- 1Department of Joint and Sports Medicine, First Affiliated Hospital, Dalian Medical University, Dalian, China
- 2Department of Nuclear Medicine, First Affiliated Hospital, Dalian Medical University, Dalian, China
Introduction: Autologous chondrocyte implantation (ACI) is a crucial method for the treatment of defects in articular cartilage. However, the extant methods for the preparation of autologous chondrocyte patch are relatively complicated and money-consuming. Therefore, an efficient, reliable, easy-to-follow, and cost-effective technique is needed to overcome constraints. This case report aims to introduce an autologous chondrocyte patch fabrication technique to repair knee joint cartilage defects and report our typical cases with a 2-year follow-up.
Case presentation: We described four cases in which patients complained of knee joint pain. According to radiological examination, the patients were diagnosed as knee joint cartilage defect. Arthroscopy and autologous chondrocyte patch implantation were performed as well as a 2-year follow up of patients. The autologous chondrocyte patch for knee joint cartilage repair was fabricated using a “sandwich” technique. The preoperative and postoperative knee function was evaluated by four subjective evaluation systems. MRI was performed for all patients to achieve more intuitionistic observation of the postoperative radiological changes of defect sites. The quality of repaired tissue was evaluated by Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART). Postoperative follow-up showed improvement in clinical and MOCART scores for all patients. However, one patient complained of knee joint pain after walking for a long time or recreational activities from 12- to 18-month postoperatively. The location of pain for this patient was not in accordance with the location of cartilage defect.
Conclusion: The patients undergoing autologous chondrocyte patch implantation demonstrated clinical improvement and good quality of repaired tissue postoperatively. The procedure is an efficient and cost-effective treatment for knee joint cartilage defect in this report. In addition, patients with osteoarthritis carry the risk of a poor outcome after the procedure, and whether to have a procedure should be considered carefully.
Introduction
A defect in articular cartilage is a common orthopedic problem, especially in the weightbearing areas of lower-extremity joints (e.g., knee). It has a high prevalence of morbidity in the general population (5%) (1, 2). The lack of vascular, nervous, or lymphatic systems hinders articular cartilage from healing. It can trigger pain, swelling, and dysfunction in the joint (3).
Autologous chondrocyte implantation (ACI) is a crucial method for the treatment of defects in articular cartilage. It can be divided into three generations (4). The first generation (ACI-P) was reported first by Brittberg et al. in 1994, and has two steps. During the first step, arthroscopy is undertaken to obtain chondrocytes, and then they are sent for culture. The second step involves injecting a suspension of cultured chondrocytes below a periosteal patch after 14–21 days (5). The second generation (ACI-C) uses a bioabsorbable collagen membrane instead of a periosteal patch. The third generation (which is based on the second generation) involves the creation of cartilage-like tissue in a biodegradable scaffold and is named ACI-M or matrix-induced autologous chondrocyte implantation (MACI) (6, 7). Several studies have revealed that ACI can lead to effective short-, medium-, and long-term outcomes (4, 7–13). However, the extant methods for the preparation of autologous chondrocyte patch are relatively complicated and money-consuming. Therefore, an efficient, reliable, easy-to-follow, and cost-effective technique is needed to overcome constraints. Therefore, we introduce an autologous chondrocyte patch fabrication technique to repair knee joint cartilage defects and report our typical cases with a 2-year follow-up for clinical and radiological outcomes in this report.
Case report
Case 1: A 26-year-old male sustained an injury to his left knee while fishing. He experienced a twisting of his knee, while a varus impaction force was applied to the slightly flexed knee. He visited the outpatient department of other hospital and was treated with conservative treatment. After 1 year, he had left knee sprain again, accompanied by severe knee joint pain and swelling. Case 2: A 36-year-old female suffered discontinuous pain in left knee that continued for at least 16 years. There was no history of obvious trauma. She had frequent knee joint pain and motion restriction in the past 1 month before hospitalisation. Case 3: A 47-year-old male fell while running 14 years ago. The right knee joint pain was worse with activity and decreased with rest. At that time, he was not receiving any examination or therapy. The right knee joint pain was aggravated in recent months before hospitalisation. Case 4: A 37-year-old male fell while running. After that, he suffered from persistent left joint pain. He could not walk up or down stairs and the quality of his life was seriously influenced.
All these four patients were diagnosed as knee joint cartilage defect after physical and MR examination during hospitalization. Demographic information (sex, age, height, weight), medical and previous history, and cartilage defect characteristics of these patients (length, width, depth, shape, localization) were documented (Supplementary Table S1).
Surgical treatments were performed for patients. The standard procedure involved two steps. The first step was knee arthroscopy in which healthy cartilage tissue was removed from the intercondylar fossa (non-weightbearing areas) and the characteristics of the cartilage defect (length, width, depth, shape, localization) were checked.
