OS and CSS of gastric SRCC patients (12). In the study by Scarinci et al., LODDS was confirmed to be superior to LNR and pN stage in predicting the short-term OS of CRC (13). Also, Scarinci et al. suggested cohort studies with larger sample size to further explore whether it could be superior to LNR and pN stage in patients with subtypes (13). SRCC is characterized by high malignancy and poor prognosis and is a rare type of CRC to which less attention has been paid (1); thus, we further explore the association between LODDS and prognosis of colorectal SRCC patients and compare the predictive capacity of LODDS with pN stage system and LNR.
This was a retrospective cohort study, and data were extracted from the Surveillance, Epidemiology and End Results (SEER) database (2004–2015, November 2018 submission), which was an authoritative source for cancer statistics in the United States (https://seer.cancer.gov/). SEER collected cancer incidence and survival data from 18 states and municipal registries, covering approximately 34.6% of the US population. SEER program was supported by the National Cancer Institute (NCI) and is freely available to the public. Therefore, informed consent from patients and approval from the Institutional Review Board of the Jiangyin Hospital of Traditional Chinese Medicine was not required for this study. All procedures involving human participants in this study were performed in accordance with the Declaration of Helsinki-2013 revision.
Participants who met all the following criteria were included: (1) SRCC diagnosed in line with the International Classification of Disease for Oncology—third version (ICD-O-3; coded as 8490/3); (2) diagnosed as primary colorectal SRCC patients; (3) age at diagnosis ≥18 years; (4) with complete pathological information, operation, and complete survival data. Exclusion criteria were as follows: (1) missed data on lymph nodes; (2) with multiple primary tumors; (3) the reported diagnosis source from autopsy or death certificate or only clinically diagnosed.
Data used in this study were extracted from the SEER 18 database (Nov 2018 Sub, 1975–2016 varying). The SEER*Stat 8.4.0 software was used to generate the case listing (https://seer.cancer.gov/data-software/documentation/seerstat/). We used data based on covariates (demographic characteristics, clinicopathological features, and treatment), prognostic variates (pN stage, LNR, and LODDS), and outcome variates (OS and CSS).
Demographic characteristics contained age (age at diagnosis), sex (male/female), race (white, black, other, unknown), marital status (single, married, unknown), and year of diagnosis (2004–2007, 2008–2011, 2012–2015). Clinicopathological characteristics contained tumor size (categorized by tertiles: <32, 32–64, >64 mm), primary site (cecum and appendix, colon, rectum), grade (I, well differentiated; II, moderately differentiated; III, poorly differentiated; IV, undifferentiated; and not stated), T stage (T1, T2, T3, and T4), and M stage (M0 and M1). Clinical characteristics contained the treatment (all assessed as categorical variables), chemotherapy (no/unknown and yes), radiation (no/unknown and yes), radiation with surgery (no/unknown, prior, and after/others), and surgery types (local/partial resection, total resection, and unspecific-underwent resection but no known the surgery site).
For comparative purposes, we classified lymph node status by pN stage, LNR, and LODDS. Based on AJCC eighth edition, pN stage was classified into N0 (no LNM), N1 (1–3 LNM), and N2 (4 and more LNM) (14). LNR was defined as the ratio between the number of PLNs and the number of TLNs, and divided into three groups: LNR 1 (≤0.32), LNR 2 (0.32–0.68), and LNR 3 (>0.68). LODDS value was calculated as follows: loge (number of PLNs + 0.5)/(number of NLNs + 0.5), where 0.5 was added to avoid an infinite number (12). Patients were divided into 3 categories: LODDS 1 (≤−3.16), LODDS 2 (−3.16–0.60) and LODDS 3 (>0.60). The cut-off values of LNR and LODDS were evaluated by x-tile software (version 3.6.1, Yale University) based on minimum P value method (15).
Outcomes were OS and CSS. OS was defined as the period from diagnosis to death from any causes. CSS was defined as the duration from diagnosis to death attributed to SRCC. The assessment of OS and CSS was according to the records in the SEER database (alive, cancer-specific death, and other death). The follow-up was ended if patients died during the follow-up period.
