- 1Department of Gastrointestinal Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2Institute of Digestive Surgery, State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Vascular Surgery, West China Hospital, Sichuan University, Chengdu, China
- 4Department of Vascular Surgery, The Affiliated Hospital of Southwest Medical University, Luzhou, China
Background: To evaluate short- and long-term outcomes of laparoscopic colectomy (LC) vs. open colectomy (OC) in patients with T4 colon cancer.
Methods: Three authors independently searched PubMed, Web of Science, Embase, Cochrane Library, and Clinicaltrials.gov for articles before June 3, 2022 to compare the clinical outcomes of T4 colon cancer patients undergoing LC or OC.
Results: This meta-analysis included 7 articles with 1,635 cases. Compared with OC, LC had lesser blood loss, lesser perioperative transfusion, lesser complications, lesser wound infection, and shorter length of hospital stay. Moreover, there was no significant difference between the two groups in terms of 5-year overall survival (5y OS), and 5-year disease-free survival (5y DFS), R0 resection rate, positive resection margin, lymph nodes harvested ≥12, and recurrence. Trial Sequential Analysis (TSA) results suggested that the potential advantages of LC on perioperative transfusion and the comparable oncological outcomes in terms of 5y OS, 5y DFS, lymph nodes harvested ≥12, and R0 resection rate was reliable and no need of further study.
Conclusions: Laparoscopic surgery is safe and feasible in T4 colon cancer in terms of short- and long-term outcomes. TSA results suggested that future studies were not required to evaluate the 5y OS, 5y DFS, R0 resection rate, positive resection margin status, lymph nodes harvested ≥12 and perioperative transfusion differences between LC and OC.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022297792.
Introduction
Colorectal cancer is both the third most commonly diagnosed cancer and the third cause of cancer-related death globally (1). In addition, colon cancers account for nearly 60%, while approximately 106,180 new cases will be confirmed in 2022 (1, 2). Among them, about 15% of colon cancer patients diagnosed with locally advanced disease (T4 stage) (3). Compared with open colectomy, the widely used minimally invasive surgical technology for colon cancer has better short-term results and comparable tumor prognosis (4–8). Moreover, based on the several large randomized controlled trials such as COLOR, CLASICC, COST, EnROL trial and several recent meta-analyses, the NCCN guidelines for colon cancer (2006) recommended that minimally invasive colectomy was considered for colon cancer and performed only by surgeons experienced in this techniques (9–20). However, since the tumor volume of T4 colorectal cancer is large and invades surrounding tissues or adjacent organs, laparoscopic (Lap) En-bloc resection is difficult and risky. Several large randomized controlled trials have compared laparoscopic and open colectomy. But in the Barcelona, ALCCaS, COST, COLOR, MRC CLASSICC, ACOSOG Z6051 trials, locally advanced colon tumors were portion of the exclusion criteria (20–25). Later, most clinical studies enrolled fewer cases of T4 colorectal cancer (20, 25–27). Therefore, there is limited evidence-based data to prove the safety and effectiveness of laparoscopic resection for T4 colon cancer. Laparoscopic T4 colorectal cancer resection is considered to be a technique demanding accuracy and its efficacy is still controversial. The American Joint Committee on Cancer (AJCC) TNM staging system and guidelines from the European Association of Endoscopic Surgery (EAES) guidelines did not recommend laparoscopic surgery for T4 colon cancer (28). However, due to the innovation and progress of laparoscopic platform and the popularization and improvement of laparoscopic technology, surgeons in some large and well-experienced centers tried to apply laparoscopic technology in T4 colorectal cancer and achieved better short-term benefits and oncological outcomes similar to open surgery (4, 6, 8, 29, 30).
Recently, three updated meta-analyses showed that LC was associated with better perioperative outcomes like a lower complication compared with OC and R0 resection rates, 5y OS, and 5y DFS for OC and LC were similar (4, 6, 8). Nevertheless, most of the cases included in the above three meta-analyses were retrospective studies, and the huge heterogeneity caused by different definitions of T4 (T4a vs. T4b, clinical T4 vs. pathological T4). In addition, with more and more statistical analysis of the accumulated literature, the possibility of observing false negative or false positive results increased (31). Trial Sequential Analysis (TSA) can overcome the above shortcomings (32, 33). Therefore, we used TSA method in meta-analysis to control the risk of type I errors.
In this study, we systematically reviewed the existing relevant literature and performed a meta-analysis comprised of TSA of the data on short- and long-term outcomes of LC vs. OC for pT4 colon cancer.
Methods
This meta-analysis was carried out according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and AMSTAR (Assessing the methodological quality of systematic reviews) Guidelines (34). Ethical consent was not applicable. The present study was registered in PROSPERO website (https://www.crd.york.ac.uk/PROSPERO/) and the Registration Number is: CRD42022297792.
Literature search
A systematic literature search was carried out in the PubMed, Web of Science, Embase, Cochrane Library, and Clinicaltrials.gov from inception to June 3, 2022 with no limit. The main terms were: (Colonic Neoplasms OR Colonic Neoplasm OR Colon Neoplasm OR Colon Cancer OR Colonic Cancer OR Colonic malignancy OR Colon tumor OR Colon tumour OR colon carcinoma) AND (Locally advanced OR T4 OR multivisceral OR advanced OR pT4 OR cT4) AND (Laparoscopy OR laparoscopic OR open OR minimally invasive OR minimal invasive). In addition, a manual search of references of relevant literatures and reviews was also conducted obtain more potential research.
Inclusion and exclusion criteria
The inclusion criteria for the meta-analysis were: (1) studies with patients with primary colon cancer; (2) clinical studies that compared LC vs. OC; and (3) raw data that included followings: conversion rate, postoperative complications, perioperative transfusion, mortality, survival, R0 resection rate, resection margin status, number of harvested lymph nodes, and recurrence. The exclusion criteria were: (1) studies did not present data of T4 tumors, (2) mix with rectal cancer or other T stage, (3) studies with no comparison cohort, (4) reviews or meta-analyses, (5) conference abstract, (6) letter, (7) study could not be retrieved.
Study selection and quality assessment
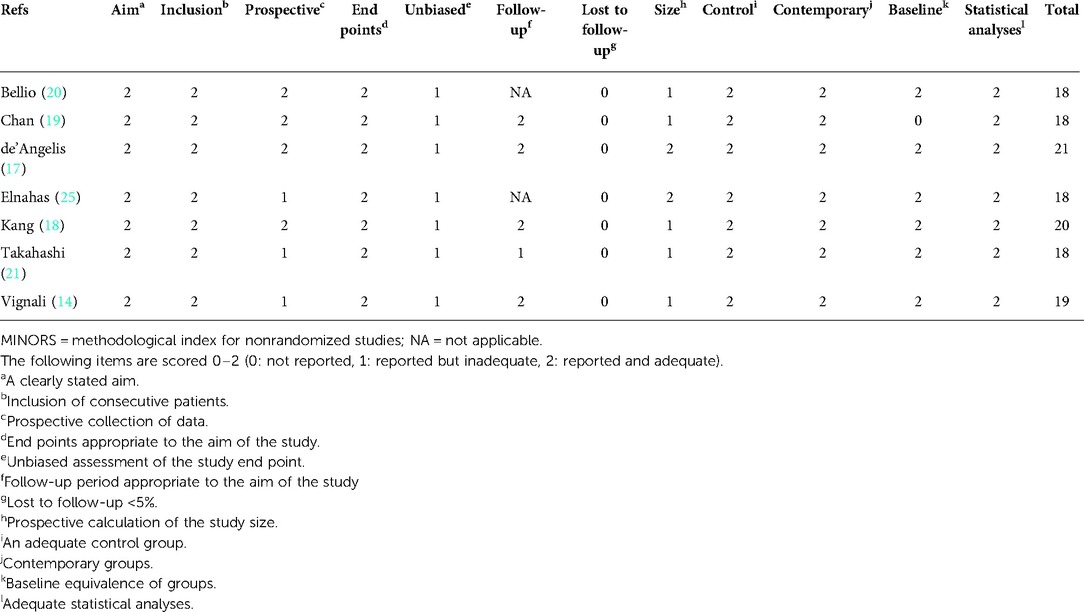
Three authors (PC, HZ and CC) independently used the Methodological Index for Non-Randomized Studies (MINORS) instrument to assess the quality of the included prospective observational studies (35). The items were scored 0 (not reported), 1(reported but not enough) or 2 (reported and enough). The full score of non- comparative research is 16 points, and the total score of comparative research is 24 points. Moreover, the Grading of Recommendations Assessment, Development, and Evaluation system (GRADE system) was used to rate the level of evidence as very low, low, moderate, or high and created a summary table with the GRADE profiler software (version 3.6.1) (36). Any differences were resolved through consensus discussion between the review group.