The healthy removed cartilage tissue was stored in Dulbecco's modified Eagle's medium (DMEM, Absin, Shanghai, China) at 4°C and sent immediately to the laboratory for cell cultivation. Chondrocytes were isolated enzymatically after digestion by 1% collagenase II (Absin, Shanghai, China) at 37°C for 4 h. Then, they were expanded using culture conditions for autologous cells. DMEM was supplemented with 10% autologous serum (separated from peripheral blood), L-glutamine, and antibiotics (penicillin and streptomycin). Cells were cultured at 37°C in an atmosphere of 5% CO2 and 95% relative humidity. The medium was removed, and a fresh medium was added every three days. A maximum of two passages were undertaken for each culture. The cartilage used for fabrication of acellular cartilage sheets was harvested from the ears of adult pigs. First, the cartilage was cut into a cylindrical shape with a diameters of 2 cm. Then, the cylindrical cartilage was cut into sheets (using a freezing microtome) of thickness 10-μm. The sheets were decellularized in 1% sodium dodecyl sulfate (SDS, Coolaber, Beijing, China) for 24 h. After decellularization, the sheets were rinsed thrice in sterile water. A vacuum freeze-drier was used for lyophilization of the sheets. The diameter of sheets was narrowed to be about 1.8 cm. An acellular cartilage sheet was placed in a culture dish, and 5-μl of the chondrocyte suspension was seeded on it. The concentrations of chondrocyte suspension was 20 × 106 cells/ml. Then, another acellular cartilage sheet was superposed on the first acellular cartilage sheet with 5-μl of the chondrocyte suspension seeded on the surface. These procedures were continued until ten sheets were stacked together. The construct was cultured at 37°C in an atmosphere of 5% CO2 and 95% relative humidity for 4 weeks. After that, the implantation was carried out. Figure 1 provides a brief summary of the construction process of implantation patch. The implantation patch was about 2 cm × 0.4 cm respectively in diameter and thickness. All constructions made by the same method in order to acquire similar sizes of implantation patches.
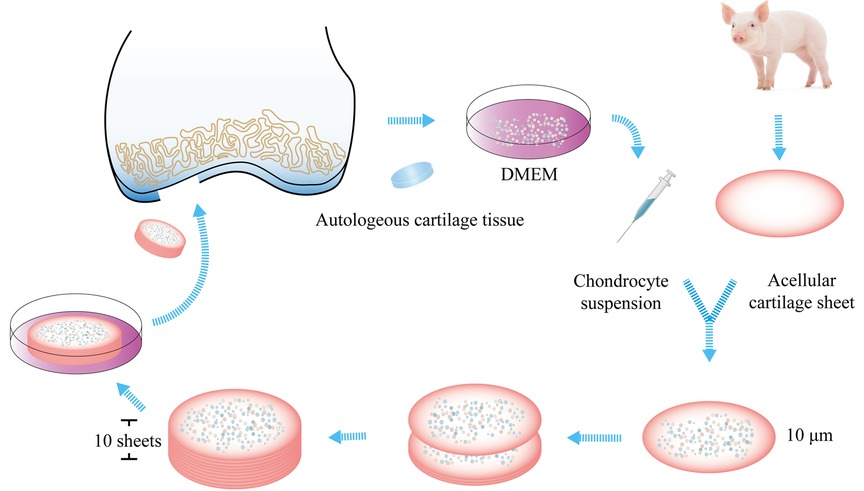
Figure 1. Fabrication of the patch. Healthy cartilage was harvested from the intercondylar fossa. Chondrocytes were isolated enzymatically. The cartilage used for the fabrication of acellular cartilage sheets was harvested from the ears of adult pigs. It was cut into sheets, and the sheets were decellularized. Chondrocytes were seeded on the sheet. Then, another acellular cartilage sheet was superposed on the first sheet, and these procedures were continued until ten sheets were stacked together. The construct was cultured at 37°C in an atmosphere of 5% CO2 and 95% relative humidity for 4 weeks before implantation.
The second step was composed of mini-arthrotomy, curettage, and implantation. During this step (Figure 2), the unhealthy cartilage and subchondral bone plate were cleaned carefully to ensure blood exudation was absent and to leave the base smooth. The patch was placed in physiologic (0.9%) saline <5 min before transplantation. It was trimmed carefully to fit the defect exactly before placed onto the defect. Then, fibrin glue was applied to the surface to fix the patch to the defect without suturing. If a subchondral cystic defect occurred, then debridement was done. A contralateral autogenous posterior iliac bone graft was made, and the bone graft was transplanted into the subchondral defect.
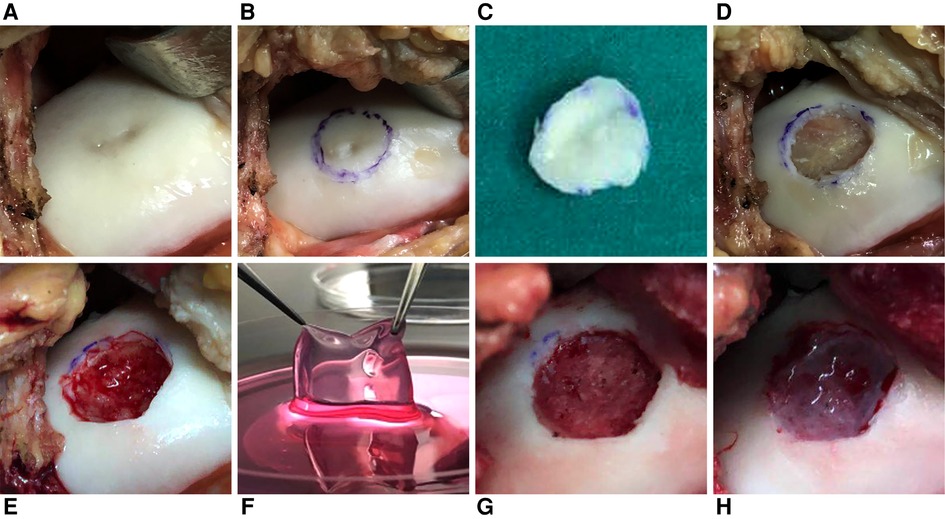
Figure 2. Mini-arthrotomy, curettage, and implantation in a patient (female; 36 years; left knee; BMI = 19.05 kg/m2; other surgery: bone grafting). (A) The cartilage defect was exposed. (B,C) The unhealthy cartilage was marked and removed. (D) The subchondral bone was exposed, and cystic degeneration could be seen. (E) The cystic degeneration in subchondral bone was removed, and blood effusion could be observed. (F) The patch was placed in 0.9% saline <5 min before transplantation. (G) A contralateral autogenous posterior iliac bone graft was made. (H) The patch was placed into the defect. Fibrin glue was applied to the surface to fix the patch to the defect without suturing.
