- 1Department of Breast Surgery, Yongchuan Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Anesthesiology, Yongchuan Hospital of Chongqing Medical University, Chongqing, China
Objective: To study the analgesic effect of breast cancer patients undergoing modified radical mastectomy (MRM) and the influence of perioperative T lymphocyte subsets by remifentanil combined with dexmedetomidine.
Methods: 80 breast patients were divided into control group and research group based on the anesthesia protocol. Patients in control group was given remifentanil for anesthesia induction and maintenance, and patients in research group was given remifentanil and dexmedetomidine for anesthesia induction and maintenance. We compared the anesthesia time, operation time, surgical blood loss, postoperative wake-up time, extubation time, incidence of adverse reactions, VAS score and T lymphocyte subsets in peripheral blood in the two groups of patients.
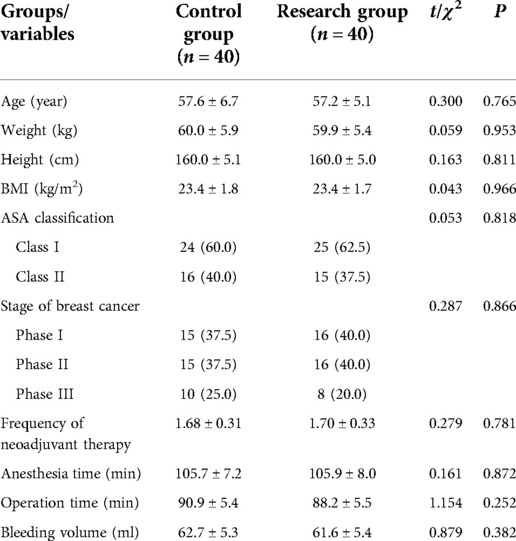
Results: The baseline data including age, height, weight and BMI, ASA classification, stage of breast cancer, frequency of neoadjuvant therapy, and surgical characteristics including anesthesia time, operation time and bleeding volume all have no significant difference between two groups (P > 0.05). Compared to control group, the time of wake up and extubation in patients of research group were all significantly decreased (P < 0.05), and significantly decreased MBP and HR after loading dose of dexmedetomidine in research group (P < 0.05). The VAS scores of patients at 4, 8, 12, 16, 20 and 24 h after surgery in the research group are all significantly lower than those in the control group (P < 0.05). Before induction of anesthesia, there was no significant difference in the ratio of CD4+, CD8+ and CD4+/CD8+ T lymphocytes in peripheral blood between the two groups (P > 0.05). At 1 h during operation and 24 h after operation, the ratio of CD4+ and CD4+/CD8+ cells in the research group was significantly higher than these of the control group (P < 0.05), while the ratio of CD8+ cells was lower than that of the control group (P < 0.05).
Conclusion: For breast cancer patients undergoing MRM, the use of remifentanil combined with dexmedetomidine can enhance postoperative analgesia and reduce postoperative immunosuppression.
Introduction
Modified radical mastectomy (MRM) is a common surgical treatment for breast cancer patients. However, immunosuppression caused by surgical anesthesia is the main cause of postoperative infection, immune escape of cancer cells, and metastasis of residual tumor cells (1–3). Anesthesia methods affect neuroendocrine stress responses and immunosuppression, affecting the body's anti-tumor and anti-inflammatory responses, which in turn are associated with cancer recurrence. Therefore, the development of better anesthesia programs is of great significance to improve the postoperative survival rate of breast cancer patients and reduce recurrence. Dexmedetomidine is an effective α2-adrenergic receptor agonist, which not only has the effect of inhibiting the high activity of the central nervous system, but also has the function of antidepressant, anxiolytic and analgesic (4). Importantly, dexmedetomidine is a commonly used adjuvant drug during surgical anesthesia (5). At the same time, dexmedetomidine as an anesthesia adjuvant drug used in MRM not only helps patients to regain consciousness after surgery, but also significantly reduces the incidence of intraoperative and postoperative complications (6, 7).
