- 1The 1st Clinical Medicine College, Gansu University of Chinese Medicine, Lanzhou, China
- 2Department of General Surgery, Gansu Provincial Hospital, Lanzhou, China
- 3School of People's Clinical Medicine, Lanzhou University, Lanzhou, China
- 4Gansu Key Laboratory of Molecular Diagnostics and Precision Medicine for Surgical Oncology, Gansu Provincial Hospital, Lanzhou, China
- 5Gansu Research Center of Prevention and Control Project for Digestive Oncology, Gansu Provincial Hospital, Lanzhou, China
- 6College of Clinical Medicine, Ningxia Medical University, Yinchuan, China
Background: Choriocarcinoma is a malignant tumour of trophoblastic origin. Most are gestational choriocarcinomas, which usually occur in women with an epithelial origin of the placental chorionic villi and are associated with pregnancy. It mainly originates in the gonads such as the ovaries and testes. However, it rarely occurs in the stomach and is known as primary choriocarcinoma (PGC).
Case presentation: A 69-year-old man complained of abdominal distention for 3 years, which worsened 1 week later. Gastroscopy showed chronic atrophic gastritis C1 (C1: indicates atrophic gastritis involving the sinus region); the pathology report of the gastroscopic specimen showed high-grade epithelial tumours in the mucosal glands. We diagnosed an occupying lesion in the stomach and performed a laparoscopically assisted distal gastrectomy and Billroth type 1 anastomosis. Postoperative pathology showed “gastric choriocarcinoma with cancerous tissue invading the entire gastric wall”. The patient was discharged on the 11th postoperative day as there were no postoperative complications. The patient was followed up until June 2022 with a good recovery and no recurrence.
Conclusion: We encountered a case of Primary Gastric Choriocarcinoma, where the cancerous tissue invades the full thickness of the gastric wall.
Background
Choriocarcinoma is a malignant tumour of trophoblastic origin (1). The vast majority of choriocarcinomas are associated with pregnancy, most commonly in the uterus, and originate mainly in the ovaries, testes and other gonads. However, primary gastric choriocarcinoma is is a rare malignancy, accounting for approximately 1% of gastric cancers (2, 3). Here, we report a case of primary gastric choriocarcinoma in which cancerous tissue invaded the entire gastric wall.
Case report
A 69-year-old male presented to our hospital complaining of abdominal distension and discomfort for 3 years, worsening for 1 week. His medical history included chronic appendicitis and he had undergone an appendectomy 2 years ago. Recently, there was no significant change in weight. One week ago, the patient felt a marked increase in abdominal distension and pain, mainly in the upper and middle abdomen, and presented to our hospital. The results of the examination were as follows: normal vital signs, fecal occult blood test (++), ALB: 33.05 g/L, Hb: 96 g/L and HCG-β: 66.12 mIU/ml. Liver and kidney function, electrolytes and blood glucose were normal. Abdominal Doppler