- 1Faculty of Medicine, University of Belgrade, Belgrade, Serbia
- 2Clinic for Neurosurgery, Clinical Center of Serbia, Belgrade, Serbia
- 3Clinic for Plastic Surgery and Burns, Military Medical Academy, Belgrade, Serbia
- 4Clinic for Neurosurgery, Military Medical Academy, Belgrade, Serbia
- 5Center for Radiology and MRI, Clinic for Neurosurgery, Clinical Center of Serbia, Belgrade, Serbia
- 6Medical Faculty of the Military Medical Academy, University of Defence, Belgrade, Serbia
- 7Institute of Hygiene, Military Medical Academy, Belgrade, Serbia
- 8School of Public Health and Health Management and Institute of Social Medicine, Faculty of Medicine, University of Belgrade, Belgrade, Serbia
Closed injuries to the peroneal nerve recover spontaneously in about a third of patients, but surgery may be needed in the remaining 2/3. The recovery after surgery is not always satisfactory and the patients may need an orthosis or a walking aid to cope with regular daily activities. This study aimed to evaluate the useful functional recovery and quality of life (QoL) in surgically treated patients with peroneal nerve (PN) injuries. The study involved 51 patients who have undergone surgical treatment due to PN injury in our department, within a 15-year period (2006–2020). Thirty patients (59%) were treated with neurolysis, 12 (23%) with nerve repair techniques, and 9 (18%) with tendon transfer (TT). Neurolysis is employed in the least extensive nerve injuries when nerve continuity is preserved and yields a motor recovery ratio of almost 80%. Nerve repairs were followed by 58.33% of patients achieving M3+ recovery, while 41.66% recovered to the useful functional state (M4 or M5) With the use of TTs, all patients recovered to the M3+, while 66.7% recovered to M4. All our results correspond to the results of previous studies. No statistically significant differences were found regarding the QoL of the groups. There is an apparent advantage of neurolysis, over nerve repair, over TT procedure, both in terms of useful functional recovery, and foot-drop-related QoL. However, when involving all aspects of QoL, these advantages diminish. The individual approach leads to optimal results in all groups of patients.
Introduction
Peripheral nerve injuries account for 2%–3% of all patients admitted to primary-level trauma centers (1). According to the previously published data, they are present in less than 2% of patients with limb injuries, while less than 1% of cases involve a peripheral nerve injury in the lower extremity (2). Although rare, the injuries may have a devastating impact on all aspects of living and significantly decrease quality of life (QoL) (3).
Peroneal nerve (PN) palsy presented as “foot drop”, is the most common mononeuropathy, but the majority of cases are related to radiculopathy and herniated discs, with favorable natural history and treatment outcomes (4), and rarely entrapment neuropathy or injury (5). Closed injuries to the PN may recover spontaneously, without specific treatment, in about a third of patients, but in the remaining 2/3, a permanent foot drop remains (6, 7). These patients are usually candidates for surgery, followed by a prolonged period of physical treatment and methods for the augmentation of nerve recovery in a multidisciplinary setting (8). The recovery is not always sufficient, and even M3 according to the Medical Research Council (MRC) scale for muscle strength (9), may end up as insufficient for some patients. Sometimes, a traditional or high-tech orthosis or walking aid may be needed to cope with regular daily activities (10).
Surgical options to treat these patients include procedures aimed at the exploration and various forms of neurolysis (external, internal, intrafascicular) (11), and nerve repair options (direct repair, grafts, or artificial conduits) (12, 13). Nerve transfers do not have the same beneficiary effect in the lower as they do in the upper extremity (14, 15), while tendon transfers (TTs) may be applied with success when recovery capacity is compromised (16, 17). Previous studies reported different results of motor recovery with the use of these techniques (18), and their combined use efficacy (19).
Motor recovery is the most commonly measured outcome, and there are some tools to asses useful functional recovery and QoL, however, with some applicable limitations. Patients treated for lower extremity nerve injuries were rarely evaluated in light of QoL before, while the foot drop is usually evaluated in terms of QoL with a focus on the need for braces, most commonly with the Stanmore system (20, 21).