Patients accepted routine rehabilitation after arthroscopy. Postoperative rehabilitation after the second step was far more critical. Initially, the patients accepted fixation using a locking hinged knee brace, and the knee was placed in extension for 2–3 days. The purpose was to prevent the patch from dislodging and allow stable adhesion between the patch and subchondral plate. After that, active and passive motions were allowed. First, non-weightbearing quadricep-strengthening exercises on a bed were recommended to patients. Then, a device to ensure continuous passive motion was used for 90–135 min daily until discharge from the hospital. The range of motion (RoM) was from 0° to 30° initially, and the upper-limit RoM was increased 5° per day until 90° (∼2 weeks). Afterward, the full RoM was allowed if patients did not feel pain. Partial-weightbearing with a walking aid within 6 weeks was allowed, after which full-weightbearing was allowed.
The preoperative and postoperative knee function was evaluated by four subjective evaluation systems: International Knee Documentation Committee Subjective Knee Evaluation Form (IKDC-SKEF) (14); Lysholm Scale (15); Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (16); Knee Injury and Osteoarthritis Outcome Score (KOOS) (17). These systems have been demonstrated to be effective and sensitive for evaluation of the repair of articular cartilage (18–24) and have been used widely for evaluation of outcomes after ACI (25, 26–33). MR examination of the operated knee joint was undertaken on a 3.0-T MR scanner (Ingenia 3.0-T CX, Philips Healthcare, Best, the Netherlands) using a sixteen-channel phased-array coil. The quality of repaired tissue was evaluated by Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART). It is a classification system established by Marlovits et al. in 2004 to analyze repaired tissue (34). Studies have indicated a correlation between the clinical outcome and MOCART score (35, 36). Some researchers consider it to be a reliable way to evaluate repaired tissue (37, 38).
Overall, the patients were all satisfied with the surgical treatments. All patients showed improvements on all clinical outcomes over the time (Figures 3A,B). As an exception, the scores of one patient were lower than those of other patients in IKDC-SKEF, Lysholm Scale, and KOOS. Accordingly, WOMAC scores were higher. His postoperative WOMAC score at 18-month was higher than that at 12-month, and was similar to the score at 6-month. He complained of knee joint pain after walking for a long time or recreational activities. The MOCART scores increased gradually after procedure (Figure 3C) for all patients. The MR images of patients are shown in Figure 4. As can be seen, the defect area decreased postoperatively, and the signal intensity of the repaired cartilage was close to that of healthy cartilage 12-month postoperatively. There was virtually no sclerosis of subchondral bone or edema 18-month postoperatively.
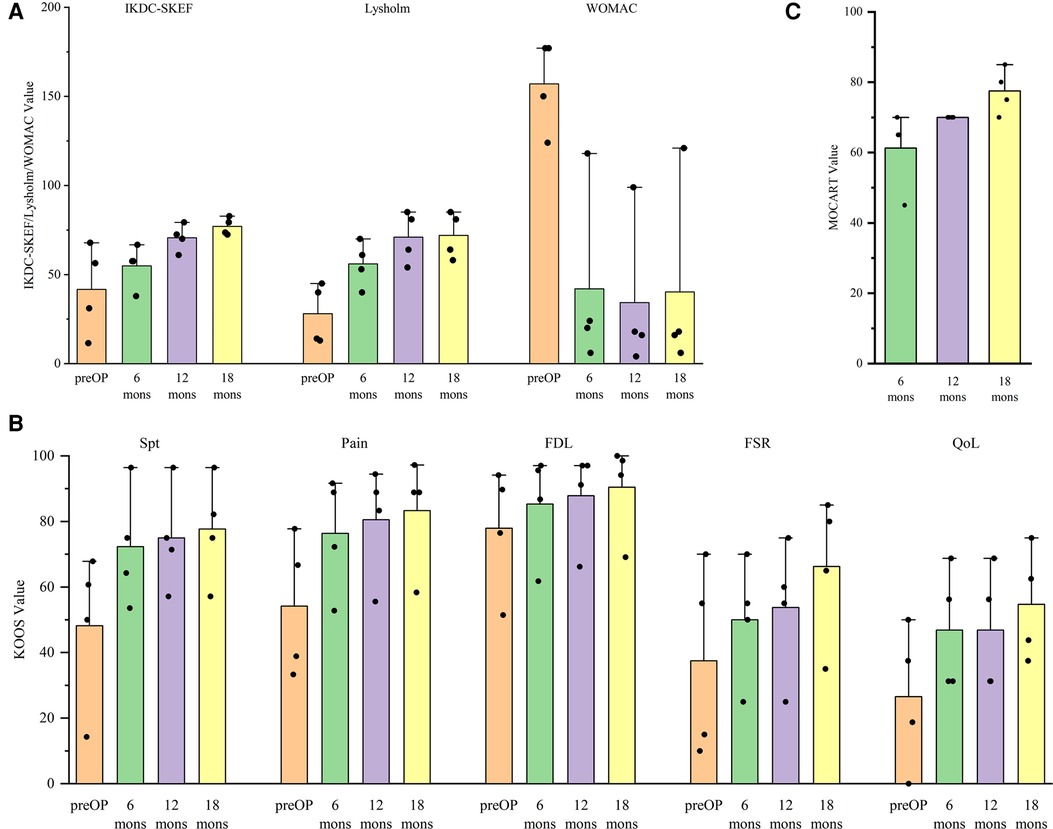
Figure 3. Variation in the trend of (A) international knee documentation committee subjective knee evaluation form (IKDC-SKEF), lysholm scale, western Ontario and mcMaster universities osteoarthritis Index (WOMAC), (B) knee injury and osteoarthritis outcome score (KOOS), and (C) magnetic resonance observation of cartilage repair tissue (MOCART) scores.