T lymphocytes are derived from bone marrow pluripotent stem cells and differentiate and mature in the thymus, and they are important immune cells in the human body, and play functions such as cellular immunity and immune regulation in the human body (8, 9). Anesthetic drugs can inhibit the excessive stress response of patients during the perioperative period, directly or indirectly affect the immune function of the body, and affect the specific immune response mediated by T lymphocytes. Previous studies have found that dexmedetomidine combined with remifentanil can achieve better anesthesia effect in patients with breast cancer after MRM, and can significantly reduce the incidence of postoperative adverse reactions in patients with breast cancer (10, 11). However, there are few studies on the effect of dexmedetomidine combined with remifentanil on peripheral blood T lymphocyte subtypes in breast cancer patients under anesthesia in patients with MRM. In this study, we designed to compare the changes of peripheral blood T lymphocyte subtypes in patients undergoing MRM for breast cancer under remifentanil anesthesia alone and remifentanil combined with dexmedetomidine anesthesia. Based on this, the purpose of this study was to study the efficacy of dexmedetomidine in improving the immunosuppressive effect of anesthesia-induced breast cancer patients undergoing MRM.
Materials and methods
Ethics statement and patients
This study was approved by the Institutional Medical Ethics Committee of our hospital, and was in accordance with the Declaration of Helsinki. All patients being included in the present study were informed about the content of this study and signed an informed consent form.
A total of 80 breast cancer patients who underwent radical surgery in our hospital from 2019 to 2021 were collected, all patients are female, they were divided into control group (n = 40) and research group (n = 40) according to the anesthesia protocol. The patients in the control group were 45–64 years old, American Society of Anesthesiologists (ASA) stage I and II are 24 case and 16 case, respectively, breast cancer stages I, II, and III are 15 case, 15 case, and 10 case, respectively. The patients in the research group were 43–64 years old, 25 case and 15 case were ASA stage I and II, respectively; and 16 case, 16 case, and 8 case were breast cancer stages I, II, and III, respectively. Inclusion criteria: (1) diagnosed with breast cancer; (2) younger than 65 years old; (3) ASA stage I-II; (4) undergoing MRM; (5) weight 45–75 kg, height 145–175 cm. Exclusion criteria: (1) Combined with other malignant tumors; (2) Combined with diseases of other tissues and organs such as heart, brain, liver, and kidney; (3) Patients with clinical stage IV breast cancer; (4) Past history of infectious diseases and drug addiction; (4) Cognitive dysfunction or other mental illness.
Anesthesia protocol
All patients were fasted before operation. After the establishment of anesthesia intravenous channel, the control group was given intravenous injection of 2–4 µg/kg remifentanil (Yichang Renfu Pharmaceutical Co., Ltd.) for induction of anesthesia, and 0.5–2 µg/kg/min remifentanil was used for anesthesia maintenance; 15 min before induction of anesthesia, patients in the research group were intravenously injected with 1 µg/kg dexmedetomidine (Jiangsu Hengrui Medicine Co., Ltd.), and then the anesthesia method was the same as control group. The other anesthetics were used in the same way in the two groups.
Data collection
According to patient electronic medical records, we collected baseline data including age, height, weight and body mass index (BMI), ASA classification, stage of breast cancer, frequency of neoadjuvant therapy. At the same, we recorded the surgical characteristics including anesthesia time, operation time and bleeding volume, and recorded the anesthesia effect related indicators including mean blood pressure (MBP) and heart rates (HR) at baseline (T0), time after loading dose (T1), induction (T2), intubation (T3), and 30 min after intubation (T4), 60 min after intubation (T5), and 90 min after intubation (T6), and 24 h after surgery (T7). Moreover, the occurrence of adverse reactions in all patients within 48 h after surgery was recorded, including nausea, vomiting, bradycardia, respiratory depression and itching.
Primary outcome
Pain assessment
We used visual analogue scale (VAS) to assess the pain of patients at 4, 8, 12, 16, 20 and 24 h after surgery. The VAS scale is the pain rating scale, which uses a visual analog method to judge the severity of pain. The scoring scale was divided into 10 equal parts using a ruler, with 0 being no pain, 1–3 being mild pain, 4–6 being moderate pain, and 7–10 being severe pain (12, 13).