This study aimed to evaluate useful functional recovery and QoL in surgically treated patients with PN injuries, as well as the evaluation of chosen general QoL inventories in terms of lower limb and PN affection.
Methods
The study involved a retrospective series of patients who have undergone surgical treatment due to the PN injury at our department in a 15-year period from January 2006 to December 2020.
All procedures performed were in accordance with the institutional ethical standards and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Inclusion criteria
• Patients with surgically treated PN injury
• Traumatic and iatrogenic nature of injury
• Common stem or branches involvement
Exclusion criteria
• Bilateral PN injury
• Associated tibial nerve injury
Out of 57 surgically treated patients fulfilling the inclusion criteria, six patients were excluded: one due to the bilateral injury, and five due to the associated tibial nerve injury.
Management
Within the preoperative evaluation, patients and injury characteristics were recorded, including the details on previous surgical interventions and associated injuries. Muscle strength was evaluated using the MRC, sensibility of the affected region using the Mackinnon–Dellon scale (MDS) (22), and the visual-analog scale (VAS) for the assessment of pain.
The individually tailored approach and decision-making on the modality of surgical treatment depended on two important features: (1) nerve continuity and (2) reinnervation capacity; based on the clinical and neurological examination, electrophysiology, imaging, and the time passed from injury to our initial examination.
Neurolysis was performed in patients with preserved continuity and reinnervation capacity (preserved efferent muscles); nerve repair with graft when the nerve was interrupted in continuity, but with preserved reinnervation capacity; and TT when there was no reinnervation capacity (usually due to the long-standing nerve lesion), regardless of the continuity.
The right timing is of utmost importance in peripheral nerve surgery. Immediate repair may be performed in open injuries with clear cuts. Open injuries involving the proximal and/or distal ends (e.g., laceration), are repaired in a delayed fashion, usually 3–4 weeks after the injury, while the initial surgery usually includes identification of the proximal and distal roots and their marking. In closed injuries, electrophysiological studies to confirm the lesion are carried out 3–4 weeks after the injury, but surgical treatment is indicated only in cases with no signs of recovery in electrophysiological studies performed 12 weeks after the injury (13). TTs use is usually not affected by the time passed from injury to surgery.
Neurolysis is performed through the popliteal approach when PN continuity is preserved. After skin incision, soft tissue dissection was performed to reveal and identify the PN. External neurolysis is performed when intraoperative findings showed that the nerve was compressed by surrounding scar/fibrous tissue. In situations when intraneural fibrosis was found, internal neurolysis is performed. After satisfactory deliberation of the nerve, hemostasis is performed and the wound is closed in a layered fashion. Usually, drainage was not needed.
The same popliteal approach is used for nerve repair as well, but, in situations when nerve continuity was not preserved. After identification and preparation of proximal and distal ends of PN, either direct (suture) or repair with various graft types was performed.
Direct repair (with 9/0 interrupted sutures) is possible when the nerve defect was less than 3 cm in length and adequate coaptation without tension could be achieved. When a longer nerve defect is found, it was repaired with sural nerve grafts, usually from the same-sided leg. Cable grafting was performed when the nerve defect was proximal to the ending branches and interfascicular autografting when the nerve defect included ending branches. The wound is then closed in a layered fashion.
For TTs, we usually used the tibialis posterior muscle tendon, which is divided into two slips. One slip is attached to the tibialis anterior muscle and the second one to the extensor digitorum longus muscle and extensor hallucis longus muscle. Suturing is performed with 2/0 sutures, followed by hemostasis and wounds closure in a layered fashion. Immobilization with above knee cast, and foot and fingers in extension, is set and kept for 6 weeks postoperatively.
After the surgery and wound healing (and plaster removal in patients with TTs), all patients were referred to physical treatment in dedicated rehabilitation centers and local health service providers for at least 6 months.
Follow-up included neurological examinations by an independent neurologist, and also functional assessment: monthly, during the first 3 months, and every 3 months later on. Postoperative recovery was recorded with MRC and the residual pain was graded on VAS of pain. The use of orthosis or walking aid was also recorded.