ultrasound showed a solid mass in the upper abdomen, which was suspected to be gastric cancer (Figure 1A). Gastroscopy revealed chronic atrophic gastritis stage C1 (C1: indicates atrophic gastritis involving the gastric sinus region) (Figure 1B). Pathological examination of the specimen taken under gastroscopy revealed high-grade intraepithelial neoplasia in the (sinus) mucosal glands (Figures 2A–D). Tumour marker examination showed normal tumour series CEA, AFP and CA199 except CA72-4: 10.07 U/ml (0–6 U/ml). MRI of the abdomen showed that the gastric sinus was occupied, with the main lesion located outside the gastric wall (Figure 3A). Biopsy consultation at another hospital showed a moderate chronic mucosal inflammation with mild intestinal metaplasia. Considering the patient's current relevant investigations and findings, we considered a gastric occupying lesion (suspected mesenchymal tumour). The patient was stable and had clear indications for surgery. We therefore performed a laparoscopic-assisted distal gastrectomy and a Billroth type 1 anastomosis. The postoperative gross specimen showed a mass measuring approximately 9 cm × 7 cm × 6 cm, which was reddish-grey in section, suggestive of solid soft tissue (Figures 4A,B). Pathology of the postoperative specimen showed PGC cancerous tissue invading the entire gastric wall (T4aN0M0). Metastases were found only in the vascular system and no other system had been identified. No tumour was found at either side of the resection margins. Microscopic examination showed marked tumour heterogeneity with visible cancerous areas, tumour cells of variable size, ovoid or polygonal in shape, with clear, lightly stained or granular cytoplasm, a single vacuolated nucleus with a distinct nucleolus, many nuclear fission signs, some cells could appear multinucleated, with abundant cytoplasm and large nuclear heterogeneity. The cells are of cytotrophoblastic and syncytial trophoblastic origin and are devoid of villous structures (Figures 4C–F). Immunohistochemistry showed CD10 (+) (Figure 5A), ck8/18 (+) (Figure 5B), CK19 (+) (Figure 5C), CKP (+) (Figure 5D) and a Ki-67 labelling index of 60% (+) (Figure 5E). Histochemical staining showed PAS (+) (Figure 5F), while immunohistochemistry showed positive for HCG antibodies (Figure 6). A postoperative blood HCG-β (β-human chorionic gonadotropin) test was performed at 66.12 mIU/ml, followed by cranial MRI and chest and abdominal CT, which showed no signs of distant metastases. The diagnosis of PGC was now almost clear. The patient was discharged on the 11th postoperative day and was followed up until June 2022, with good recovery and no recurrence.
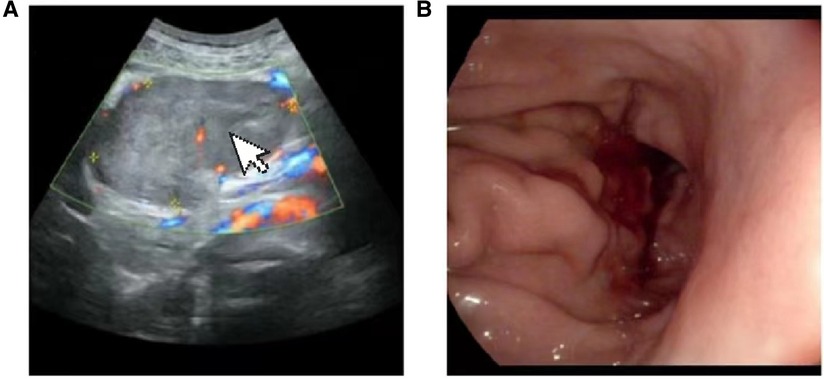
Figure 1. (A) Doppler ultrasound:upper abdominal solid mass. (B) Gastroscopy: chronic atrophic gastritis C1: gastric antrum mass (to be examined).
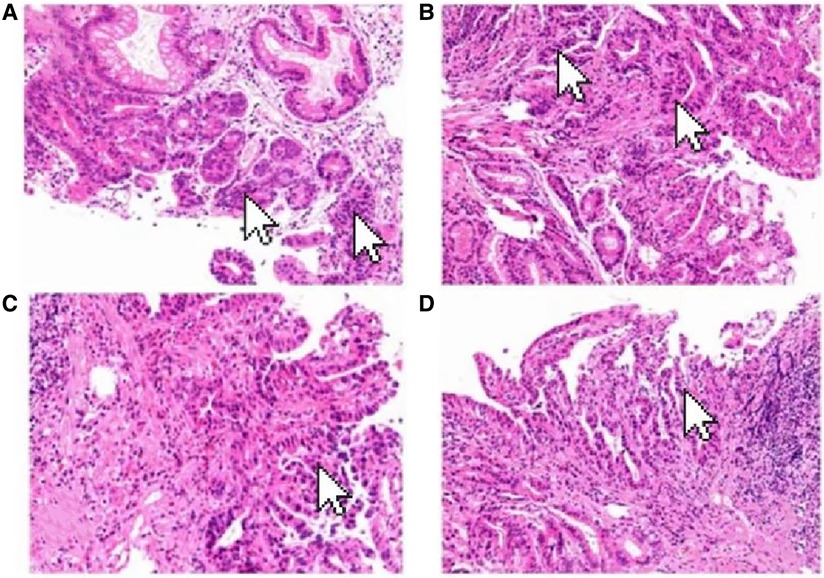
Figure 2. Pathological examination of the specimen taken under gastroscopy revealed high-grade intraepithelial neoplasia in the (sinus) mucosal glands (arrow). (HE×100).