Peripheral blood T lymphocytes
Before induction of anesthesia (preoperative), 1 h during operation (intraoperative) and 24 h after operation (postoperative), we collected 5 ml of peripheral blood from patients, centrifuged the peripheral blood mononuclear cells of patients by density gradient centrifugation, and then added CD3-PE antibody, CD4-TITC antibody and CD8-APC antibody respectively and incubated for half an hour in the dark. Finally, T lymphocyte subtypes were analyzed by flow cytometry.
Statistical analysis
SPSS 20.0 (SPSS Inc., Chicago, USA) was used to analyze the data in this study. The chi-square test was used to compare the difference of count data between the two groups, and the Student’s t-test was used to compare the difference of the measurement data between the two groups. P < 0.05 indicates that the difference is statistically significant.
Results
Demographic and surgical characteristics
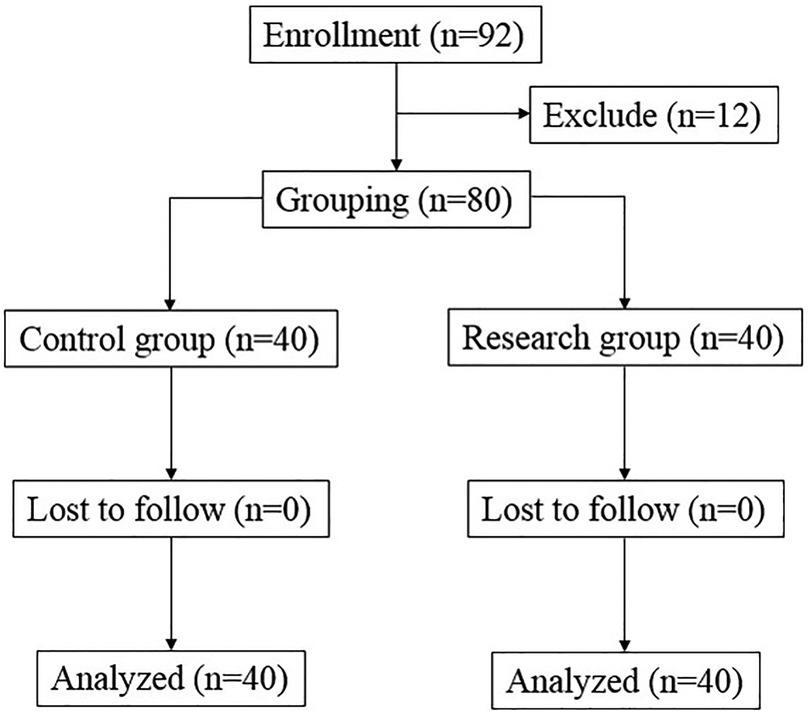
There were 92 breast cancer patients being valuated for eligibility, and 12 patients dropped out during the study, and finally 80 patients were involved in the clinical observation experiment. According to the anesthesia protocol, those 80 breast cancer patients were divided into two group: Control group (n = 40) and Research group (n = 40) (Figure 1). The baseline data including age, height, weight and BMI, ASA classification, stage of breast cancer, frequency of neoadjuvant therapy, and surgical characteristics including anesthesia time, operation time and bleeding volume all have no significant difference between two groups (Table 1).
Anesthesia effect related indicators
The time of wake up and extubation in patients of research group were all significantly lower than those in patients of control group (Figure 2). At the same time, the two groups were also comparable with respect to their baseline MBP (Figure 3A) and heart rates HR (Figure 3B) before surgery. Compared to control group, decreased MBP and HR after loading dose of dexmedetomidine in research group (Figures 3A,B).