QoL evaluation was performed when no further recovery was expected. Since there is no dedicated tool to asses QoL in patients with PN injuries, we opted for the use of three questionaries including the Ulm questionnaire as a dedicated tool for peripheral nerve injuries (23), the Short Form 36 (SF-36) health survey as a general QoL inventory (24), and Stanmore questionnaire focusing on foot-drop correction (25).
Statistical analysis
In the case of normally distributed variables, mean and standard deviation are shown in the tables, while the differences are tested using the t-test; in case of two groups, and ANOVA in the case of more than two groups. For variables not falling under the normal distribution, median, minimum, and maximum values are reported in tables, while the differences between groups are tested using the Man–Whitney–Wilcoxon test, for two groups, or the Kruskal–Wallis test in case more than two groups are present. Categorical variables are presented by the number of observations and the percentage, while χ2 or Fisher test is used to compare frequencies between groups. All data were analyzed using R 3.4.2. [R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria].
This case series has been reported in line with the PROCESS Guideline (26).
Results
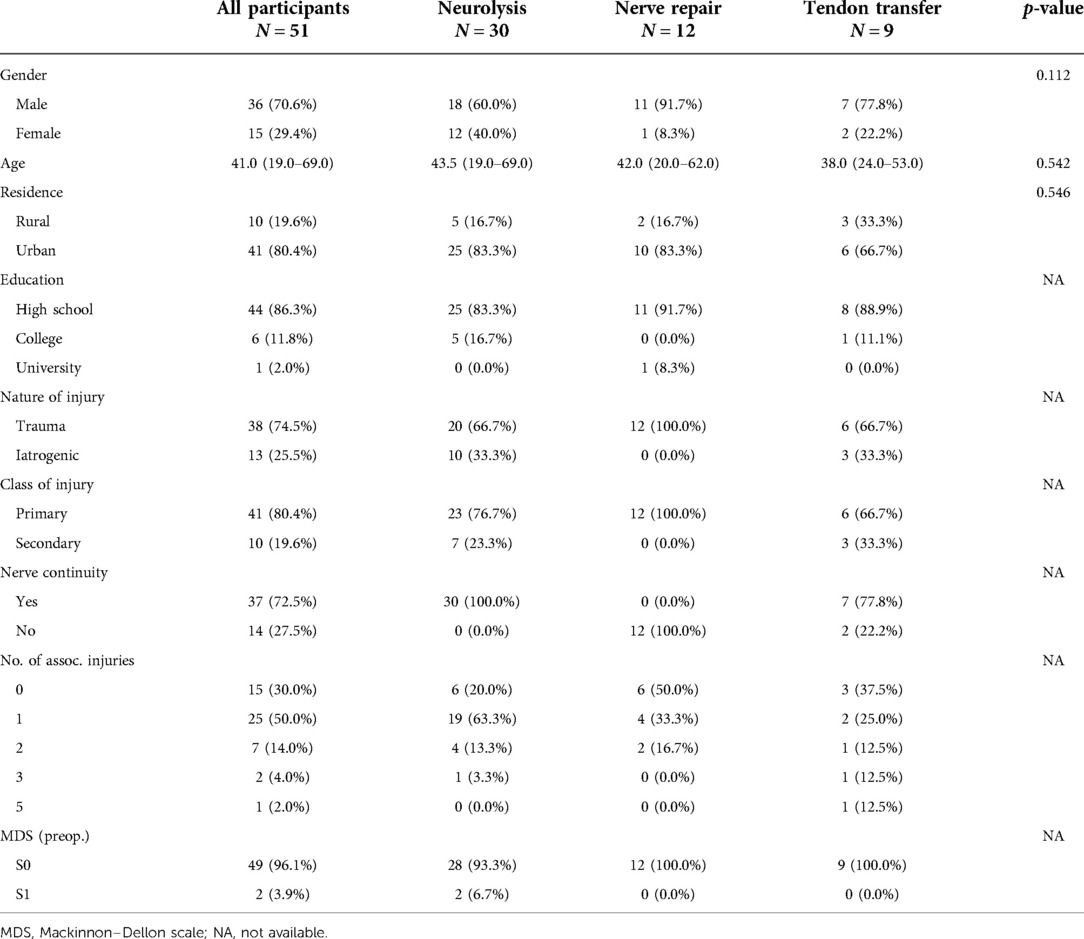
This study included 51 patients: 30 (59%) were treated with neurolysis, 12 (23%) with nerve repair, and 9 (18%) with TT. Patients were mostly male (70%) with a median age of 41 (19–69) years old, with an urban residence (80%), and medium education (86%). All patients were Caucasian. Table 1 shows the sociodemographic characteristics and preoperative status of the patients by surgery class.