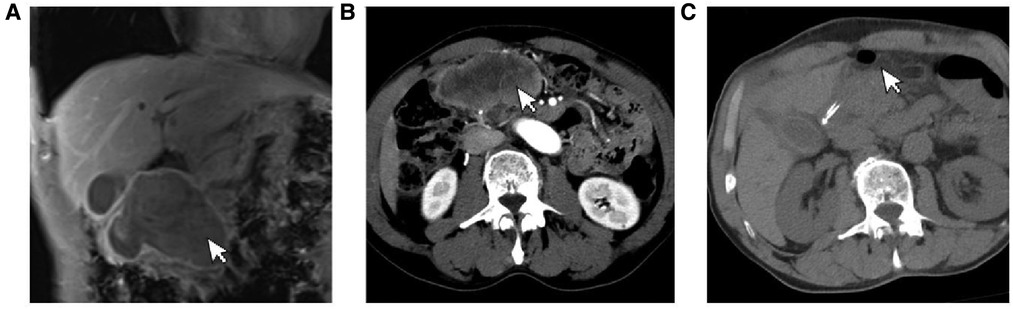
Figure 3. (A) The gastric antrum occupies space, the main lesion is located outside the gastric wall. (B) Patient's preoperative CT showed obvious lesions. (C) Postoperative CT of the patient showed significant resection of the lesion.
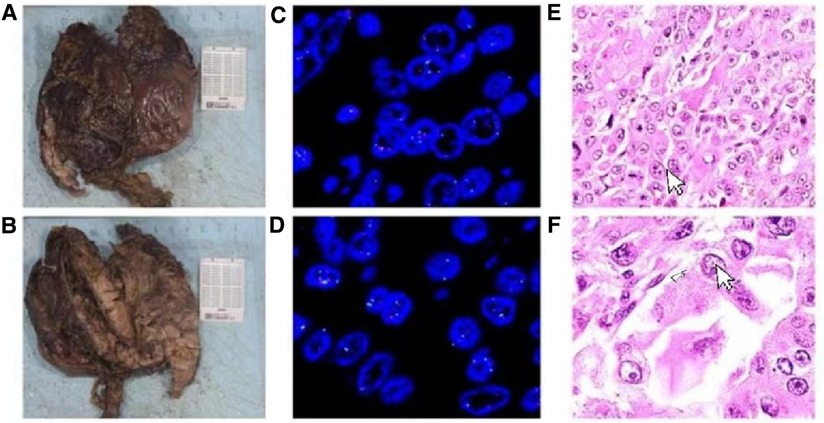
Figure 4. (A,B) Specimen: the size of the mass is about 9 cm × 7 cm × 6 cm, and the surface of the slice is red and gray, indicating solid, soft tissue. (C-F) Primary choriocarcinoma of the stomach, with Ovoid or polygonal, with hyaline, lightly stained or granular cytoplasm and a single vacuolated nucleus (HE ×100).