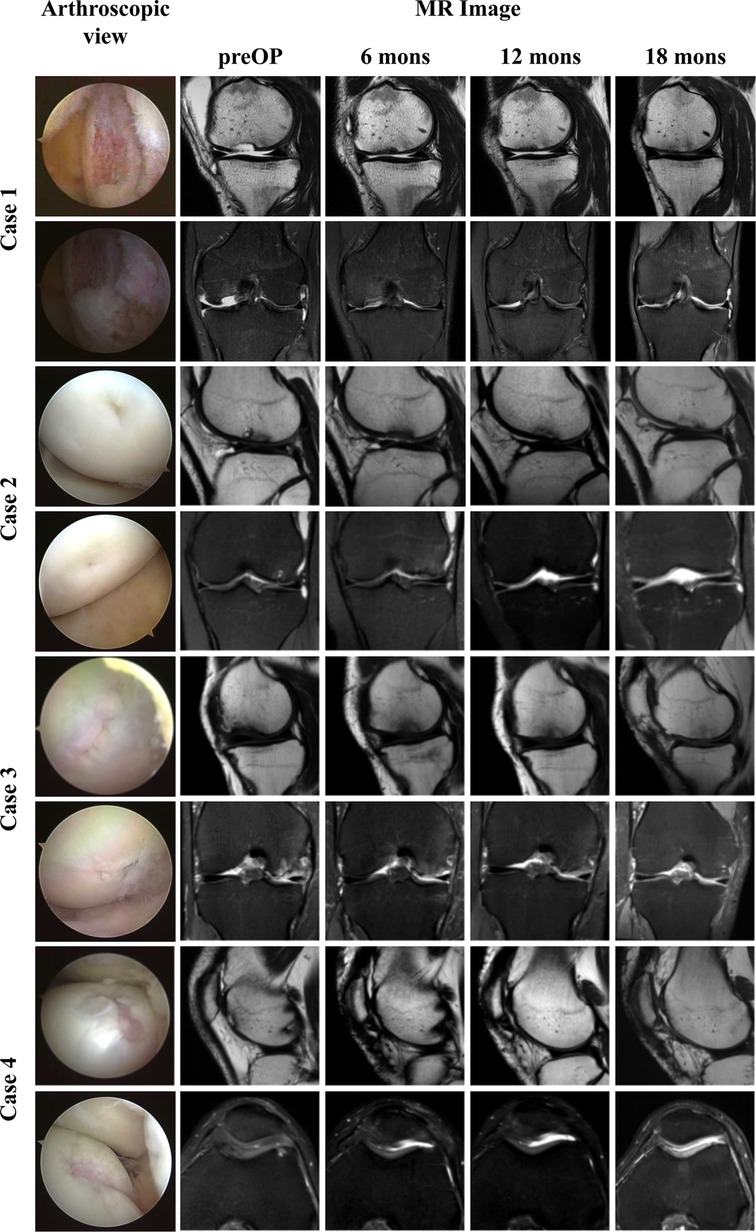
Figure 4. Arthroscopic view and MR images of four patients. The first column: arthroscopic view of cartilage defect. The second to fifth columns: Sagittal T2-weighted, coronal, or axial PD-weighted MR images of four patients from pre to 18 months after procedure.
Discussion
The improvements in IKDC-SKEF, Lysholm Scale, WOMAC, and KOOS were demonstrated postoperatively in all patients. The patients expressed satisfaction with the functional postoperative recovery after autologous chondrocyte patch implantation.
As seen from Figure 3B, the function score in sport and recreation (FSR) and quality of life (QoL) were lower than other parts in KOOS at all times. This means that the time needed for patients to return to sports and recreational activities after the procedure was longer than that for returning to activities of daily living. It still needs a long time to return to a higher QoL level. Pestka et al. and Erdle et al. showed that returning to low-intensity and moderate-intensity activities was feasible, but returning to high-intensity or identical-intensity activities to that before surgery was infeasible in 1 year (39, 40). Ebert et al. and Erggelet et al. advised their patients to return to contact and competitive activities 12-month postoperatively (41, 42). Furthermore, Niethammer et al. compared the rehabilitation process postoperatively. They found that patients who returned to sporting activities 12-month postoperatively showed significantly better clinical outcomes than those who returned to sporting activities before 12-month postoperatively (43). However, Kreuz et al. concluded that moderate-intensity sporting activities are an essential component of rehabilitation but should be undertaken at least 2–3 years after the surgical procedure (44). All of the above studies demonstrated that patients should not return to high-intensity activities too earlier. It may help surgeons and patients determine the appropriate time for returning to sporting activities postoperatively.
The MOCART scores increased gradually after the surgical procedure. These data are in accordance with the trend reported by Zak et al. and Niemeyer and colleagues (45, 46). It illustrated that the repaired cartilage tissue progressed towards a healthy morphology. MR examination is a non-invasive way to evaluate articular cartilage and has a sensitivity of ≤96% (47). MR features can show cartilage status directly. The MOCART score is a quantitative indicator to evaluate cartilage. However, Siebold et al. pointed out that a low MOCART score did not denote poor clinical results but, instead, we should not evaluate cartilage status using the MOCART score only (4).