Categorical data were reported as number (n) and proportion (%), and compared using the Chi-square or nonparametric test. Continuous data in normal distribution were reported as mean ± standard deviation (mean ± SD) and compared using the independent-sample t test. Continuous data in skew distribution were expressed as median and quartile [M (Q1, Q3)] and compared using the independent-sample Wilcoxon rank sum test. Univariate Cox proportional hazard model (for OS) and univariate Cox competing risk model (for CSS) were used to identify the significant factors influencing OS and CSS. Association between pN stage, LNR, and LODDS with OS and CSS was explored using univariate and multivariate Cox proportional hazard model and Cox competing risk model, respectively, and results were shown as hazard ratio (HR) with 95% confidence interval (95% CI). Prediction capacity of the pN stage system, LNR, and LODDS in OS and CSS was evaluated by calculating the C-index. The prognostic capacity of the pN stage system, LNR, and LODDS was also assessed according to the number of retrieved lymph nodes (TLNs < 12 or TLNs ≥ 12) and neoadjuvant radiotherapy (with or without). Statistical analyses were performed using SPSS 26.0 software for Mac (IBM, Armonk, NY, United States). P <0.05 was considered statistically significant.
We extracted 8,701 patients with primary colorectal SRCC from the SEER database. Of these, 2,482 patients were excluded because they were younger than 18 years (n = 30) and had incomplete data on tumor size (n = 272), pathological stage (n = 2170), and the operation (n = 10). Of the remaining 6,219 patients, we further excluded 4,021 patients due to missing data on lymph nodes (n = 2,359), with multiple primary tumors (n = 1,662), and with diagnosis source from autopsy or death certificate or only clinically diagnosed (n = 0). Finally, a total of 2198 colorectal SRCC patients were included in our study (Figure 1). These patients were composed of 1,101 men (50.09%) and 1,097 women (49.91%), and the mean age was 63.47 ± 16.29 years. The median survival time of alive patients, patients with SRCC-specific death, and patient died from other causes was 56 months (range 27–96), 13 months (range 6–24), and 20 months (range 5.00–58.00), respectively. Age, year of diagnosis, tumor size categories, primary site, AJCC T stage, AJCC N stage, AJCC M stage, LNR categories, LODDS categories, chemotherapy, surgery types, and survival months were significantly different among the groups. Characteristics of the included patients are summarized in Table 1.
Supplementary Table S1 shows that age, married status, tumor size, AJCC T stage, AJCC M stage, chemotherapy, and primary site were significant factors affecting the OS of colorectal SRCC patients. Marriage status, tumor size, AJCC T stage, AJCC M stage, chemotherapy, surgery types, and primary site were significant factors affecting the CSS of colorectal SRCC patients. Considering many studies have reported radiotherapy as an important influencing factor of the prognosis in colorectal SRCC (16, 17), radiation sequence with surgery was also considered as a covariate in this study.
In the univariate analysis, higher pN stage, LNR, and LODDS were associated with the worse OS and CSS (all P < 0.001). After adjusting for age, married status, tumor size, AJCC T stage, AJCC M stage, chemotherapy, primary site, and radiation sequence with surgery, pN stage (N1: HR = 2.04, 95% CI: 1.69–2.46; N2: HR = 3.40, 95% CI: 2.86–4.03), LNR (LNR 2: HR = 1.96, 95% CI: 1.72–2.26; LNR 3: HR = 2.97, 95% CI: 2.60–3.39), and LODDS (LODDS 2: HR = 2.51, 95% CI: 2.06–3.04; LODDS 3: HR = 5.08, 95% CI: 4.13–6.26) showed similar results in the OS. Also, N1, N2, LNR 2, LNR 3, LODDS 2, and LODDS 3 were associated with higher risk of worse CSS after adjusting for married status, tumor size, AJCC T stage, AJCC M stage, chemotherapy, surgery types, primary site, and radiation sequence with surgery, with HR of 2.46 (95% CI: 1.94–3.10), 3.90 (95% CI: 3.11–4.87), 2.02 (95% CI: 1.74–2.34), 2.70 (95% CI: 2.33–3.13), 3.21 (95% CI: 2.48–4.15), and 5.64 (95% CI: 4.29–7.43), respectively (Table 2).
Table 3 shows the predictive performance of pN stage, LNR, and LODDS in the OS and CSS. LODDS (C-index: 0.704, 95% CI: 0.690–0.718) had a better performance in predicting the OS than LNR (C-index: 0.657, 95% CI: 0.643–0.671) and pN stage (C-index: 0.643, 95% CI: 0.629–0.657). We also found the better performance of LODDS in the CSS, with C-index of 733 (95%CI: 0.719–0.747), which was higher than 0.713 (95% CI: 0.697–0.729) of LNR and 0.667 (95% CI: 0.651–0.683) of pN stage.