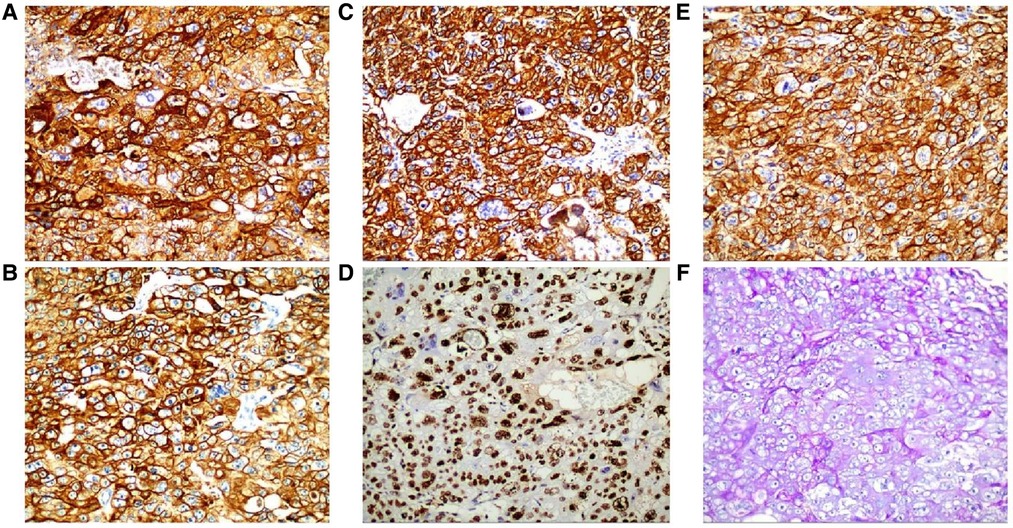
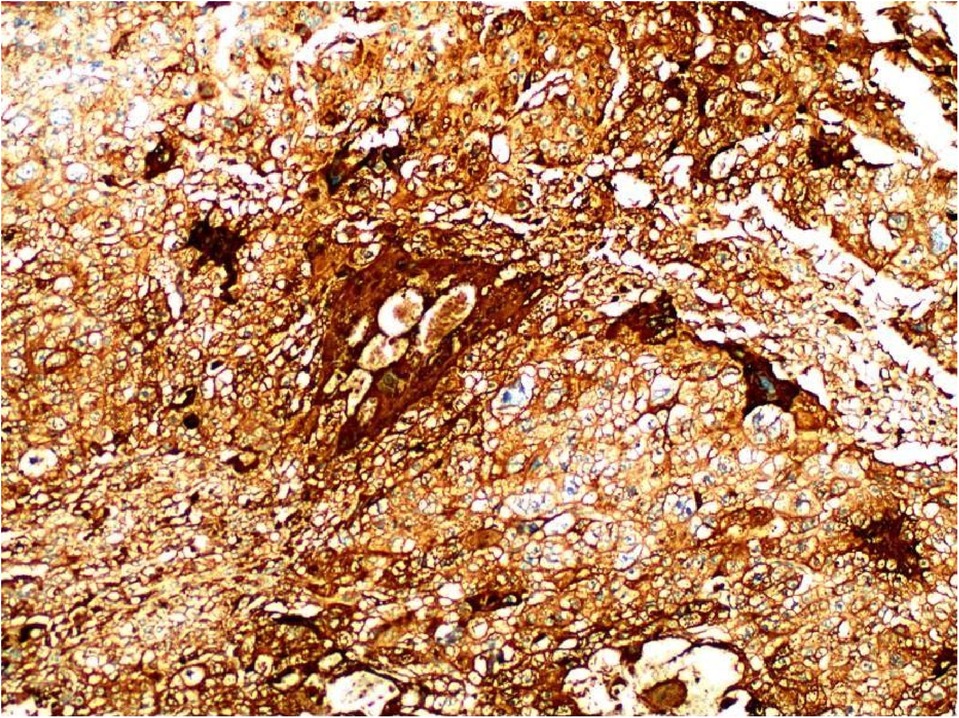
Figure 5. Immunohistochemistry: CD10 (+) (A), ck8/18 (+) (B), CK19 (+) (C), CKP (+) (D) and Ki-67 labelling index of 60% (+) (E) (HE ×200). Histochemical staining: PAS (+) (F) (SP ×200).