In the present study, the mean total cost of procedure was 57693.2 (range, 55905.2–61644.2) CNY. This figure included the fees for preoperative consultation and preparation, surgical items (anesthesia, surgical supplies, duration of use of the operating room, drugs, and hospital fees), cell processing, and implantation patch fabrication. The costs of this procedure are significantly higher than those of knee arthroscopy (chondroplasty and debridement), microfracture (MFx), osteochondral transplantation (OCT), or osteochondral allograft (OCA) (6, 25, 48). However, arthroscopic chondroplasty and debridement cannot fundamentally tackle the problem. MFx leads to the synthesis of fibrocartilage instead of hyaline cartilage. The biomechanical property of the fibrocartilage is inferior to that of hyaline cartilage, which leads to a worse long-term outcome (4, 8, 9, 49–54). Donor-area complications (e.g., pain, discomfort, and formation of secondary bone defects) cannot be ignored after OCT (55).
Although ACI can lead to the synthesis of hyaline cartilage, the different generation of ACI have different advantages and disadvantages. Samuelson et al. demonstrated that ACI-P and ACI-C are cost-effective, and that the latter is marginally more cost-effective than the former (56). Their results are similar to those of Schrock and colleagues (25). Everhart et al. pointed out that the cost of MACI is similar or slightly higher than that of ACI-C (48). Several studies have pointed out that MACI can lead to satisfactory and reliable clinical outcomes (7, 9, 26, 27, 30, 57). It has other advantages: homogeneous distribution; biocompatibility; appropriateness for large cartilage defects; relatively simple production process; easy to model; can be produced in differently sized and shaped membranes; straightforward surgical procedure; use of fibrin glue instead of suturing (6). Most importantly, it has been approved by the Chinese government. According to the three generations of ACI, only ACI-P and MACI have been approved in China. The patch in this study was easy to prepare and not technically demanding. Due to the disadvantages of ACI-P (e.g., complex surgical procedure, highly invasive, inhomogeneous distribution, weak biomechanical property), we believe that our procedure is an excellent way to treat patients with a focal articular-cartilage defect in knee joint.
Interestingly, the changes of WOMAC score of one patient were different from those of other patients. He complained of knee joint pain after walking for a long time or recreational activities from 12- to 18-month postoperatively. During a recent follow-up (18-month postoperatively), we undertook a comprehensive examination of the operated knee. We discovered that the location of the knee joint pain for this patient was not in accordance with the location of the cartilage defect. We found hyperosteogeny according to preoperative radiography (Supplementary Figure S1). The hyperosteogeny and appearance of osteoarthritis became more marked with time.
There are likely two explanations for this problem. First, the indications for the procedure should be stricter. Even though the patient was aged only 46 years, the appearance of osteoarthritis was present preoperatively. Although the clinical outcome was good, it did not proceed as expected. Niemeyer et al. considered that diffuse lesions (e.g., osteoarthritis) should not be included in the indication for ACI (54). Conversely, Minas and collaborators considered that patients with early-stage osteoarthritis can accept ACI (58). Rosenberger et al. pointed out that the failure rates of ACI in older and younger patient groups are similar if the cartilage defect is focal (59). Second, the progress of intrinsic osteoarthritis for this patient influenced the effect postoperatively. Osteoarthritis progression may be fast in some patients, even if the procedure has been done and the defect has been repaired. A focal cartilage defect is a risk factor for osteoarthritis development (54). The procedure can be a powerful tool for delaying osteoarthritis progression, but intrinsic osteoarthritis cannot be delayed (58). Therefore, patients aged >40 years with osteoarthritis may carry the risk of a poor clinical outcome after the procedure, and whether to have a procedure should be considered carefully.
Conclusion
Patients undergoing autologous chondrocyte patch (fabricated via the sandwich technique) implantation demonstrated clinical improvement and good quality of repaired tissue postoperatively. The procedure is an efficient and cost-effective treatment for knee joint cartilage defect in this report. In addition, patients with osteoarthritis carry the risk of a poor outcome after the procedure, and whether to have a procedure should be considered carefully. Large studies with long-term follow-up are also needed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The ethics committee of First Affiliated Hospital of Dalian Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors contributed to the study conception and design. Design of case report, patients examination, data collection, and analysis were performed by LW, HL, YC, and CS. QC and JH performed follow-up of the patients. The first draft of the manuscript was written by LW. WZ and KT critically reviewed and edited the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Supported by National Natural Science Foundation of China (No. 81601901); Natural Science Foundation of Liaoning (No. 2019-MS-079, No. 20170540285) for patient's treatment costs and follow-up MR examination charges.
Acknowledgments
Thanks to our operation team WZ, JL, YZ, JZ, and KT, who have been working hard for many years, it is impossible to complete the study without the team.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1015091/full#supplementary-material.