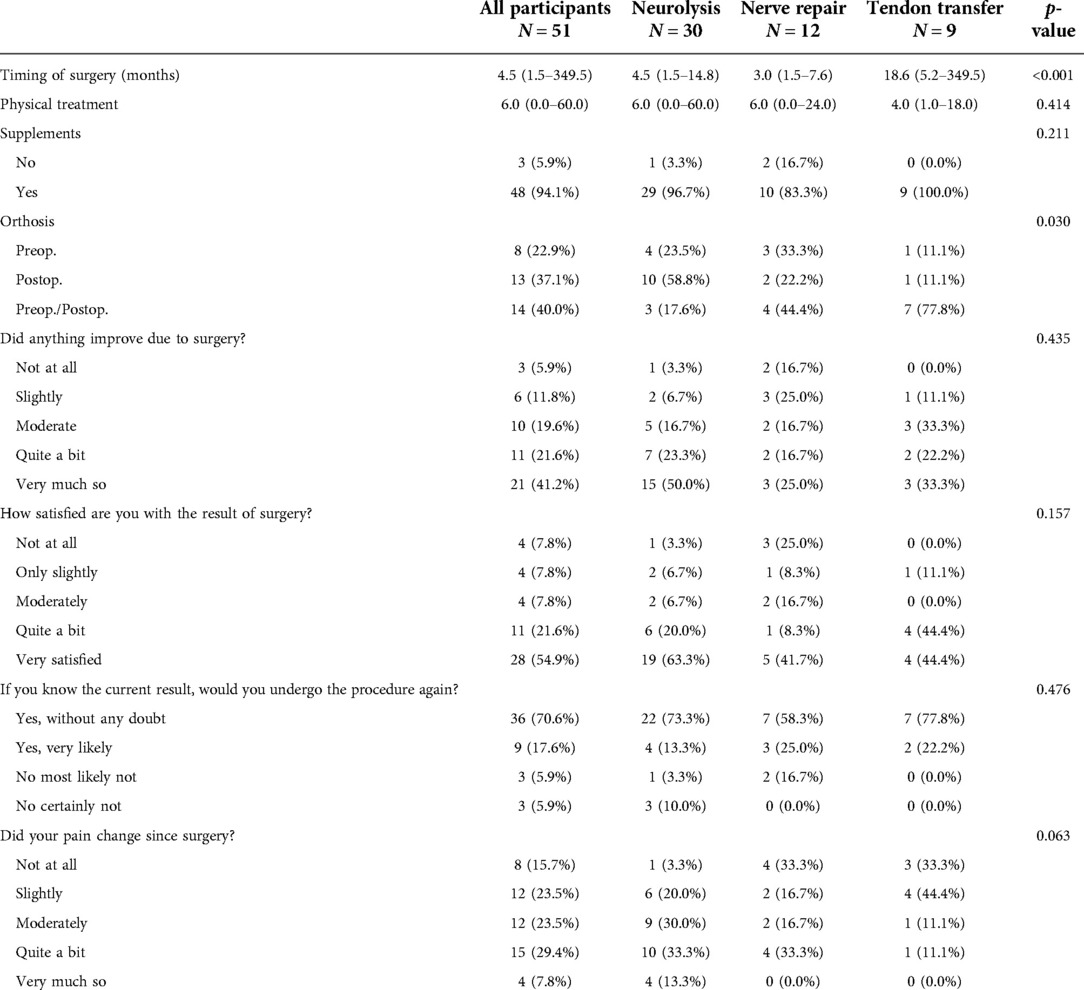
Characteristics of the surgery and postsurgical treatment, and of the Ulm questionnaire are presented in Table 2. The majority of patients reported that they had experienced an improvement due to the surgery (63%), that they were satisfied with the results of the surgical treatment (76%), most would undergo the surgery again if they had known the results (88%), and just above one-third of patients noticed a significant pain relief (37%).