Discussion and conclusions
Primary gastric choriocarcinoma was first described by Davidsohn in 1905 (4) and accounts for approximately 0.8% of all gastric cancers (5). PGC is most commonly seen in older men, with a male to female ratio of 2.3:1 (6). The tumour is mainly located in the gastric sinus, followed by the gastric body and least in the cardia (7, 8). The clinical presentation is similar to that of gastric adenocarcinoma, with only 25% of patients developing choriocarcinoma (9). However, primary gastric choriocarcinoma is more likely to cause gastrointestinal bleeding than other tumours (10). Very few cases of primary gastric choriocarcinoma have been reported in China, most of which are associated with elevated serum β-HCG (human chorionic gonadotropin) levels, nausea, vomiting and gynaecomastia (7). The pathogenesis of the disease is unclear and several theories exist. The most accepted mechanism is Pick's theory of dedifferentiation, which states that primary gastric choriocarcinoma originates from the reverse differentiation of gastric adenocarcinoma. This theory suggests that existing gastric adenocarcinoma cells differentiate into embryonic stages and become trophoblast cells, which in turn differentiate into choriocarcinoma (11). Its diagnosis remains very difficult. The diagnosis of PGC must be made with great caution and the following conditions must be met: firstly, the presence of a tumour in the stomach must be excluded from any other occult primary site; secondly, the morphology of the tumour is similar to that of choriocarcinoma in other sites and the tumour cells express HCG by immunohistochemistry; and finally, HCG is elevated in serum and urine preoperatively but decreases to normal levels after gastrectomy and chemotherapy. Therefore, when preoperative gastroscopy reveals a malignant gastric ulcer, a tissue biopsy is required to improve the preoperative diagnosis. The diagnosis of primary gastric choriocarcinoma is then made postoperatively based on pathological features and immunohistochemical findings. In this case the preoperative gastroscopic pathology showed only high-grade intraepithelial neoplasia of the mucosal glands, yet the postoperative pathology report showed gastric choriocarcinoma. The serum HCG level decreased from 66.12 miu/ml postoperatively to 42.86 miu/ml (one week postoperatively). We then performed various imaging examinations such as cranial MRI and chest CT to exclude other tumours. We eventually combined the imaging findings, postoperative pathological features, immunohistochemical results and serological tests to make a final diagnosis of primary gastric choriocarcinoma. Its histopathology was rapid in most patients with PGC, with a poor prognosis. Most patients survive for 6 months (12) and usually die of blood transmission within the first year of diagnosis. Lymph nodes, liver, peritoneum and lung are common sites of metastasis (5). Poor prognostic factors affecting overall survival are mainly liver metastases, which usually lead to liver failure, residual tumour after surgery, and lack of chemotherapy (13). Due to its combination of gastric and choriocarcinoma characteristics, there is no standard treatment protocol. For early stage patients, postoperative chemotherapy is the treatment of choice after gastrectomy and lymph node dissection (13). For patients with locally advanced disease, systemic chemotherapy can be followed by radical gastrectomy combined with postoperative chemotherapy. For patients with advanced cancer, systemic chemotherapy is primarily used. Previous case reports have described (5) the use of VIP, BEP (bleomycin, etoposide, and cisplatin), EMA/CO (eto-poside, methotrexate, actinomycin D, cyclophosphamide, and vincristine), and fluorouracil plus cisplatin, but their efficacy was poor. However, Ceilesh A et al. (2) found that VIP chemotherapy regimens were effective in the early treatment of metastatic primary gastric choriocarcinoma. Picazo (14) found that two patients who received chemotherapy had a mean survival of 120 days. Monitoring of serum HCG levels may help to assess the effectiveness of treatment and tumour recurrence. As a rare disease, PGC is particularly important to diagnose early and accurately through various tests as its clinical presentation is very similar to that of other tumours. To date, there is no standard treatment for patients with PGC, so it is important to explore standard treatment options. In this case, the postoperative blood HCG-β test was 66.12 mIU/ml: since then, the HCG-β level has been measured regularly (1 week postoperative: 42.86 mIU/ml; 2 months postoperative: 15.56 mIU/ml), showing a gradual decrease and a stable trend, and together with imaging examinations (cranial MRI, chest and abdominal CT), suggesting that there are no signs of recurrence or metastasis, but close follow-up is still needed. The patient should be followed up closely.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the patient and legal guardian for the publication of any identifiable data/care reports.
Author contributions
ZXS wrote the paper. YYK provided the cases. YYK provided the images. YXJ reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Funded by key projects in the National Scientific Research Cultivation Program, No.: 2019-216; Gansu Innovation Base and Talent Project, No.: 20JR10RA433; Key research and development projects of Gansu Provincial Science and Technology Plan, No.: 21YF5WA027; Gansu Province Health Industry Scientific Research Plan, No.: GSWSKY2020-45; Gansu Provincial People's Hospital-Internal Scientific Research Youth Project, No.: 20GSSY4-12.