References
1. Wu S, Kai Z, Wang D, Tao L, Zhang P, Wang D, et al. Allogenic chondrocyte/osteoblast-loaded β-tricalcium phosphate bioceramic scaffolds for articular cartilage defect treatment. Artif Cells Nanomed Biotechnol. (2019) 47:1570–6. doi: 10.1080/21691401.2019.1604534
2. Zhou Y, Li H, Xiang D, Shao J, Fu Q, Han Y, et al. The clinical efficacy of arthroscopic therapy with knee infrapatellar fat pad cell concentrates in treating knee cartilage lesion: a prospective, randomized, and controlled study. J Orthop Surg Res. (2021) 16:87. doi: 10.1186/s13018-021-02224-9
3. Niethammer T, Holzgruber M, Gülecyüz M, Weber P, Pietschmann M, Müller P. Matrix based autologous chondrocyte implantation in children and adolescents: a match paired analysis in a follow-up over three years post-operation. Int Orthop. (2017) 41:343–50. doi: 10.1007/s00264-016-3321-1
4. Siebold R, Suezer F, Schmitt B, Trattnig S, Essig M. Good clinical and mri outcome after arthroscopic autologous chondrocyte implantation for cartilage repair in the knee. Knee Surg Sports Traumatol Arthrosc. (2018) 26:831–9. doi: 10.1007/s00167-017-4491-0
5. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. (1994) 331:889–95. doi: 10.1056/nejm199410063311401
6. Zhang C, Cai Y, Lin X. Autologous chondrocyte implantation: is it likely to become a saviour of large-sized and full-thickness cartilage defect in young adult knee? Knee Surg Sports Traumatol Arthrosc. (2016) 24:1643–50. doi: 10.1007/s00167-015-3643-3
7. Niethammer T, Altmann D, Holzgruber M, Goller S, Fischer A, Müller P. Third generation autologous chondrocyte implantation is a good treatment option for athletic persons. Knee Surg Sports Traumatol Arthrosc. (2021) 29:1215–23. doi: 10.1007/s00167-020-06148-5
8. Lopez-Alcorocho J, Aboli L, Guillen-Vicente I, Rodriguez-Iñigo E, Guillen-Vicente M, Fernández-Jaén T, et al. Cartilage defect treatment using high-density autologous chondrocyte implantation: two-year follow-up. Cartilage. (2018) 9:363–9. doi: 10.1177/1947603517693045
9. Barié A, Kruck P, Sorbi R, Rehnitz C, Oberle D, Walker T, et al. Prospective long-term follow-up of autologous chondrocyte implantation with periosteum versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. (2020) 48:2230–41. doi: 10.1177/0363546520928337
10. Kim M, Park J, Jeon Y, Jeon Y. Clinical, radiological, and histological outcomes after the fibrin-matrix autologous chondrocyte implantation for chondral lesions of the knee in patients more than 50 years old: a prospective case series with minimum 2-year follow-up. J Orthop Surg. (2020) 28:2309499019893509. doi: 10.1177/2309499019893509
11. Sumida Y, Nakamura K, Feil S, Siebold M, Kirsch J, Siebold R. Good healing potential of patellar chondral defects after all-arthroscopic autologous chondrocyte implantation with spheroids: a second-look arthroscopic assessment. Knee Surg Sports Traumatol Arthrosc. (2022) 30:1535–42. doi: 10.1007/s00167-021-06584-x
12. Biant L, Bentley G, Vijayan S, Skinner J, Carrington R. Long-term results of autologous chondrocyte implantation in the knee for chronic chondral and osteochondral defects. Am J Sports Med. (2014) 42:2178–83. doi: 10.1177/0363546514539345
13. Niemeyer P, Feucht M, Fritz J, Albrecht D, Spahn G, Angele P. Cartilage repair surgery for full-thickness defects of the knee in Germany: indications and epidemiological data from the German cartilage registry (knorpelregister dgou). Arch Orthop Trauma Surg. (2016) 136:891–7. doi: 10.1007/s00402-016-2453-5
14. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. (1982) 10:150–4. doi: 10.1177/036354658201000306
15. Osti L, Papalia R, Del Buono A, Amato C, Denaro V, Maffulli N. Good results five years after surgical management of anterior cruciate ligament tears, and meniscal and cartilage injuries. Knee Surg Sports Traumatol Arthrosc. (2010) 18:1385–90. doi: 10.1007/s00167-009-1035-2
16. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of womac: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. (1988) 15:1833–40.3068365
17. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee injury and osteoarthritis outcome score (koos)–development of a self-administered outcome measure. J Orthop Sports Phys Ther. (1998) 28:88–96. doi: 10.2519/jospt.1998.28.2.88
18. Mithoefer K, Acuna M. Clinical outcomes assessment for articular cartilage restoration. J Knee Surg. (2013) 26:31–40. doi: 10.1055/s-0032-1333308
19. de Windt T, Welsch G, Brittberg M, Vonk L, Marlovits S, Trattnig S, et al. Correlation between magnetic resonance imaging and clinical outcomes after knee cartilage repair: letter to the editor. Am J Sports Med. (2013) 41:NP48–50. doi: 10.1177/0363546513510140
20. Engelhart L, Nelson L, Lewis S, Mordin M, Demuro-Mercon C, Uddin S, et al. Validation of the knee injury and osteoarthritis outcome score subscales for patients with articular cartilage lesions of the knee. Am J Sports Med. (2012) 40:2264–72. doi: 10.1177/0363546512457646