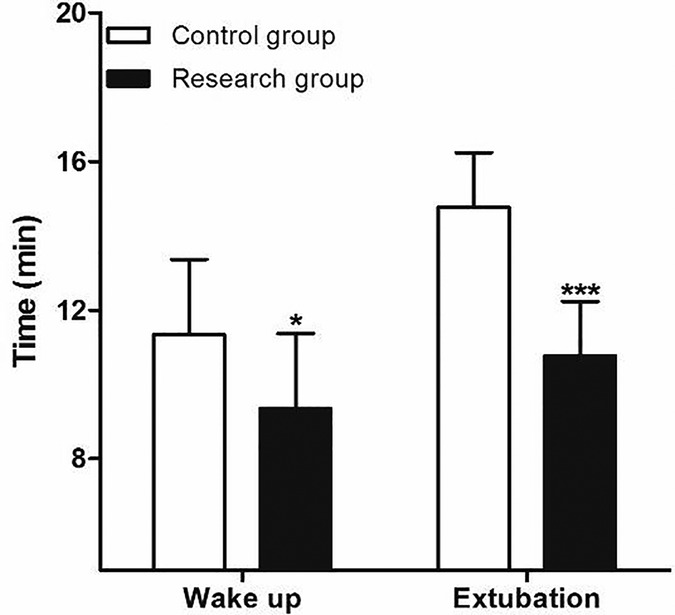
Figure 2. Comparison of postoperative the time of waken up and extubation between two group. Compared with Control group, *P < 0.05 and ***P < 0.001.
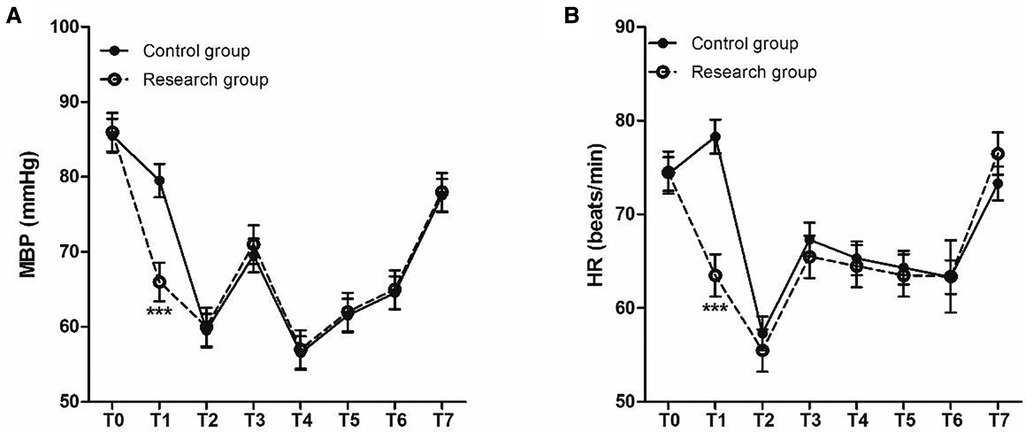
Figure 3. Perioperative HR and MBP at different time points. (A) MBP (mmHg) and (B) HR (beats/min). T0, baseline; T1, time after loading dose; T2, induction; T3, intubation; T4–T6, 30, 60, and 90 min after intubation; T7, 24 h after surgery.
VAS score for the analgesic effect
The VAS scores of patients at 4, 8, 12, 16, 20 and 24 h after surgery in the research group are all significantly lower than those in the control group (P < 0.001) (Figure 4).
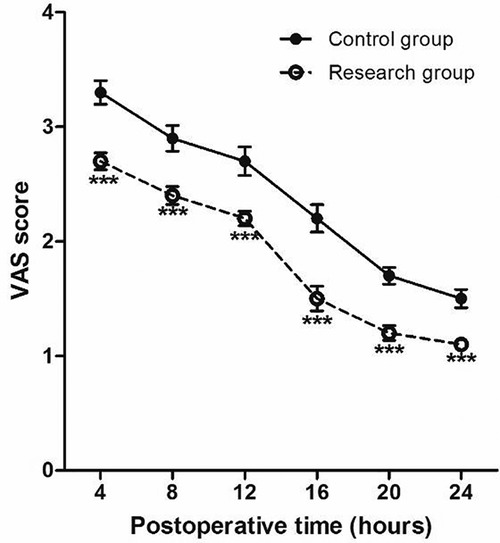
Figure 4. Perioperative VAS score at different time points. Compared with Control group, *P < 0.05 and ***P < 0.001.