Figure 1 shows the comparison between the preoperative and postoperative functional status according to the MRC scale. Thirty-three of the 51 included patients achieved useful functional recovery (M4 or M5), and 18 remained without significant improvement (p < 0.001) (≦M3). Neurolysis yields good results with an M3+ ratio nearing 80%, while useful functional recovery was achieved in 22 (73.3%) of 30 patients. Nerve repairs were followed by 58.33% of patients achieving M3+, while 41.66% recovered to a useful functional state. With the use of TT, all patients recovered to the M3+, while 66.7% recovered to M4.
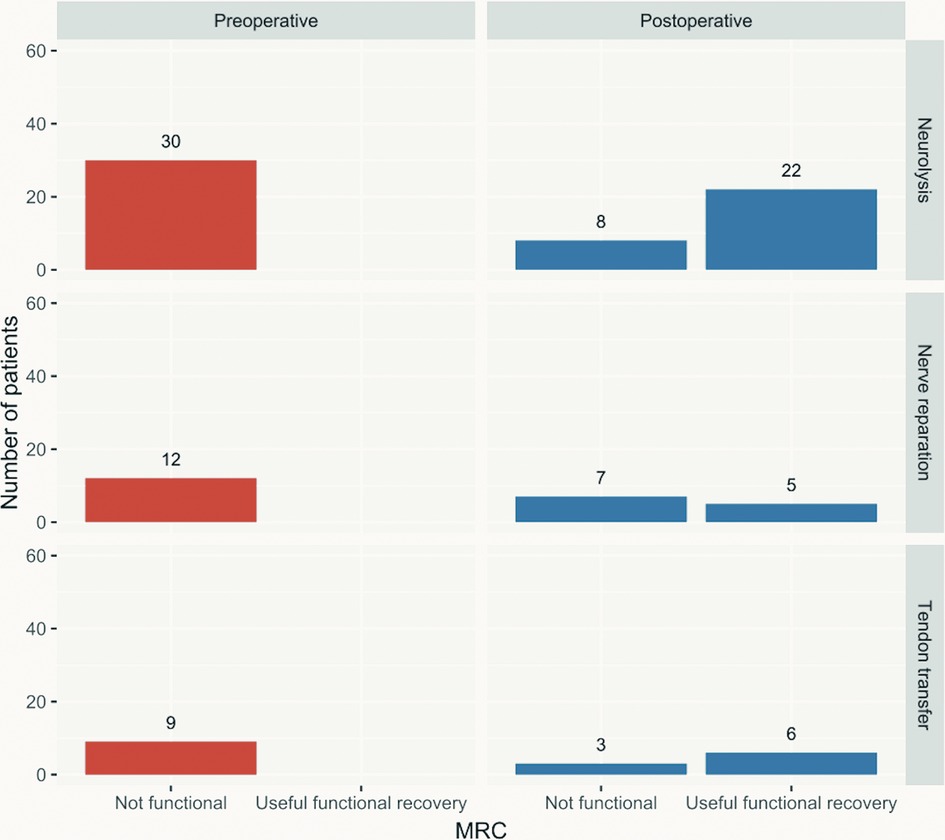
Figure 1. Preoperative vs. postoperative useful functional recovery according to the MRC scale by surgical technique applied. MRC, Medical Research Council.
Figure 2 shows the differences between the preoperative and postoperative VAS in patients treated with different surgical approaches. The dashed lines represent the difference in single patients. Overall, in the majority of patients, the pain intensity reduced significantly; although, in each class of surgery, there were cases where the pain remained the same or even increased. The reduction in pain, as a difference in VAS scores, was statistically significant [Friedman test, χ2(1) = 31.8, p < 0.001].

Figure 2. VAS pain scale difference between preoperative and postoperative status by class of surgery. VAS, visual-analog scale.