Data extraction
Three researchers (PC, HZ and CC) used structured tables to extract data from each study and input the data into the database. The extracted items contained: author, publication year, study period, country, Single or multicenter study, sample size, gender, age, body mass index(BMI), American Society of Anesthesiologists (ASA), Tumor, Node, Metastasis (TNM) staging classification, neoadjuvant therapy, adjuvant chemotherapy, inclusion and exclusion criteria, median follow-up, conversion rate, operation time (min), blood loss (ml), length of hospital stay (days), soft diet start (days), complications, wound infection, intra-abdominal abscess, ileus, anastomotic leakage, perioperative transfusion, diverting stoma, mortality rate, 5y OS, 5y DFS, R0 resection rate, positive resection margin, lymph nodes harvested ≥12, recurrence.
Follow-up plans
The follow-up plans were similar in the 3 studies that evaluated long-term results. Patients were followed up at 3 monthly intervals for the first 2 years and every 6 months thereafter. Physical examination and carcinoembryonic antigen (CEA) were routinely performed, whereas abdominal and chest CT scans were performed with an average interval of 6 months. Colonoscopy was carried out once a year or when abnormalities were detected during any follow-up visit. An 18-FDG PET scan was performed if recurrence was suspected. Biopsies were selectively performed.
Statistical analysis
Analysis was performed using Review Manager (version 5.4.1). Meta-analysis was conducted in which two or more studies assessed the same risk factor in a comparable manner (37). The inverse-variance method and the Mantel-Haenszel estimator were used to calculate pooled mean difference (MD) values and odds ratios (ORs), respectively. MD and pooled ORs were used for continuous variables and dichotomous variables respectively. For continuous variables, if the study only provides median and range values or means and range values, the method described in the previous study was used to calculate the means and standard deviations (38). For the survival endpoints, relative risk (RR) with the corresponding 95% CIs were applied. Statistical heterogeneity was assessed using the Higgins I2value (39).
The thresholds of low, medium and high heterogeneity(I2) are 25%, 50% and 75%, respectively. A random-effects model was used for all outcomes (40). Publication bias was evaluated through the funnel plots in Review Manager. P < 0.05 was considered statistically significant.
Trial sequential analysis
TSA was used to evaluate the statistical reliability of data in the cumulative meta-analysis. It controlled the α and β Value for repetitive testing on the accumulating data. TSA was a tool to assess whether the currently available evidence is sufficiently conclusive (41). Meta-analysis of small samples may increase the risk of false-positive results, resulting in wrong conclusions. To avoid false-negative/positive results, we performed a TSA using the TSA software (version 0.9.5.10, Copenhagen Trial Unit, Denmark). TSA was performed for both dichotomous and continuous outcomes, in which a 20% relative risk reduction, a low-risk-based MD, a type I error (α = 0.05, two-sided), and a type II error (β = 0.20, power of 80%) were applied to calculate optimal information size.
Results
Selected studies and baseline characteristics
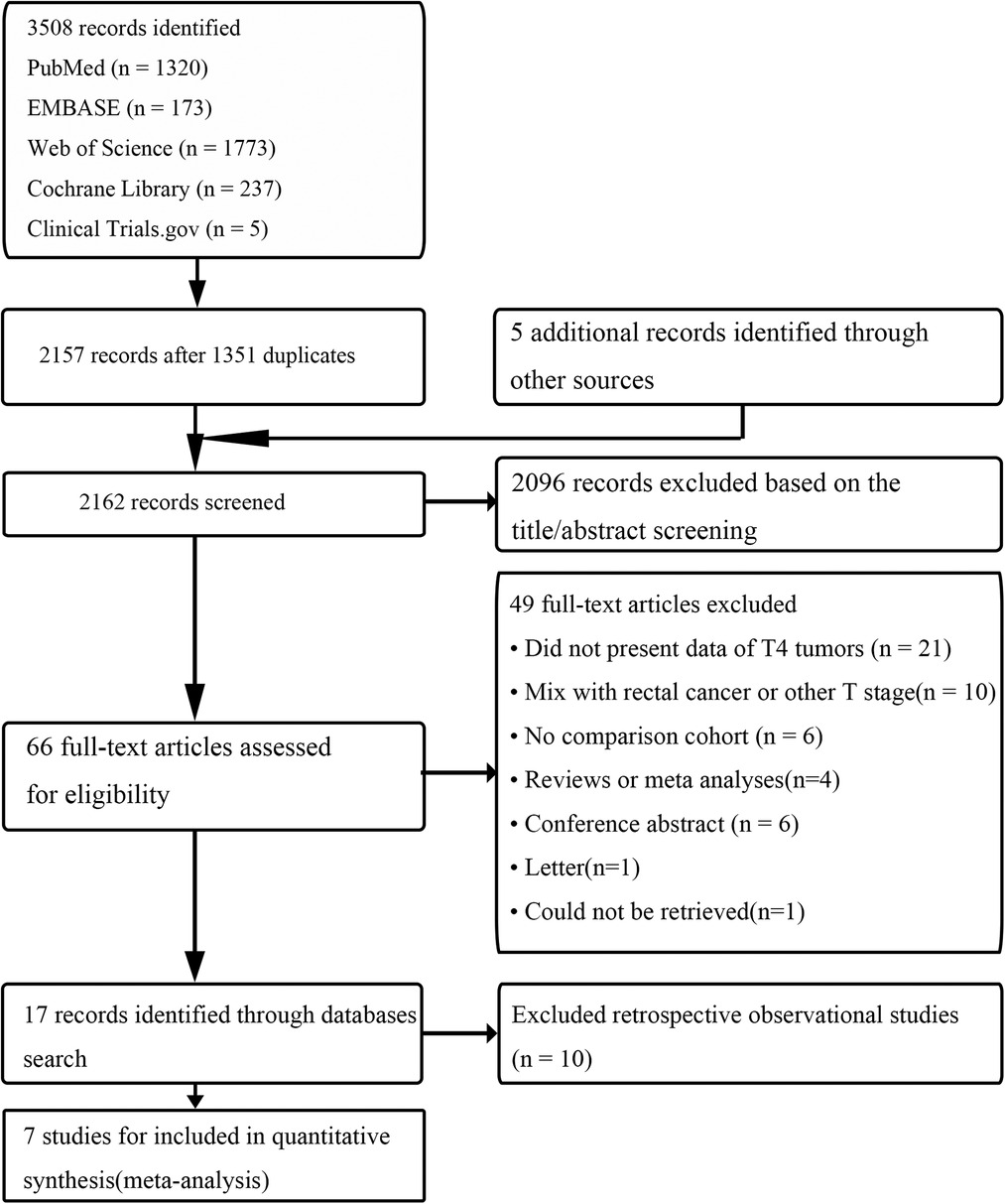
According to the literature search and selection strategy, a total of 7 prospective observational studies that included 1,635 cases with pT4 colon cancer resection (863 LC and 772 OC) were enrolled in this meta-analysis (Figure 1) (42–48). Demographic and clinical characteristics of 7 prospective observational studies were shown in Table 1. Quality assessment of studies was shown in Table 2; 7 studies had a score of >18 points based on MINORS. Meta-analysis for LC vs. OC was shown in Table 3.