Adverse reactions
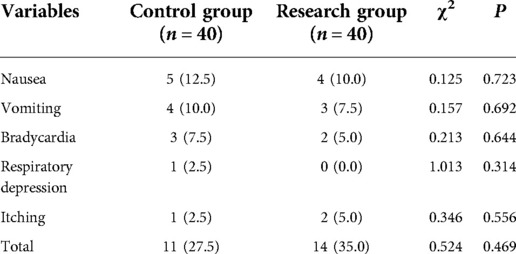
Within 48 h after surgery, nausea, vomiting, bradycardia, respiratory depression and itching occurred in 5 case, 4 case, 3 case, 1 case and 1 case in the control group, respectively. However, nausea, vomiting, bradycardia, respiratory depression and itching occurred in 4 case, 3 case, 2 case, 0 case and 2 case in the research group, respectively. There is no significant difference between the control group and research group in the adverse reactions at 48 h after surgery (35.0% vs. 27.5%, P > 0.05) (Table 2).
Peripheral blood T lymphocyte subsets
The two groups were also comparable with respect to their baseline CD4+ (Figure 5A), CD8 + T (Figure 5B) and CD4+/CD8+ (Figure 5C) T lymphocyte (P > 0.05). At 1 h during the operation (intraoperative) and 24 h after the operation (postoperative), the CD4+ and CD4+/CD8+ T cells in the research group were significantly higher than those in the control group (P < 0.001), while the CD8+ T cells were significantly lower than those in the control group(P < 0.001) (Figure 5).
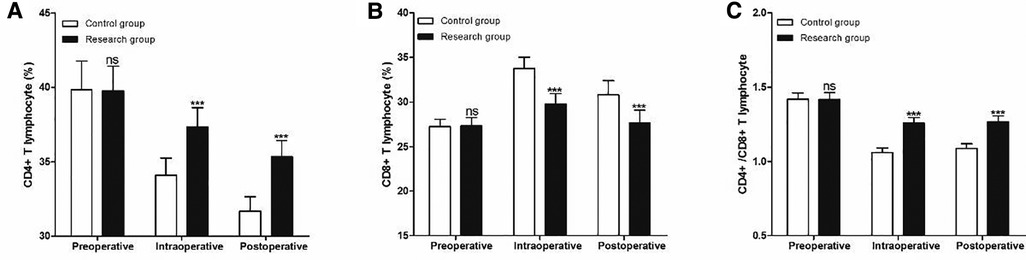
Figure 5. Perioperative peripheral blood T lymphocyte subsets at different timepoints. (A) CD4 T lymphocyte, (B) CD8 T lymphocyte and (C) CD4+/CD8+ T lymphocyte. Compared with Control group, ns P > 0.05 and ***P < 0.001.
Discussion
Breast cancer is one of the most common malignancies, with approximately 2.2 million new cases of breast cancer and more than 680,000 deaths worldwide each year (14). In China, there are 420,000 new cases of breast cancer and 120,000 deaths from breast cancer each year (15, 16). There are five main treatments for breast cancer, including surgery, radiotherapy, chemotherapy, targeted therapy and immunotherapy. Among them, preoperative neoadjuvant chemoradiotherapy combined with surgery has become a classic treatment strategy for patients with advanced breast cancer (17, 18). However, immunosuppression caused by surgical anesthesia in breast cancer patients undergoing MRM will not only increase the risk of postoperative infection, but also increase the risk of tumor cell immune escape and increase the probability of postoperative recurrence (19, 20). Therefore, a scientific and appropriate anesthesia scheme is of great significance to the survival and postoperative recovery of patients after radical resection of adenocarcinoma.