21. Greco N, Anderson A, Mann B, Cole B, Farr J, Nissen C, et al. Responsiveness of the international knee documentation committee subjective knee form in comparison to the western Ontario and mcmaster universities osteoarthritis index, modified cincinnati knee rating system, and short form 36 in patients with focal articular cartilage defects. Am J Sports Med. (2010) 38:891–902. doi: 10.1177/0363546509354163
22. Hambly K, Griva K. Ikdc or koos? Which measures symptoms and disabilities most important to postoperative articular cartilage repair patients? Am J Sports Med. (2008) 36:1695–704. doi: 10.1177/0363546508317718
23. Collins N, Prinsen C, Christensen R, Bartels E, Terwee C, Roos E. Knee injury and osteoarthritis outcome score (koos): systematic review and meta-analysis of measurement properties. Osteoarthr Cartil. (2016) 24:1317–29. doi: 10.1016/j.joca.2016.03.010
24. Howe T, Dawson L, Syme G, Duncan L, Reid J. Evaluation of outcome measures for use in clinical practice for adults with musculoskeletal conditions of the knee: a systematic review. Man Ther. (2012) 17:100–18. doi: 10.1016/j.math.2011.07.002
25. Schrock J, Kraeutler M, Houck D, McQueen M, McCarty E. A cost-effectiveness analysis of surgical treatment modalities for chondral lesions of the knee: microfracture, osteochondral autograft transplantation, and autologous chondrocyte implantation. Orthop J Sports Med. (2017) 5:2325967117704634. doi: 10.1177/2325967117704634
26. Marlovits S, Aldrian S, Wondrasch B, Zak L, Albrecht C, Welsch G, et al. Clinical and radiological outcomes 5 years after matrix-induced autologous chondrocyte implantation in patients with symptomatic, traumatic chondral defects. Am J Sports Med. (2012) 40:2273–80. doi: 10.1177/0363546512457008
27. Ebert J, Fallon M, Zheng M, Wood D, Ackland T. A randomized trial comparing accelerated and traditional approaches to postoperative weightbearing rehabilitation after matrix-induced autologous chondrocyte implantation: findings at 5 years. Am J Sports Med. (2012) 40:1527–37. doi: 10.1177/0363546512445167
28. Kreuz P, Müller S, Freymann U, Erggelet C, Niemeyer P, Kaps C, et al. Repair of focal cartilage defects with scaffold-assisted autologous chondrocyte grafts: clinical and biomechanical results 48 months after transplantation. Am J Sports Med. (2011) 39:1697–705. doi: 10.1177/0363546511403279
29. Pascual-Garrido C, Slabaugh M, L'Heureux D, Friel N, Cole B. Recommendations and treatment outcomes for patellofemoral articular cartilage defects with autologous chondrocyte implantation: prospective evaluation at average 4-year follow-up. Am J Sports Med. (2009) 37(Suppl 1):33S–41S. doi: 10.1177/0363546509349605
30. Kraeutler M, Belk J, Carver T, McCarty E. Is delayed weightbearing after matrix-associated autologous chondrocyte implantation in the knee associated with better outcomes? A systematic review of randomized controlled trials. Orthop J Sports Med. (2018) 6:2325967118770986. doi: 10.1177/2325967118770986
31. Riboh J, Cvetanovich G, Cole B, Yanke A. Comparative efficacy of cartilage repair procedures in the knee: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. (2017) 25:3786–99. doi: 10.1007/s00167-016-4300-1
32. Mundi R, Bedi A, Chow L, Crouch S, Simunovic N, Sibilsky Enselman E, et al. Cartilage restoration of the knee: a systematic review and meta-analysis of level 1 studies. Am J Sports Med. (2016) 44:1888–95. doi: 10.1177/0363546515589167
33. DiBartola A, Wright B, Magnussen R, Flanigan D. Clinical outcomes after autologous chondrocyte implantation in adolescents’ knees: a systematic review. Arthroscopy. (2016) 32:1905–16. doi: 10.1016/j.arthro.2016.03.007
34. Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. (2004) 52:310–9. doi: 10.1016/j.ejrad.2004.03.014
35. de Windt T, Welsch G, Brittberg M, Vonk L, Marlovits S, Trattnig S, et al. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am J Sports Med. (2013) 41:1695–702. doi: 10.1177/0363546512473258
36. Versier G, Dubrana F. Treatment of knee cartilage defect in 2010. Orthop Traumatol Surg Res. (2011) 97:S140–53. doi: 10.1016/j.otsr.2011.09.007
37. Schreiner M, Raudner M, Röhrich S, Zalaudek M, Weber M, Kaiser G, et al. Reliability of the mocart (magnetic resonance observation of cartilage repair tissue) 2.0 knee score for different cartilage repair techniques-a retrospective observational study. Eur Radiol. (2021) 31:5734–45. doi: 10.1007/s00330-021-07688-1
38. Schreiner M, Raudner M, Marlovits S, Bohndorf K, Weber M, Zalaudek M, et al. The mocart (magnetic resonance observation of cartilage repair tissue) 2.0 knee score and atlas. Cartilage. (2021) 13:571S–87S. doi: 10.1177/1947603519865308
39. Erdle B, Herrmann S, Porichis S, Uhl M, Ghanem N, Schmal H, et al. Sporting activity is reduced 11 years after first-generation autologous chondrocyte implantation in the knee joint. Am J Sports Med. (2017) 45:2762–73. doi: 10.1177/0363546517716920
40. Kreuz P, Steinwachs M, Erggelet C, Lahm A, Krause S, Ossendorf C, et al. Importance of sports in cartilage regeneration after autologous chondrocyte implantation: a prospective study with a 3-year follow-up. Am J Sports Med. (2007) 35:1261–8. doi: 10.1177/0363546507300693