Short-term outcomes
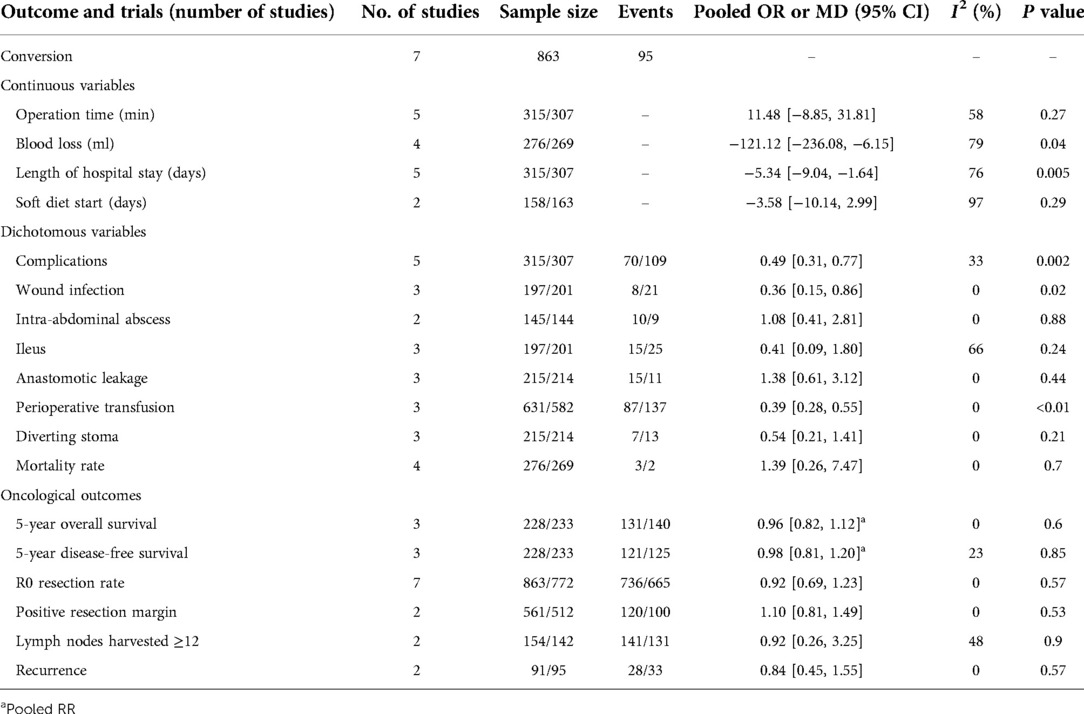
Table 3 showed the results of meta-analysis for all outcomes. All 7 clinical studies reported data on conversion rate with a pooled rate of 11% (95 cases) (42–48). The conversion rate ranged from 7.1 to 28.2% in the LC group. The pooled results showed no significant difference in the operation time between the two groups (MD = 11.48, 95% CI, −8.85 to 31.81, P = 0.27). The pooled results showed a significant reduction (MD = −121.12 ml, 95% CI, −236.08 to −6.15, P = 0.04) in blood loss among the LC group. LC group showed a significantly lower hospital stay than OC group (MD = −5.34 days, 95% CI, −9.04 to −1.64, P =0.005). LC group showed a shorter trend duration than OC group in terms of the number of days to the soft diet start (MD = − 3.58, 95% CI, −10.14 to 2.99, P = 0.29).
The morbidity rates of LC group ranged from 13.5% to 28.3%, while the morbidity rates of OC group ranged from 27.1% to 52.6%. The overall complications significantly decreased in LC group compared to OC group (OR = 0.49, 95% CI, 0.31–0.77, P = 0.002, Figure 2). Among overall complication, the pooled results showed a significant reduction in wound infection among the LC group (OR = 0.36, 95% CI, 0.15–0.86, P = 0.02). In addition, the incidence of perioperative transfusion in the LC group was lower than that in the OC group (OR = 0.39, 95% CI, 0.28–0.55, P < 0.01). The pooled results showed no significant differences in terms of intra-abdominal abscess (OR = 1.08, 95% CI, 0.41–2.81, P = 0.88), ileus (OR = 0.41, 95% CI, 0.09–1.80, P = 0.24), anastomotic leakage (OR = 1.38, 95% CI, 0.61–3.12, P = 0.44), diverting stoma (OR = 0.54, 95% CI, 0.21–1.41, P = 0.21), mortality (OR = 1.39, 95% CI, 0.26 = 7.47, P = 0.7), R0 resection rate (OR = 0.92, 95% CI, 0.69–1.23, P = 0.57), positive resection margin (OR = 1.10, 95% CI, 0.81–1.49, P = 0.53), and lymph nodes harvested ≥12 (OR = 0.92, 95% CI, 0.26–3.25, P = 0.9).
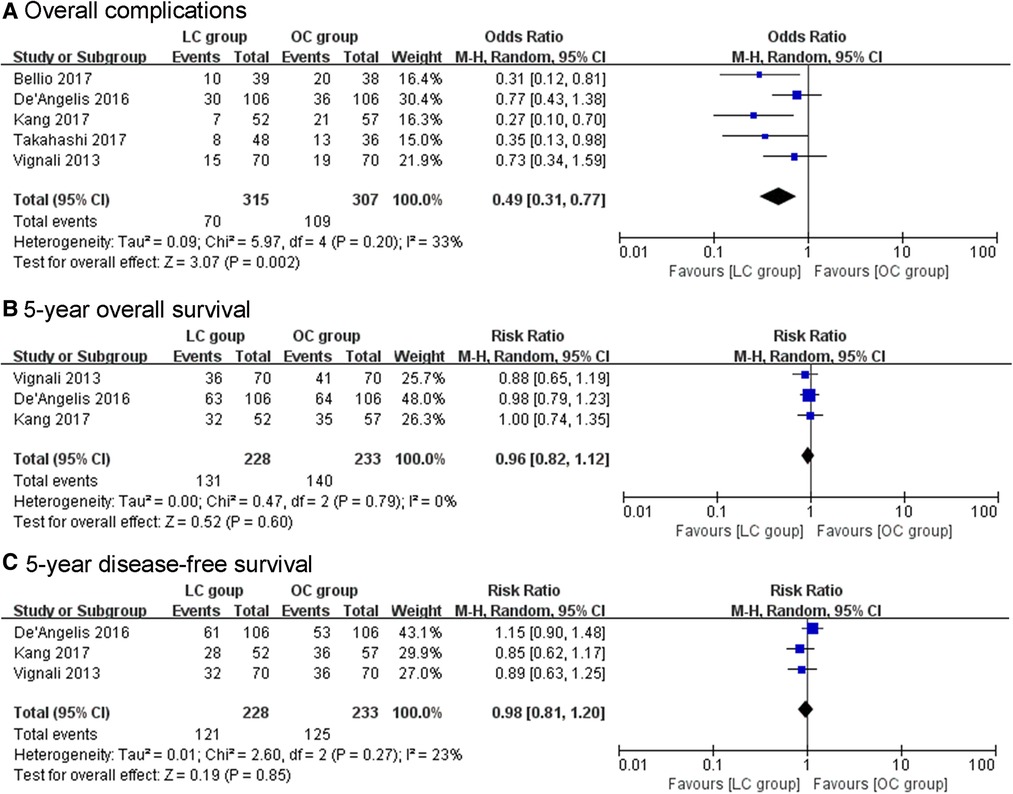
Figure 2. (A) The pooled results showed significant decrease in overall complications with LC compared with OC. (B, C) The pooled results showed no significant difference in 5-year overall survival and 5-year disease-free survival between the treatment groups.