Dexmedetomidine is a highly selective α2 adrenergic receptor agonist, which has the advantages of rapid onset of action, short duration of action, sedative and analgesic effects, and no respiratory depression, and is a widely used anesthesia adjuvant drug (9, 10). In this study, we found that the anesthesia time, operation time, and blood loss during the operation did not increase in the research group with additional dexmedetomidine for anesthesia, indicating that dexmedetomidine does not affect the surgical process of patients undergoing MRM for breast cancer, which is consistent with the results in the study by Das et al. (21). Das et al. found that the addition of dexmedetomidine can not only significantly reduce the consumption of anesthetics in patients undergoing MRM for breast cancer, but also significantly shorten the postoperative breathing satisfaction time, eye opening time and extubation time. In addition, both this study and the study of Das et al. found that the incidence of postoperative adverse reactions in patients with dexmedetomidine anesthesia was lower, indicating that the addition of dexmedetomidine for anesthesia can not only improve the anesthesia effect, but also is safe and effective.
In addition, in this study, we also used the VAS scale to evaluate the pain status of patients at different times after surgery, and the results found that the VAS scores of the patients in the research group were significantly lower than those in the control group within 24 h after surgery, it shows that the postoperative analgesia effect of the patients in the research group was better, which is basically consistent with the research results of Liu et al. (22). A meta-analysis of 12 clinical studies reported that dexmedetomidine was a favorable anesthetic adjuvant in breast cancer surgery, which can relieve postoperative pain (22). However, the results of the study by Yang et al. was different from this study, and they found that the VAS scores of breast cancer patients with dexmedetomidine added at 2, 8 and 24 h after surgery were not significantly different from those of control patients (23). Therefore, the effect of dexmedetomidine on postoperative pain in patients with MRM for breast cancer may be related to the time of pain assessment, the included population, and the subject of the assessment.
T lymphocytes are a key component of the human immune system. By making appropriate activation responses to relevant antigens, they lead and coordinate various immune responses of the immune system to ensure that the body effectively removes invading pathogens or diseased cells, and avoids immune diseases occur on its own (24, 25). CD is short for leukocyte differentiation antigen, CD3 is a class of antigens on the surface of T lymphocytes, and CD3+ refers to mature T lymphocytes. CD3 binds to the T cell receptor and can transmit antigen signals to T lymphocytes (26, 27). CD4+ lymphocytes, namely helper T cells, have the function of assisting humoral and cellular immunity (28, 29). CD8+ lymphocytes, namely cytotoxic T cells, have the function of killing target cells and are activated by direct binding of MHCI to antigens (30, 31). In this study, we found that the ratio of CD4+ and CD4+/CD8+ cells in the research group was significantly higher than these of the control group, while the ratio of CD8+ cells was lower than that of the control group at 1 h during operation and 24 h after operation, indicating dexmedetomidine can significantly reduce anesthesia-induced immunosuppression in patients undergoing MRM for breast cancer.