41. Zak L, Kleiner A, Albrecht C, Tichy B, Aldrian S. Third-generation autologous chondrocyte implantation at the knee joint using the igor scaffold: a case series with 2-year follow-up. Orthop J Sports Med. (2021) 9:2325967120969237. doi: 10.1177/2325967120969237
42. Niemeyer P, Laute V, Zinser W, Becher C, Kolombe T, Fay J, et al. A prospective, randomized, open-label, multicenter, phase iii noninferiority trial to compare the clinical efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology versus arthroscopic microfracture for cartilage defects of the knee. Orthop J Sports Med. (2019) 7:2325967119854442. doi: 10.1177/2325967119854442
43. Azer N, Winalski C, Minas T. MR imaging for surgical planning and postoperative assessment in early osteoarthritis. Radiol Clin North Am. (2004) 42:43–60. doi: 10.1016/s0033-8389(03)00157-x
44. Samuelson E, Brown D. Cost-effectiveness analysis of autologous chondrocyte implantation: a comparison of periosteal patch versus type i/iii collagen membrane. Am J Sports Med. (2012) 40:1252–8. doi: 10.1177/0363546512441586
45. Saris D, Vanlauwe J, Victor J, Almqvist K, Verdonk R, Bellemans J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. (2009) 37(Suppl 1):10S–9S. doi: 10.1177/0363546509350694
46. Niemeyer P, Uhl M, Salzmann G, Morscheid Y, Südkamp N, Madry H. Evaluation and analysis of graft hypertrophy by means of arthroscopy, biochemical mri and osteochondral biopsies in a patient following autologous chondrocyte implantation for treatment of a full-thickness-cartilage defect of the knee. Arch Orthop Trauma Surg. (2015) 135:819–30. doi: 10.1007/s00402-015-2194-x
47. Niemeyer P, Salzmann G, Hirschmüller A, Südkamp N. Factors that influence clinical outcome following autologous chondrocyte implantation for cartilage defects of the knee. Z Orthop Unfall. (2012) 150:83–8. doi: 10.1055/s-0030-1270894
48. Everhart J, Campbell A, Abouljoud M, Kirven J, Flanigan D. Cost-efficacy of knee cartilage defect treatments in the United States. Am J Sports Med. (2020) 48:242–51. doi: 10.1177/0363546519834557
49. Perera J, Gikas P, Bentley G. The present state of treatments for articular cartilage defects in the knee. Ann R Coll Surg Engl. (2012) 94:381–7. doi: 10.1308/003588412x13171221592573
50. Johnson L. Arthroscopic abrasion arthroplasty: a review. Clin Orthop Relat Res. (2001) (391 Suppl):S306–17. doi: 10.1097/00003086-200110001-00028
51. Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. (2009) 37:33–41. doi: 10.1177/0363546508323256
52. Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. (1999) (365):149–62. doi: 10.1097/00003086-199908000-00020
53. Kreuz P, Kalkreuth R, Niemeyer P, Uhl M, Erggelet C. Treatment of a focal articular cartilage defect of the talus with polymer-based autologous chondrocyte implantation: a 12-year follow-up period. J Foot Ankle Surg. (2017) 56:862–4. doi: 10.1053/j.jfas.2017.03.001
54. Niemeyer P, Albrecht D, Andereya S, Angele P, Ateschrang A, Aurich M, et al. Autologous chondrocyte implantation (aci) for cartilage defects of the knee: a guideline by the working group “clinical tissue regeneration” of the German society of orthopaedics and trauma (dgou). Knee. (2016) 23:426–35. doi: 10.1016/j.knee.2016.02.001
55. Jia B, Yang H, Zhang Z, Qu X, Jia X, Wu Q, et al. Biodegradable zn-sr alloy for bone regeneration in rat femoral condyle defect model: in vitro and in vivo studies. Bioact Mater. (2021) 6:1588–604. doi: 10.1016/j.bioactmat.2020.11.007
56. Siebold R, Karidakis G, Feil S, Fernandez F. Second-look assessment after all-arthroscopic autologous chondrocyte implantation with spheroides at the knee joint. Knee Surg Sports Traumatol Arthrosc. (2016) 24:1678–85. doi: 10.1007/s00167-015-3822-2
57. Erggelet C, Kreuz P, Mrosek E, Schagemann J, Lahm A, Ducommun P, et al. Autologous chondrocyte implantation versus aci using 3d-bioresorbable graft for the treatment of large full-thickness cartilage lesions of the knee. Arch Orthop Trauma Surg. (2010) 130:957–64. doi: 10.1007/s00402-009-0957-y
58. Rosenberger R, Gomoll A, Bryant T, Minas T. Repair of large chondral defects of the knee with autologous chondrocyte implantation in patients 45 years or older. Am J Sports Med. (2008) 36:2336–44. doi: 10.1177/0363546508322888
Keywords: cartilage defect, autologous chondrocyte patch implantation, sandwich technique, cartilage repair, ACI, MACI
Citation: Wang L, Li H, Cao Y, Song C, Chen Q, Hao J, Zhang W and Tian K (2022) Four cases report: Treatment of knee joint cartilage defects using autologous chondrocyte patch implantation. Front. Surg. 9:1015091. doi: 10.3389/fsurg.2022.1015091
Received: 9 August 2022; Accepted: 18 October 2022;
Published: 8 November 2022.
Edited by:
Paphon Sa-ngasoongsong, Mahidol University, ThailandReviewed by:
Wenfu Lai, Taipei Medical University, TaiwanOsvaldo Mazza, Bambino Gesù Children's Hospital (IRCCS), Italy
© 2022 Wang, Li, Cao, Song, Chen, Hao, Zhang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kang Tian ZG11LXRpYW5rYW5nQG91dGxvb2suY29t Weiguo Zhang ZGxtZWR1QG91dGxvb2suY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Le Wang1,†
Le Wang1,† Qi Chen
Qi Chen Weiguo Zhang
Weiguo Zhang Kang Tian
Kang Tian