Oncological outcomes
The pooled results showed no significant difference in 5y OS between the LC group and OC group (RR = 0.96, 95% CI, 0.82–1.12, P = 0.6, Figure 2). Also, no significant difference was found in the rate of 5y DFS between the groups (RR = 0.98, 95% CI, 0.81–1.20, P = 0.85, Figure 2). There was no significant between-group difference in terms of recurrence (OR = 0.84, 95% CI, 0.45–1.55, P = 0.57) between the two groups.
Trial sequential analyses
The potential false-positive errors of the meta-analysis were found in the length of hospital stay (days) (Figure 3A), blood loss (Figure 3B), operation time (Figure 3C) and complications (Figure 3E), the TSA results showed that the cumulative Z-curve crossed the conventional boundary but did not cross the futility boundaries or the trial sequential monitoring boundary (TSMB). Therefore, more trials are needed before drawing a definite conclusion. For mortality (Figure 3F), recurrence (Figure 4E), and positive resection margin status (Figure 4F), neither the TSMB nor the traditional boundary was crossed, indicating the lack of specific evidence and the need for more research. For perioperative transfusion (Figure 3D), the cumulative Z-curve crossed the TSMB and the traditional boundary, indicating conclusive evidence in the LC group compared with the OC group. The meta-analyses of 5y OS (Figure 4A) and 5y DFS (Figure 4B) did not differ statistically significant; the cumulative Z-curve crossed neither the traditional boundary nor the TSMB, but it crossed the futility boundaries, suggesting no statistical significance between-group difference and no need of further study. The cumulative Z-curve of lymph nodes harvested ≥12 (Figure 4C) and R0 resection rate (Figure 4D) crossed the RIS, suggesting firm evidence of no statistical significance in the LC group compared with OC group.

Figure 3. Trial sequential analysis (TSA). The adjusted required information size was calculated using α = 0.05 (two-sided), β = 0.20 (power 80%), and an empirical mean difference. (A) Hospital stay (days); (B) Blood loss; (C) Operation time; (D) Perioperative transfusion; (E) Complication; (F) Mortality.
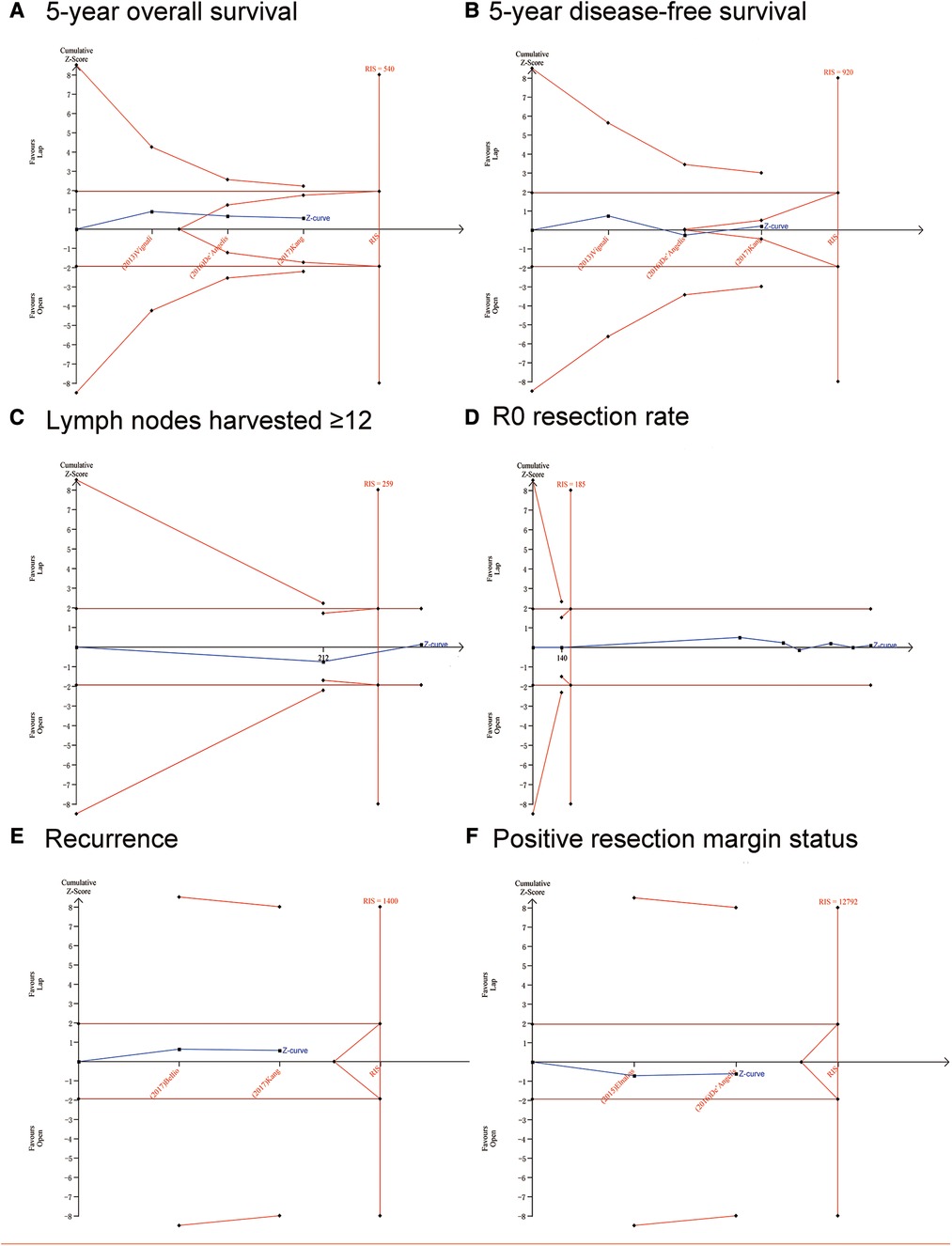
Figure 4. Trial sequential analysis (TSA). The adjusted required information size was calculated using α = 0.05 (two-sided), β = 0.20 (power 80%), and an empirical mean difference. (A) 5-year overall survival; (B) 5-year disease-free survival; (C) Lymph nodes harvested ≥12; (D) R0 resection rate; (E) Recurrence; (F) Positive resection margin status.