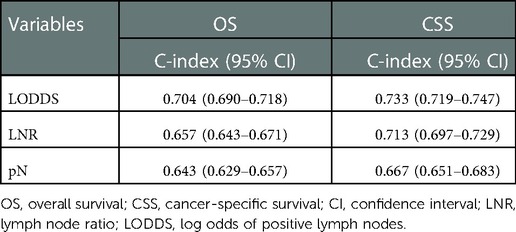
Table 3. Predictive capacity of pN stage, LNR, and LODDS in the OS and CSS of colorectal SRCC patients.
Table 4 displays the predictive capacity of pN stage, LNR, and LODDS in the OS and CSS based on retrieved lymph nodes. In patients with number of retrieved lymph nodes <12, LODDS had a better predictive capacity in the OS and CSS, with C-index of 0.663 (95% CI: 0.641–0.691) and 0.711 (95% CI: 0.681–0.741), respectively. In patients with number of retrieved lymph nodes ≥12, the results remained similar, with C-index of 0.713 (95% CI: 0.701–0.731) for OS and 0.739 (95% CI: 0.721–0.751) for CSS.
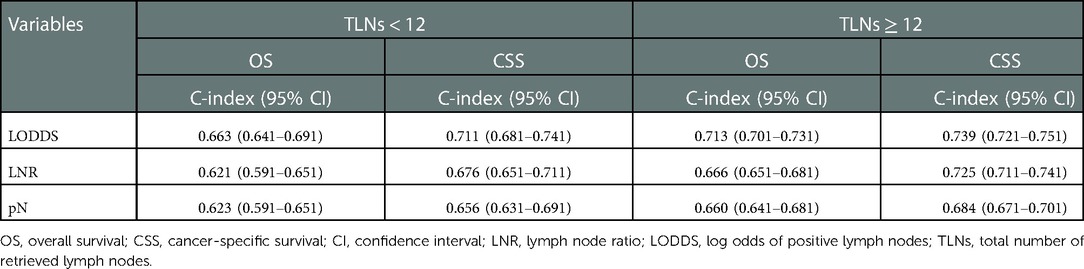
Table 4. Prognostic efficacy of pN stage, LNR, and LODDS in the OS and CSS of colorectal SRCC patients based on retrieved lymph nodes.
The prognosis of CRC has gained much attention (18, 19), but few studies explored the prognosis of colorectal SRCC, which is a rare and special subtype of CRC and characterized by a poorer prognosis (1). In this study, we found that LODDS, LNR, and pN stage were associated with the OS and CSS of colorectal SRCC patients. LODDS had a better performance to predict the OS and CSS than LNR and pN stage in colorectal SRCC patients. Similar results were found in patients with TLNs either <12 or ≥12.
LNM is an important factor affecting the prognosis of CRC and affects therapeutic decisions (5). AJCC pN stage and LNR are both reported as prognostic factors in CRC (6, 8), but AJCC pN stage is limited by TLNs and LNR did not consider the effect of NLNs (7, 11). LODDS has been reported to be less influenced by TLNs and takes into account the number of NLNs (9). Scarinci et al. have reported the association of LODDS, LNR, and pN stage with the OS in CRC (13). Similarly, our study has found that LODDS, LNR, and pN stage were associated with OS and CSS of colorectal SRCC patients.
Several studies have shown the strong prognostic ability of LODDS in SRCC (12, 20). In a cohort study, Wang et al. found that, compared with the pN stage, LODDS was a highly reliable index with a good predictive performance for the OS and CSS of patients with esophageal SRCC (20). Xu et al. enrolled 1,365 patients with gastric SRCC and developed a prognostic nomogram based on LODDS, which provided a more satisfying predictive capacity both in the OS and CSS than AJCC TNM stage alone (12). Scarinci et al. has reported that the predictive performance of LODDS was superior to LNR and pN stage in the OS of CRC patients and suggested a future study to verify their findings in the subtype of CRC (13). In this study, we found that LODDS outmatched the pN stage and LNR for the prediction of OS and CSS in colorectal SRCC, which made up the gap of the study reported by Scarinci et al. These findings clearly showed that LODDS could accurately predict the survival of colorectal SRCC patients. Therefore, LODDS may be a useful and scientific tool to evaluate lymph node dissection and to consider ratio-based index into the prognosis of colorectal SRCC patients and was recommended to examine LNM in practical use.