Conclusion
In conclusion, adding dexmedetomidine to anesthetize patients with MRM for breast cancer can not only enhance the anesthesia effect of remifentanil, but also reduce postoperative pain and the incidence of postoperative adverse reactions, and reduce postoperative immunosuppression by regulating T lymphocyte subsets.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by This study was approved by the ethics committee of our hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YZ, WJ are the mainly responsible for the writing, research design of the article. The corresponding author is XL, and she is responsible for ensuring that the descriptions are accurate and agreed by all authors. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Karmakar MK, Samy W, Lee A, Li JW, Chan WC, Chen PP, et al. Survival analysis of patients with breast cancer undergoing a modified radical mastectomy with or without a thoracic paravertebral block: a 5-year follow-up of a randomized controlled trial. Anticancer Res. (2017) 37:5813–20. doi: 10.21873/anticanres.12024
2. Adesoye T, Lucci A. Current surgical management of inflammatory breast cancer. Ann Surg Oncol. (2021) 28:5461–7. doi: 10.1245/s10434-021-10522-z
3. Marinkovic M, Djordjevic N, Djordjevic L, Ignjatovic N, Djordjevic M, Karanikolic V. Assessment of the quality of life in breast cancer depending on the surgical treatment. Support Care Cancer. (2021) 29:3257–66. doi: 10.1007/s00520-020-05838-7
4. Li R, Lai IK, Pan JZ, Zhang P, Maze M. Dexmedetomidine exerts an anti-inflammatory effect via α2 adrenoceptors to prevent lipopolysaccharide-induced cognitive decline in mice. Anesthesiology. (2020) 133:393–407. doi: 10.1097/ALN.0000000000003390
5. Afonso J, Reis F. Dexmedetomidine: current role in anesthesia and intensive care. Rev Bras Anestesiol. (2012) 62:118–33. doi: 10.1016/S0034-7094(12)70110-1
6. Lee S. Dexmedetomidine: present and future directions. Korean J Anesthesiol. (2019) 72:323–30. doi: 10.4097/kja.19259
7. Kaye AD, Chernobylsky DJ, Thakur P, Siddaiah H, Kaye RJ, Eng LK, et al. Dexmedetomidine in enhanced recovery after surgery (ERAS) protocols for postoperative pain. Curr Pain Headache Rep. (2020) 24:21. doi: 10.1007/s11916-020-00853-z
8. Amersfoort J, Kuiper J. T cell metabolism in metabolic disease-associated autoimmunity. Immunobiology. (2017) 222:925–36. doi: 10.1016/j.imbio.2017.03.001
9. Bektas A, Schurman SH, Sen R, Ferrucci L. Human T cell immunosenescence and inflammation in aging. J Leukoc Biol. (2017) 102:977–88. doi: 10.1189/jlb.3RI0716-335R
10. Fan W, Xue H, Sun Y, Yang H, Zhang J, Li G, et al. Dexmedetomidine improves postoperative patient-controlled analgesia following radical mastectomy. Front Pharmacol. (2017) 8:250. doi: 10.3389/fphar.2017.00250
11. Lee JM, Han HJ, Choi WK, Yoo S, Baek S, Lee J. Immunomodulatory effects of intraoperative dexmedetomidine on T helper 1, T helper 2, T helper 17 and regulatory T cells cytokine levels and their balance: a prospective, randomised, double-blind, dose-response clinical study. BMC Anesthesiol. (2018) 18:164. doi: 10.1186/s12871-018-0625-2
12. Shafshak TS, Elnemr R. The visual analogue scale versus numerical rating scale in measuring pain severity and predicting disability in low back pain. J Clin Rheumatol. (2021) 27:282–5. doi: 10.1097/RHU.0000000000001320
13. Dode A, Mehdi M, Pryss R, Schlee W, Probst T, Reichert M, et al. Using a visual analog scale (VAS) to measure tinnitus-related distress and loudness: investigating correlations using the Mini-TQ results of participants from the TrackYourTinnitus platform. Prog Brain Res. (2021) 263:171–90. doi: 10.1016/bs.pbr.2020.08.008
14. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
15. Jian W, Shao K, Qin Q, Wang X, Song S, Wang X. Clinical and genetic characterization of hereditary breast cancer in a Chinese population. Hered Cancer Clin Pract. (2017) 15:19. doi: 10.1186/s13053-017-0079-4
16. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. (2021) 134:783–91. doi: 10.1097/CM9.0000000000001474