GRADE of the outcomes
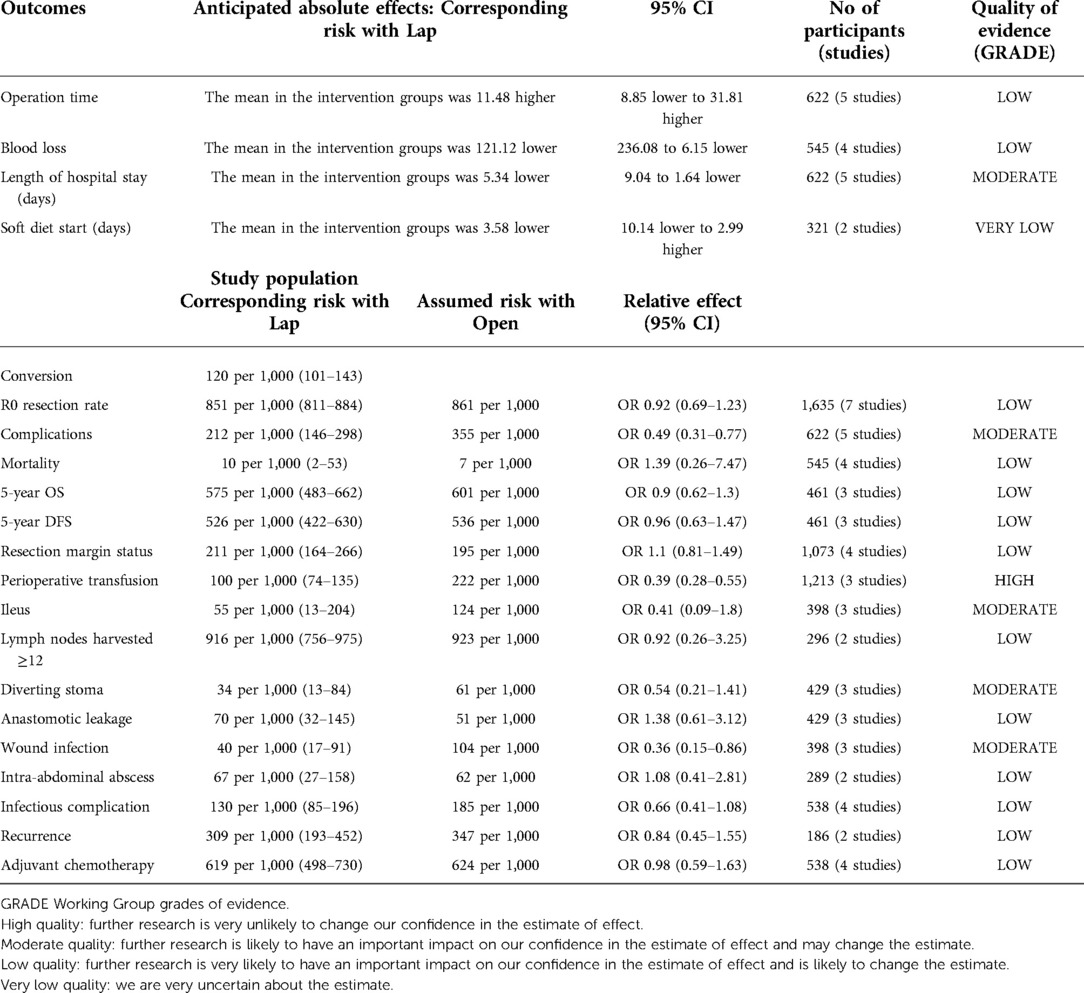
The GRADE system was applied to synthesize and evaluate the evidence level for the outcomes (Table 4). The power of evidence was moderate in length of hospital stay, complications, ileus, diverting stoma, and wound infection, while it was low in operation time, blood loss, R0 resection rate, mortality, 5y OS, 5y DFS, lymph nodes harvested ≥12, anastomotic leakage, intra-abdominal abscess, infectious complication, recurrence, and adjuvant chemotherapy. The level of evidence was high in perioperative transfusion and very low in soft diet start.
Evaluation of publication bias
A funnel plot of R0 resection rate was applied to visually assess publication bias in this meta-analysis. The funnel plot of R0 resection rate suggested a lack of publication bias (Figure 5).
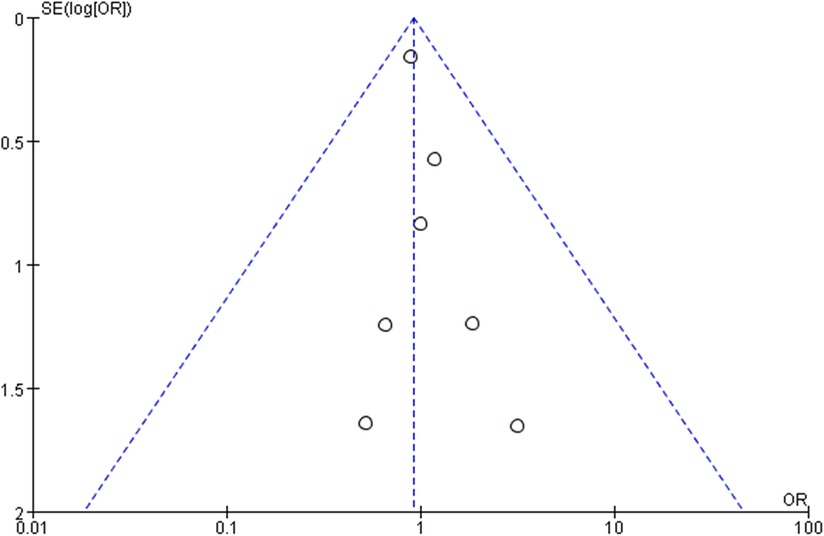
Figure 5. The bilateral symmetry shaped funnel plot of R0 resection rate indicated a lack of publication bias.
Discussion
The safety and oncological outcomes of LC for pT4 colon cancer remain controversial. In the meta-analysis, we included 7 prospective observational studies comparing the efficacy of LC with OC for colon cancer, all of which are scored as high-quality studies based on the MINORS scores (42–48). The results showed that LC could be performed with lesser perioperative transfusion, lesser blood loss, lesser complications, lesser wound infection, and shorter length of hospital stay. Furthermore, there was no significant difference between the two groups in terms of 5y OS, 5y DFS, R0 resection rate, positive resection margin, lymph nodes harvested ≥12, and recurrence. Based on the TSA results, the current evidence for the potential advantages of LC on perioperative transfusion appeared reliable and conclusive. Meanwhile, TSA results suggested that the comparable outcomes in terms of 5y OS, 5y DFS, lymph nodes harvested ≥12, and R0 resection rate drawn from this meta-analysis were reliable and no need of further study.
The perioperative short-term outcomes were significantly more superior in the LC group for colon cancer in terms of lesser blood loss, lesser perioperative transfusion, lesser complications, lesser wound infection, and shorter length of hospital stay. Moreover, the evidence of the advantage of LC on perioperative blood transfusion seemed to be reliable and decisive. Based on the existing literature, LC for T4a colon tumor might be safe, but it should be performed cautiously for T4b colon cancer requiring multivisceral resection (MVR) (6, 49, 50). However, several study groups have reported the safety and effectiveness of Lap MVR (47, 51, 52). Both studies considered that patients with urinary tract invasion were not suitable for lap MVR because the technical complexity and possibility of complications outweighed the gains (47, 48). Nevertheless, with the maturity of laparoscopic surgery technology, especially with the appearance of robotic surgery, ureterectomy and anastomosis had accumulated rich experience. Therefore, this technology depends, at least to some extent, on the technology of urologists in each hospital.