Some observational studies have found that adequate evaluation of TLNs (≥12) is associated with the increased survival (21–23). However, half of the CRC patients receive inadequate lymph node assessment (7). Herein, we performed subgroup analysis to explore the prediction capacity of LODDS, LNR, and pN based on the TLNs. In colorectal SRCC patients with TLNs < 12, the predictive performance of LODDS was superior to LNR and pN stage in the OS and CSS. We found similar results in the patients with TLNs ≥12. Our findings further confirmed the accuracy of LODDS in the prediction of OS and CSS.
There are some strengths in our study. First, our data are extracted from the SEER database, which contains a large, validated, representative sample of the US population. Second, our study makes up for the gap in the prediction capacity of LODDS in colorectal SRCC, which indicates that LODDS may be a useful and scientific tool to evaluate lymph node dissection and predict the prognosis of colorectal SRCC patients. In addition, our study has some limitations. First, this is a retrospective study, which may cause selection bias and information bias. Second, important factors associated with the prognosis of colorectal SRCC, such as pathological data, nutritional status, and lifestyle, were not recorded in the SEER database. Third, neoadjuvant therapy may affect postoperative LNMs, but chemotherapy time is not recorded and radiotherapy sequence of most patients (89.49%) was recorded as no/unknown in the database. Therefore, patients undergoing neoadjuvant therapy cannot be accurately distinguished, which limits us to explore the effect of neoadjuvant therapy on the results. However, we adjust the relevant information on chemotherapy and radiotherapy to minimize its impact on the results. Fourth, external validation is not performed due to the lack of a sufficient sample size; further studies in the actual clinical samples are needed to validate our findings.
Our study found the better predictive capacity of LODDS than LNR and pN stage in the OS and CSS in colorectal SRCC, indicating that LODDS may be a useful and scientific tool to assess the prognosis of colorectal SRCC patients in the clinic.
Publicly available datasets were analyzed in this study. This data can be found here: SEER database, https://seer.cancer.gov/.
Ethical approval was not provided for this study on human participants because the data used in this study was from a public database. The patients/participants provided their written informed consent to participate in this study.
XH and WM designed the study. XH wrote the manuscript. XH, LJ, and JW collected, analyzed, and interpreted the data. WM critically reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1019454/full#supplementary-material.
1. Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers (Basel). (2020) 12:1544. doi: 10.3390/cancers12061544
2. Weng MT, Chao KH, Tung CC, Chang HC, Shih IL, Lin BR, et al. Characteristics of primary signet ring cell carcinoma of colon and rectum: a case control study. BMC Gastroenterol. (2022) 22:173. doi: 10.1186/s12876-022-02258-1
3. Zhu L, Ling C, Xu T, Zhang J, Zhang Y, Liu Y, et al. Clinicopathological features and survival of signet-ring cell carcinoma and mucinous adenocarcinoma of right colon, left colon, and rectum. Pathol Oncol Res. (2021) 27:1609800. doi: 10.3389/pore.2021.1609800
4. Sun Y, Yu D, Zhong J, Lin Y, Cheng N, Lin H, et al. Para-aortic lymph node dissection in left-sided colorectal cancer: risk factors, prognostic impact, and therapeutic value. J Surg Oncol. (2022) 125:1251–9. doi: 10.1002/jso.26829
5. Kiehl L, Kuntz S, Höhn J, Jutzi T, Krieghoff-Henning E, Kather JN, et al. Deep learning can predict lymph node status directly from histology in colorectal cancer. Eur J Cancer. (2021) 157:464–73. doi: 10.1016/j.ejca.2021.08.039
6. Chen K, Collins G, Wang H, Toh JWT. Pathological features and prognostication in colorectal cancer. Curr Oncol. (2021) 28:5356–83. doi: 10.3390/curroncol28060447