Acknowledgements
ZXS would like to thank everyone who has helped through the researching and preparing of this article, especially thank Professor Xiaojun Yang, who reviewed the manuscript and Yan, who provided the case and images. They would also like to sincerely thank their friends and family who have given considerable encouragement and financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wierzchniewska A, Romanowicz-Makowska H. Kosmówczak [choriocarcinoma]. Ginekol Pol. (1995) 66(9):537–40.8778012
2. Ceilesh A, Burroughs S, Majeed N, Klein L. Primary gastric choriocarcinoma: a case report of early successful treatment outcome. JCO Oncol Pract. (2020) 16(9):608–10. doi: 10.1200/OP.20.00008
3. Yoon JH, Kim MS, Kook EH, Ahn SH, Jeong SY, Han MS, et al. Primary gastric choriocarcinoma: two case reports and review of the literatures. Cancer Res Treat. (2008) 40(3):145–50. doi: 10.4143/crt.2008.40.3.145
4. Davidson C. Chorionepitherion und magenkrebs, eine seltene vershmelzung zweierbosartiger geschwulste. Carite Ann. (1905) 29:426–37.
5. Kobayashi A, Hasebe T, Endo Y, Sasaki S, Konishi M, Sugito M, et al. Primary gastric choriocarcinoma: two case reports and a pooled analysis of 53 cases. Gastric Cancer. (2005) 8(3):178–85. doi: 10.1007/s10120-005-0332-9
6. Takahashi K, Tsukamoto S, Saito K, Ohkohchi N, Hirayama K. Complete response to multidisciplinary therapy in a patient with primary gastric choriocarcinoma. World J Gastroenterol. (2013) 19(31):5187–94. doi: 10.3748/wjg.v19.i31.5187
7. Jindrak K, Bochetto JF, Alpert LI. Primary gastric choriocarcinoma: case report with review of world literature. Hum Pathol. (1976) 7(5):595–604. doi: 10.1016/s0046-8177(76)80105-0
8. Noguchi T, Takeno S, Sato T, Takahashi Y, Uchida Y, Yokoyama S. A patient with primary gastric choriocarcinoma who received a correct preoperative diagnosis and achieved prolonged survival. Gastric Cancer. (2002) 5(2):112–7. doi: 10.1007/s101200200019
9. Shastri A, Daver NG, Hayes TG. Primary gastric chorioadenocarcinoma: a needle in a haystack. Rare Tumors. (2011) 3(2):e19. doi: 10.4081/rt.2011.e19
10. Seckl MJ, Sebire NJ, Fisher RA, Golfier F, Massuger L. Sessa C; ESMO guidelines working group. Gestational trophoblastic disease: eSMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2013) 24(Suppl 6):vi39–50. doi: 10.1093/annonc/mdt345
11. Pick L. Uber die chorioepthelahnlich metastasierende from des magencarcinomas [in German]. Klin Wochenschr. (1926) 5:1728.
12. Baraka BA, Al Kharusi SS, Al Bahrani BJ, Bhathagar G. Primary gastric chorioadenocarcinoma. Oman Med J. (2016) 31(5):381–3. doi: 10.5001/omj.2016.75
13. Raghavapuram R, Veerankutty FH, Anandakumar M. Primary choriocarcinoma of the stomach. A case report and review of the literature. Indian J Surg Oncol. (2016) 7(1):119–23. doi: 10.1007/s13193-016-0494-4
Keywords: choriocarcinoma, primary gastric choriocarcinoma, pathology, treatment, case report
Citation: Xusheng Z, Yuke Y, Yun M, Huijun G, Jiangshan P, Xueqin D and Xiaojun Y (2022) Primary gastric choriocarcinoma: A case report. Front. Surg. 9:1009119. doi: 10.3389/fsurg.2022.1009119
Received: 1 August 2022; Accepted: 10 October 2022;
Published: 2 November 2022.
Edited by:
Tomoyuki Abe, Higashi-Hiroshima Medical Center, Japan© 2022 Xusheng, Yuke, Yun, Huijun, Jiangshan, Xueqin and Xiaojun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Xiaojun eWFuZ3hqbWRAYWxpeXVuLmNvbQ==
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Zhang Xusheng1,2
Zhang Xusheng1,2