However, several studies reported that MVR was related to high postoperative morbidity and increased risk of conversion (49, 50, 53). Some studies have pointed out that preoperative conversion was associated with poor postoperative outcomes, such as increased postoperative complication rate and mortality, and even a poor prognosis (54, 55). However, studies have reported that conversion has been divided into two types: (I) strategic conversion, which is a prescient decision to avoid complications; (II) reactive conversion, i.e., laparotomy due to unexpected surgical difficulties or complications (56, 57). It is well known that strategic conversion can bring better results than reactive conversion (56). Takahashi et al. reported that except for one case of reactive conversion due to intraoperative bleeding, the other five cases were strategic conversion. The results showed that the reported postoperative complication rate was relatively low, the patient's hospital stay was not prolonged, and the oncological results were favorable. This suggested that strategic conversion might not have a significant unfavorable effect on short- and long-term outcomes.
Another worry is the risk of R1 resection-insufficient clearance of cancer tissue. Our meta-analysis results showed that there were no differences in the R0 resection rate treated with laparoscopic surgery and the evidence of comparable R0 resection rates was reliable. R0 resection was very important for the cancer treatment of T4 patients, and it was also the principal factor affecting the survival after MVR (47, 49, 58). Some people worried that choosing the lap method might threaten the implementation of R0 (48). The COLOR trial, about 20% of T4 patients detected a microscope positive edge (R1), compared to 1% of T3 patients; However, the open group had little superiority (T4, 17.6%; T3, 1.0%) (59). Takahashi et.al reported that the R1 rate in lap group did not increase and two patients in lap group underwent R1 palliative resection for stage IV patients (47). Therefore, the risk of R1 resection in the treatment of T4 tumors with Lap method had not been fully confirmed.
Although the oncological safety of LC in the treatment of colon cancer has been confirmed, there were scarce data on LC in the treatment of T4 colon cancer. In this meta-analysis and TSA of 7 prospective observational studies, there was no significant difference in 5y OS, 5y DFS between two group patients, which was in line with previous studies (4, 60). The above results showed that the oncological results of LC for pT4 colon cancer are acceptable.
Our results also revealed that laparoscopic surgery did not increase the recurrence rate of T4 colon cancer patients when compared with open surgery. Consistent with our research, several large meta-analysis and original research had confirmed this conclusion (4, 8, 61, 62). However, after literature search, there were still several reports that laparoscopic surgery could increase the peritoneal and trocar recurrence rate of T4 colon cancer patients (63–67). Wang et al. reported that laparoscopic colectomy for T4 colon cancer had a higher peritoneal recurrence rate than open surgery (18.1 vs. 10.6 percent; RR 1.56, 1.23–1.99; P = 0.0003) (66). In addition, a review published in 1998 showed that trocar recurrence seemed to be secondary to a variety of factors, including pneumoperitoneum, laparoscopic instruments, biologic properties of the tumor, local trauma, and individual surgical skills (63). Therein, careful patient selection in operative approach for T4 colon cancer is needed especially for patients at high risk of intraperitoneal tumor spread.
Recently, there had also been studies on the safety and effectiveness of robot approaches for T4 colon cancer (68–70). An NCDB propensity score-matched analysis of open, laparoscopic, and robotic approaches demonstrated that compared with T4 colon cancer open resection, laparoscopic and robot-assisted surgery had achieved better tumor prognosis and survival rate and robot-assisted surgery was significantly associated with a lower conversion rate compared with laparoscopic surgery (69). This case-matching study demonstrated the safety of using minimally invasive techniques in T4 colon cancers (69). Further multicenter, large-sample randomized controlled trials are needed to verify these results.
Our present meta-analysis has several advantages. The search methodology and inclusion criteria were rigorous, with a systematic literature search to determine the relevant prospective observational studies without restrictions. Further, TSA integrated information indicators and effect indicators, which was more conservative and might be more accurate. In the evaluation setting of non-significant results, TSA could help determine whether “more studies needed” to reduce uncertainty when cumulative Z-curve did not cross the futility boundary. However, this study has some shortcomings. First, there were no randomized controlled trials and no information on quality of life in the literature included in this meta-analysis. Second, because different literatures had different definitions of T4 (T4a vs. T4b, clinical T4 vs. pathological T4), there was heterogeneity between the studies.
Conclusion
In conclusion, laparoscopic surgery is as acceptable as open surgery for T4 colon cancer in terms of the conversion rate, R0 resection rate, short-term and oncological outcomes. laparoscopic surgery is an innovative and promising approach for the treatment of T4 colon cancer. TSA results demonstrated that further research is not needed to evaluate the 5y OS, 5y DFS, R0 resection rate, positive resection margin status, lymph nodes harvested ≥12 and perioperative transfusion differences between two techniques. Additional multicenter, large-sample randomized controlled trials to evaluate the safety and effectiveness of robot and laparoscope technology for T4 colon cancer are needed in the future.
Author contributions
LY and PC: participated in the conception and design of this meta-analysis. PC and HZ: conducted the literature searching and performed a quality evaluation of included studies by MINORS. PC, HZ and CC: acquired the data. PC, HZ and XQ: performed the statistical analysis and interpretation of data. PC: wrote the manuscript. LY and ZZ: reviewed the manuscript. LY: revised and supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Key Research and Development Program of China [grant no. 2016YFC0906000 (2016YFC0906003)]; National Natural Science Foundation of China [grant no. 81472304]; Sichuan Science and Technology Program [grant no. 2017JY0020]; and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (grant no. 2016105, ZYGD20006)
Acknowledgments
The authors thank the reviewers for their helpful comments on this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
2. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. (2019) 16:713–32. doi: 10.1038/s41575-019-0189-8
3. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. (2014) 383:1490–502. doi: 10.1016/s0140-6736(13)61649-9
4. Feinberg AE, Chesney TR, Acuna SA, Sammour T, Quereshy FA. Oncologic outcomes following laparoscopic vs. open resection of pT4 colon cancer: a systematic review and meta-analysis. Dis Colon Rectum. (2017) 60:116–25. doi: 10.1097/dcr.0000000000000641
5. Kumamoto T, Toda S, Matoba S, Moriyama J, Hanaoka Y, Tomizawa K, et al. Short- and long-term outcomes of laparoscopic multivisceral resection for clinically suspected T4 colon cancer. World J Surg. (2017) 41:2153–9. doi: 10.1007/s00268-017-3976-9
6. Klaver CEL, Kappen TM, Borstlap WAA, Bemelman WA, Tanis PJ. Laparoscopic surgery for T4 colon cancer: a systematic review and meta-analysis. Surg Endosc. (2017) 31:4902–12. doi: 10.1007/s00464-017-5544-7
7. Yamanashi T, Nakamura T, Sato T, Naito M, Miura H, Tsutsui A, et al. Laparoscopic surgery for locally advanced T4 colon cancer: the long-term outcomes and prognostic factors. Surg Today. (2018) 48:534–44. doi: 10.1007/s00595-017-1621-8
8. Liu ZH, Wang N, Wang FQ, Dong Q, Ding J. Oncological outcomes of laparoscopic vs. open surgery in pT4 colon cancers: a systematic review and meta-analysis. Int J Surg. (2018) 56:221–33. doi: 10.1016/j.ijsu.2018.06.032
9. Rondelli F, Trastulli S, Avenia N, Schillaci G, Cirocchi R, Gullà N, et al. Is laparoscopic right colectomy more effective than open resection? A meta-analysis of randomized and nonrandomized studies. Colorectal Disease. (2012) 14:e447–469. doi: 10.1111/j.1463-1318.2012.03054.x
10. Ohtani H, Tamamori Y, Arimoto Y, Nishiguchi Y, Maeda K, Hirakawa K. A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and open colectomy for colon cancer. J Cancer. (2012) 3:49–57. doi: 10.7150/jca.3621