7. Li Destri G, Di Carlo I, Scilletta R, Scilletta B, Puleo S. Colorectal cancer and lymph nodes: the obsession with the number 12. World J Gastroenterol. (2014) 20:1951–60. doi: 10.3748/wjg.v20.i8.1951
8. Zanghì A, Cavallaro A, Lo Menzo E, Curella Botta S, Lo Bianco S, Di Vita M, et al. Is there a relationship between length of resection and lymph-node ratio in colorectal cancer? Gastroenterol Rep. (2021) 9:234–40. doi: 10.1093/gastro/goz066
9. Xu T, Zhang L, Yu L, Zhu Y, Fang H, Chen B, et al. Log odds of positive lymph nodes is an excellent prognostic factor for patients with rectal cancer after neoadjuvant chemoradiotherapy. Ann Transl Med. (2021) 9:637. doi: 10.21037/atm-20-7590
10. Dinaux AM, Leijssen L, Bordeianou LG, Kunitake H, Amri R, Berger DL. Outcomes of persistent lymph node involvement after neoadjuvant therapy for stage III rectal cancer. Surgery. (2018) 163:784–8. doi: 10.1016/j.surg.2017.10.021
11. Sun Y, Zhang Y, Huang Z, Chi P. Prognostic implication of negative lymph node count in ypN+ rectal cancer after neoadjuvant chemoradiotherapy and construction of a prediction nomogram. J Gastrointest Surg. (2019) 23:1006–14. doi: 10.1007/s11605-018-3942-3
12. Xu Z, Jing J, Ma G. Development and validation of prognostic nomogram based on log odds of positive lymph nodes for patients with gastric signet ring cell carcinoma. Chin J Cancer Res. (2020) 32:778–93. doi: 10.21147/j.issn.1000-9604.2020.06.11
13. Scarinci A, Di Cesare T, Cavaniglia D, Neri T, Colletti M, Cosenza G, et al. The impact of log odds of positive lymph nodes (LODDS) in colon and rectal cancer patient stratification: a single-center analysis of 323 patients. Updates Surg. (2018) 70:23–31. doi: 10.1007/s13304-018-0519-3
14. Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. (2018) 25:1454–5. doi: 10.1245/s10434-018-6462-1
15. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. (2004) 10:7252–9. doi: 10.1158/1078-0432.Ccr-04-0713
16. Ling CR, Wang R, Wang MJ, Ping J, Zhuang W. Prognosis and value of preoperative radiotherapy in locally advanced rectal signet-ring cell carcinoma. Sci Rep. (2017) 7:45334. doi: 10.1038/srep45334
17. Kong X, Zhang X, Huang Y, Tang L, Peng Q, Li J. Characteristics and prognostic factors of colorectal mucinous adenocarcinoma with signet ring cells. Cancer Manag Res. (2017) 9:573–80. doi: 10.2147/CMAR.S149582
18. Zhang JX, Qin MB, Ye Z, Peng P, Li SM, Song Q, et al. Association of tricellulin expression with poor colorectal cancer prognosis and metastasis. Oncol Rep. (2020) 44:2174–84. doi: 10.3892/or.2020.7773
19. Takagi Y, Sakai N, Yoshitomi H, Furukawa K, Takayashiki T, Kuboki S, et al. High expression of Krüppel-like factor 5 is associated with poor prognosis in patients with colorectal cancer. Cancer Sci. (2020) 111:2078–92. doi: 10.1111/cas.14411
20. Wang F, Gao SG, Xue Q, Tan FW, Gao YS, Mao YS, et al. Log odds of positive lymph nodes is a better prognostic factor for oesophageal signet ring cell carcinoma than N stage. World J Clin Cases. (2021) 9:24–35. doi: 10.12998/wjcc.v9.i1.24
21. Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol. (2002) 26:179–89. doi: 10.1097/00000478-200202000-00004
22. Sarli L, Bader G, Iusco D, Salvemini C, Mauro DD, Mazzeo A, et al. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer. (2005) 41:272–9. doi: 10.1016/j.ejca.2004.10.010
Keywords: log odds of positive lymph nodes, lymph node ratio, pN stage, colorectal signet ring cell carcinoma, SEER
Citation: Hu X, Jiang L, Wu J and Mao W (2023) Prognostic value of log odds of positive lymph nodes, lymph node ratio, and N stage in patients with colorectal signet ring cell carcinoma: A retrospective cohort study. Front. Surg. 9:1019454. doi: 10.3389/fsurg.2022.1019454
Received: 15 August 2022; Accepted: 28 November 2022;
Published: 5 January 2023.
Edited by:
Peng Liu, Sun Yat-sen University, ChinaReviewed by:
Salem Baldi, Axbio Biotechnology (Shenzhen) Co., Ltd. Shenzhen, China© 2023 Hu, Jiang, Wu and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weida Mao TWFvd2QtZG9jMTIzQGhvdG1haWwuY29t
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.