17. Poggio F, Bruzzone M, Ceppi M, Pondé NF, La Valle G, Del Mastro L, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta- analysis. Ann Oncol. (2018) 29:1497–508. doi: 10.1093/annonc/mdy127
18. Nguyen TT, Hoskin TL, Day CN, Degnim AC, Jakub JW, Hieken TJ, et al. Decreasing use of axillary dissection in node-positive breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. (2018) 25:2596–602. doi: 10.1245/s10434-018-6637-9
19. Cui X, Zhu C, Chen P, Qu M, Zhang B, Li H. Effect of pectoral nerve block type II under general anesthesia on the immune function of patients with breast cancer. Am J Surg. (2020) 220:938–44. doi: 10.1016/j.amjsurg.2020.03.008
20. Pérez-González O, Cuéllar-Guzmán LF, Soliz J, Cata JP. Impact of regional anesthesia on recurrence, metastasis, and immune response in breast cancer surgery: a systematic review of the literature. Reg Anesth Pain Med. (2017) 42:751–6. doi: 10.1097/AAP.0000000000000662
21. Das R, Das RK, Sahoo S, Nanda S. Role of dexmedetomidine as an anaesthetic adjuvant in breast cancer surgery as a day-care procedure: a randomised controlled study. Indian J Anaesth. (2018) 62:182–7. doi: 10.4103/ija.IJA_752_17
22. Liu C, Wang W, Shan Z, Zhang H, Yan Q. Dexmedetomidine as an adjuvant for patients undergoing breast cancer surgery: a meta-analysis. Medicine (Baltimore). (2020) 99:e23667. doi: 10.1097/MD.0000000000023667
23. Yang H, Jia G, Wang Y. Influence of dexmedetomidine on the effect of anesthesia in patients with radical mastectomy and on the T-lymphocyte subsets. Oncol Prog. (2018) 16:495–9. doi: 10.11877/j.issn.1672-1535.2018.16.04.28
24. Khedri M, Samei A, Fasihi-Ramandi M, Taheri RA. The immunopathobiology of T cells in stress condition: a review. Cell Stress Chaperones. (2020) 25:743–52. doi: 10.1007/s12192-020-01105-0
25. Xiao F, Liu X, Guo SW. Platelets and regulatory T cells may induce a type 2 immunity that is conducive to the progression and fibrogenesis of endometriosis. Front Immunol. (2020) 11:610963. doi: 10.3389/fimmu.2020.610963
26. Jenner RG, Townsend MJ, Jackson I, Sun K, Bouwman RD, Young RA, et al. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc Natl Acad Sci USA. (2009) 106:17876–81. doi: 10.1073/pnas.0909357106
27. Jamwal DR, Marati RV, Harrison CA, Midura-Kiela MT, Figliuolo Paz VR, Besselsen DG, et al. Total CD3 T cells are necessary and sufficient to induce colitis in immunodeficient mice with dendritic cell-specific deletion of TGFbR2: a novel IBD model to study CD4 and CD8 T-cell interaction. Inflamm Bowel Dis. (2020) 26:229–41. doi: 10.1093/ibd/izz191
28. Mauro C, Smith J, Cucchi D, Coe D, Fu H, Bonacina F, et al. Obesity-induced metabolic stress leads to biased effector memory CD4+ T cell differentiation via PI3K p110δ-akt-mediated signals. Cell Metab. (2017) 25:593–609. doi: 10.1016/j.cmet.2017.01.008
29. Sharma S, Pettus J, Gottschalk M, Abe B, Gottlieb P, Teyton L. Single-Cell analysis of CD4 T cells in type 1 diabetes: from mouse to man, how to perform mechanistic studies. Diabetes. (2019) 68:1886–91. doi: 10.2337/dbi18-0064
30. Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, et al. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. (2008) 68:2972–83. doi: 10.1158/0008-5472.CAN-08-0045
Keywords: breast cancer, radical mastectomy, dexmedetomidine, remifentanil, perioperative T lymphocyte subsets
Citation: Zhang Y, Jiang W and Luo X (2022) Remifentanil combined with dexmedetomidine on the analgesic effect of breast cancer patients undergoing modified radical mastectomy and the influence of perioperative T lymphocyte subsets. Front. Surg. 9:1016690. doi: 10.3389/fsurg.2022.1016690
Received: 11 August 2022; Accepted: 13 October 2022;
Published: 8 November 2022.
Edited by:
Armando Orlandi, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Hiroshi Morimatsu, Okayama University, JapanTevfiktolga Sahin, İnönü University, Turkey
© 2022 Zhang, Jiang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi Luo NzAwODA3QGhvc3BpdGFsLmNxbXUuZWR1LmNu
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Yanjun Zhang1
Yanjun Zhang1 Xi Luo
Xi Luo