11. Montie JE, Clark PE, Eisenberger MAJJotNCCNJ. National comprehensive cancer network. Colon Cancer Guidel. (2006).
12. Kuhry E, Schwenk W, Gaupset R, Romild U, Bonjer J. Long-term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treat Rev. (2008) 34:498–504. doi: 10.1016/j.ctrv.2008.03.011
13. Kennedy RH, Francis EA, Wharton R, Blazeby JM, Quirke P, West NP, et al. Multicenter randomized controlled trial of conventional vs. laparoscopic surgery for colorectal cancer within an enhanced recovery programme: enrol. J Clin Oncol. (2014) 32:1804–11. doi: 10.1200/jco.2013.54.3694
14. Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK mrc clasicc trial group. J Clin Oncol. (2007) 25:3061–8. doi: 10.1200/jco.2006.09.7758
15. Jackson TD, Kaplan GG, Arena G, Page JH, Rogers SO Jr. Laparoscopic vs. open resection for colorectal cancer: a metaanalysis of oncologic outcomes. J Am Coll Surg. (2007) 204:439–46. doi: 10.1016/j.jamcollsurg.2006.12.008
16. Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, et al. Long-term follow-up of the medical research council clasicc trial of conventional vs. laparoscopically assisted resection in colorectal cancer. Br J Surg. (2013) 100:75–82. doi: 10.1002/bjs.8945
17. Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the cost study group trial. Ann Surg. (2007) 246:655–62; discussion 662-654. doi: 10.1097/SLA.0b013e318155a762
18. Di B, Li Y, Wei K, Xiao X, Shi J, Zhang Y, et al. Laparoscopic vs. open surgery for colon cancer: a meta-analysis of 5-year follow-up outcomes. Surg Oncol. (2013) 22:e39–43. doi: 10.1016/j.suronc.2013.03.002
19. Deijen CL, Vasmel JE, de Lange-de Klerk ESM, Cuesta MA, Coene PLO, Lange JF, et al. Ten-year outcomes of a randomised trial of laparoscopic vs. open surgery for colon cancer. Surg Endosc. (2017) 31:2607–15. doi: 10.1007/s00464-016-5270-6
20. Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, et al. Survival after laparoscopic surgery vs. open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. (2009) 10:44–52. doi: 10.1016/s1470-2045(08)70310-3
21. Nelson H, Sargent DJ, Wieand HS, Fleshman J, Anvari M, Stryker SJ, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. (2004) 350:2050–9. doi: 10.1056/NEJMoa032651
22. Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, et al. Short-term endpoints of conventional vs. laparoscopic-assisted surgery in patients with colorectal cancer (mrc clasicc trial): multicentre, randomised controlled trial. Lancet (London, England). (2005) 365:1718–26. doi: 10.1016/s0140-6736(05)66545-2
23. Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, et al. The long-term results of a randomized clinical trial of laparoscopy-assisted vs. open surgery for colon cancer. Ann Surg. (2008) 248:1–7. doi: 10.1097/SLA.0b013e31816a9d65
24. Bagshaw PF, Allardyce RA, Frampton CM, Frizelle FA, Hewett PJ, McMurrick PJ, et al. Long-term outcomes of the australasian randomized clinical trial comparing laparoscopic and conventional open surgical treatments for colon cancer: the australasian laparoscopic colon cancer study trial. Ann Surg. (2012) 256:915–9. doi: 10.1097/SLA.0b013e3182765ff8
25. Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, et al. Effect of laparoscopic-assisted resection vs open resection of stage ii or iii rectal cancer on pathologic outcomes: the acosog z6051 randomized clinical trial. Jama. (2015) 314:1346–55. doi: 10.1001/jama.2015.10529
26. Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, et al. Open vs. laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (corean trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. (2010) 11:637–45. doi: 10.1016/s1470-2045(10)70131-5
27. Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, et al. Open vs. laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (corean trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. (2014) 15:767–74. doi: 10.1016/s1470-2045(14)70205-0
28. Mathis KL, Nelson H. Controversies in laparoscopy for colon and rectal cancer. Surg Oncol Clin N Am. (2014) 23:35–47. doi: 10.1016/j.soc.2013.09.006
29. Shukla PJ, Trencheva K, Merchant C, Maggiori L, Michelassi F, Sonoda T, et al. Laparoscopic resection of T4 colon cancers: is it feasible? Dis Colon Rectum. (2015) 58:25–31. doi: 10.1097/dcr.0000000000000220
30. de'Angelis N, Landi F, Vitali GC, Memeo R, Martínez-Pérez A, Solis A, et al. Multicentre propensity score-matched analysis of laparoscopic vs. open surgery for T4 rectal cancer. Surg Endosc. (2017) 31:3106–21. doi: 10.1007/s00464-016-5332-9
31. Borm GF, Donders AR. Updating meta-analyses leads to larger type i errors than publication bias. J Clin Epidemiol. (2009) 62:825–830.e810. doi: 10.1016/j.jclinepi.2008.08.010
32. Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. (2008) 61:64–75. doi: 10.1016/j.jclinepi.2007.03.013
33. Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol. (2009) 38:276–86. doi: 10.1093/ije/dyn179
34. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. Br Med J. (2009) 339:b2535. doi: 10.1136/bmj.b2535
35. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
36. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
37. Scholz AF, Oldroyd C, McCarthy K, Quinn TJ, Hewitt J. Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. Br J Surg. (2016) 103:e21–28. doi: 10.1002/bjs.10062
38. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
39. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
40. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
41. Bobo Z, Xin W, Jiang L, Quan W, Liang B, Xiangbing D, et al. Robotic gastrectomy vs. laparoscopic gastrectomy for gastric cancer: meta-analysis and trial sequential analysis of prospective observational studies. Surg Endosc. (2019) 33:1033–48. doi: 10.1007/s00464-018-06648-z
42. Bellio G, Lo Cicero A, Barbieri V, Tarchi P, Casagranda B NDEM. Is T4 colon cancer still an absolute contraindication to laparoscopic surgery? Minerva Chir. (2017) 72:483–90. doi: 10.23736/s0026-4733.17.07378-3
43. Chan DK, Tan KK. Laparoscopic surgery should be considered in T4 colon cancer. Int J Colorectal Dis. (2017) 32:517–20. doi: 10.1007/s00384-016-2702-7
44. de'Angelis N, Vitali GC, Brunetti F, Wassmer CH, Gagniere C, Puppa G, et al. Laparoscopic vs. Open surgery for T4 colon cancer: a propensity score analysis. Int J Colorectal Dis. (2016) 31:1785–97. doi: 10.1007/s00384-016-2646-y
45. Elnahas A, Sunil S, Jackson TD, Okrainec A, Quereshy FA. Laparoscopic vs. open surgery for T4 colon cancer: evaluation of margin status. Surg Endosc. (2016) 30:1491–6. doi: 10.1007/s00464-015-4360-1
46. Kang J, Baik SH, Lee KY, Sohn SK. Outcomes of laparoscopic surgery in pathologic T4 colon cancers compared to those of open surgery. Int J Colorectal Dis. (2017) 32:531–8. doi: 10.1007/s00384-016-2720-5
47. Takahashi R, Hasegawa S, Hirai K, Hisamori S, Hida K, Kawada K, et al. Safety and feasibility of laparoscopic multivisceral resection for surgical T4b colon cancers: retrospective analyses. Asian J Endosc Surg. (2017) 10:154–61. doi: 10.1111/ases.12355
48. Vignali A, Ghirardelli L, Di Palo S, Orsenigo E, Staudacher C. Laparoscopic treatment of advanced colonic cancer: a case-matched control with open surgery. Colorectal Dis. (2013) 15:944–8. doi: 10.1111/codi.12170
49. Hoffmann M, Phillips C, Oevermann E, Killaitis C, Roblick UJ, Hildebrand P, et al. Multivisceral and standard resections in colorectal cancer. Langenbeck's Arch Surg. (2012) 397:75–84. doi: 10.1007/s00423-011-0854-z
50. Mohan HM, Evans MD, Larkin JO, Beynon J, Winter DC. Multivisceral resection in colorectal cancer: a systematic review. Ann Surg Oncol. (2013) 20:2929–36. doi: 10.1245/s10434-013-2967-9
51. Kim KY, Hwang DW, Park YK, Lee HS. A single surgeon's experience with 54 consecutive cases of multivisceral resection for locally advanced primary colorectal cancer: can the laparoscopic approach be performed safely? Surg Endosc. (2012) 26:493–500. doi: 10.1007/s00464-011-1907-7
52. Nagasue Y, Akiyoshi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, et al. Laparoscopic vs. open multivisceral resection for primary colorectal cancer: comparison of perioperative outcomes. J. Gastrointest. Surg. (2013) 17:1299–305. doi: 10.1007/s11605-013-2222-5
53. Nakafusa Y, Tanaka T, Tanaka M, Kitajima Y, Sato S, Miyazaki K. Comparison of multivisceral resection and standard operation for locally advanced colorectal cancer: analysis of prognostic factors for short-term and long-term outcome. Dis Colon Rectum. (2004) 47:2055–63. doi: 10.1007/s10350-004-0716-7
54. Marusch F, Gastinger I, Schneider C, Scheidbach H, Konradt J, Bruch HP, et al. Importance of conversion for results obtained with laparoscopic colorectal surgery. Dis Colon Rectum. (2001) 44:207–14; discussion 214-206. doi: 10.1007/bf02234294
55. Clancy C, O'Leary DP, Burke JP, Redmond HP, Coffey JC, Kerin MJ, et al. A meta-analysis to determine the oncological implications of conversion in laparoscopic colorectal cancer surgery. Colorectal Disease. (2015) 17:482–90. doi: 10.1111/codi.12875
56. Yang C, Wexner SD, Safar B, Jobanputra S, Jin H, Li VK, et al. Conversion in laparoscopic surgery: does intraoperative complication influence outcome? Surg Endosc. (2009) 23:2454–8. doi: 10.1007/s00464-009-0414-6
57. Blikkendaal MD, Twijnstra AR, Stiggelbout AM, Beerlage HP, Bemelman WA, Jansen FW. Achieving consensus on the definition of conversion to laparotomy: a delphi study among general surgeons, gynecologists, and urologists. Surg Endosc. (2013) 27:4631–9. doi: 10.1007/s00464-013-3086-1
58. Valentini V, Coco C, Rizzo G, Manno A, Crucitti A, Mattana C, et al. Outcomes of clinical T4m0 extra-peritoneal rectal cancer treated with preoperative radiochemotherapy and surgery: a prospective evaluation of a single institutional experience. Surgery. (2009) 145:486–94. doi: 10.1016/j.surg.2009.01.007
59. Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, et al. Laparoscopic surgery vs. open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. (2005) 6:477–84. doi: 10.1016/s1470-2045(05)70221-7
60. Völkel V, Draeger T, Gerken M, Klinkhammer-Schalke M, Fürst A. Long-term oncologic outcomes after laparoscopic vs. Open colon cancer resection: a high-quality population-based analysis in a southern German district. Surg Endosc. (2018) 32:4138–47. doi: 10.1007/s00464-018-6158-4
61. Sueda T, Tei M, Nishida K, Yoshikawa Y, Matsumura T, Koga C, et al. Oncological outcomes following laparoscopic surgery for pathological T4 colon cancer: a propensity score-matched analysis. Surg Today. (2021) 51:404–14. doi: 10.1007/s00595-020-02106-3
62. Podda M, Pisanu A, Morello A, Segalini E, Jayant K, Gallo G, et al. Laparoscopic vs. open colectomy for locally advanced T4 colonic cancer: meta-analysis of clinical and oncological outcomes. Br J Surg. (2022) 109:319–31. doi: 10.1093/bjs/znab464
63. Schaeff B, Paolucci V, Thomopoulos J. Port site recurrences after laparoscopic surgery. A review. Dig Surg. (1998) 15:124–34. doi: 10.1159/000018605
64. Nagata H, Kawai K, Hata K, Tanaka T, Nozawa H, Ishihara S. Laparoscopic surgery for T4 colon cancer: a risk factor for peritoneal recurrences? Surgery. (2020) 168:119–24. doi: 10.1016/j.surg.2020.02.026
65. Huynh C, Minkova S, Kim D, Stuart H, Hamilton TD. Laparoscopic vs. open resection in patients with locally advanced colon cancer. Surgery. (2021) 170:1610–5. doi: 10.1016/j.surg.2021.07.027
66. Wang X, Zheng Z, Xie Z, Chi P. Laparoscopic colectomy for T4 colon cancer conferred an increased risk of peritoneal recurrence: a meta-analysis. Asian J Surg. (2022) 45:2285–6. doi: 10.1016/j.asjsur.2022.05.010
67. Kwong MLM, Sampah MES, Bello BL, Sugarbaker PH. Port site metastases after minimally invasive resection for colorectal cancer: a retrospective study of 13 patients. Surg Oncol. (2019) 29:20–4. doi: 10.1016/j.suronc.2019.02.008
68. Crolla R, Tersteeg JJC, van der Schelling GP, Wijsman JH, Schreinemakers JMJ. Robot-assisted laparoscopic resection of clinical T4b tumours of distal sigmoid and rectum: initial results. Surg Endosc. (2018) 32:4571–8. doi: 10.1007/s00464-018-6210-4
69. Parascandola SA, Horsey ML, Hota S, Sparks AD, Tampo MMT, Kim G, et al. Surgical resection of T4 colon cancers: an ncdb propensity score-matched analysis of open, laparoscopic, and robotic approaches. J Robot Surg. (2021) 15:701–10. doi: 10.1007/s11701-020-01166-4
Keywords: laparoscopic colectomy, open colectomy, T4 colon cancer, survival, meta-analysis
Citation: Chen P, Zhou H, Chen C, Qian X, Yang L and Zhou Z (2022) Laparoscopic vs. open colectomy for T4 colon cancer: A meta-analysis and trial sequential analysis of prospective observational studies. Front. Surg. 9:1006717. doi: 10.3389/fsurg.2022.1006717
Received: 29 July 2022; Accepted: 10 October 2022;
Published: 1 November 2022.
Edited by:
Andrea Laurenzi, IRCCS Azienda Ospedaliero-Universitaria di Bologna, ItalyReviewed by:
Olivier Glehen, Hospices Civils de Lyon, FranceGianni Lazzarin, Abano Terme Hospital, Italy
© 2022 Chen, Zhou, Chen, Qian, Yang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lie Yang bGllXzIyMkAxNjMuY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Peng Chen1,2,†
Peng Chen1,2,† Lie Yang
Lie Yang Zongguang Zhou
Zongguang Zhou