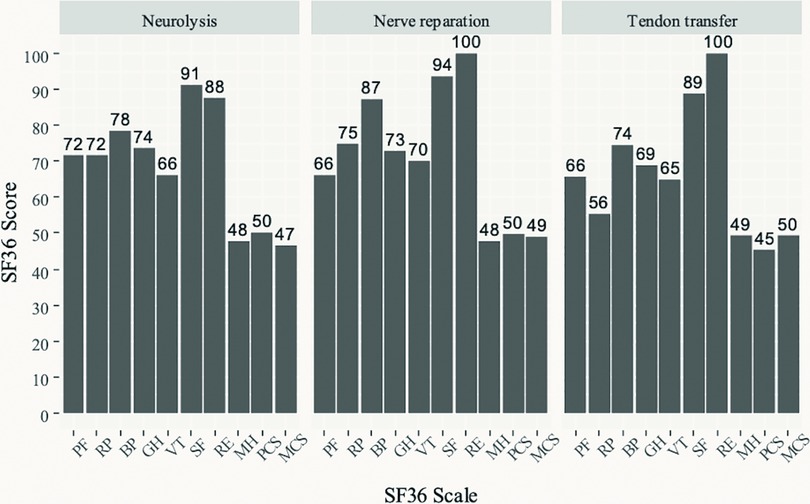
Figure 3 shows the results of the SF-36 QoL questionnaire in the three groups of patients. The highest (best) scores across the three groups are seen in the social functioning (SF) and role-emotional (RE) scales, followed by pain index (BP). Lower scores are seen on the physical functioning (PF), role-physical (RP), general health (GH) perceptions, and vitality (VT) scales. The lowest scores are seen on the mental health (MH) scale. Standardized physical component scales had values of 50 for neurolysis and nerve reparation, and 45 for TT, while for the standardized mental component scales the values were 47, 49, and 50, for neurolysis, nerve reparation and TT, respectively.
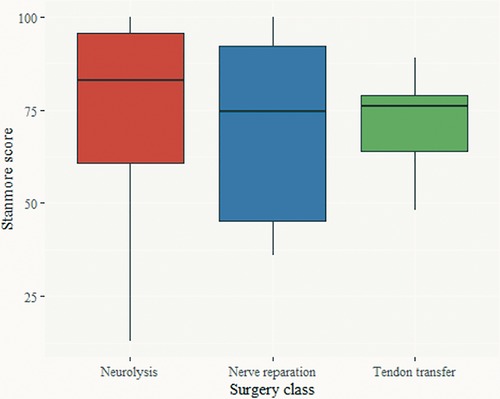
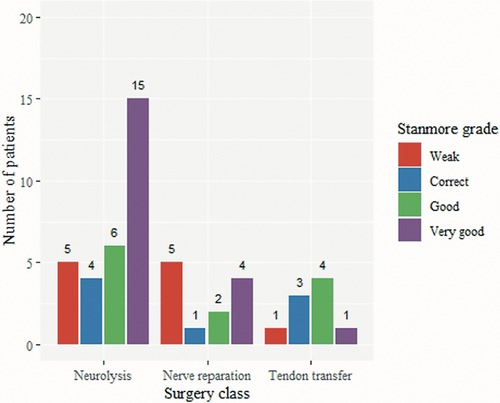
Figure 4 shows the Stanmore score by surgery class. There was no statistically significant difference in the Stanmore score (ANOVA, F = 0.419, p = 0.66), or the Stanmore grades (Figure 5) (weak, correct, good, and very good) among the three surgical treatment modalities.
Discussion
The study evaluated the outcomes and QoL of 51 patients with PN injuries, who received one of three surgical treatment options (neurolysis, nerve repair, or TT), based on preoperative characteristics and individually tailored approaches.
PN injuries require surgical treatment in approximately 2/3 of cases (6), with the usual indication for surgery being more than 3 months after injury without recovery for closed injuries, and immediate or as soon-as-possible repair for open (sharp injuries or lacerations) (27). Timing for surgery is established as the most important factor that predisposes the recovery potential and also plays a major role in the choice of surgical technique (p < 0.001), together with the nerve continuity status and nature of injury (28).
These same principles were applied to our patients, although we have no data on patients who recovered or started to recover during the 3 months period, as these were not referred to the neurosurgical department. On the other hand, some patients reported for an initial exam when the reinnervation capacity was lost (more than 12 months without recovery after the injury, and with obvious target muscles atrophy). In these cases, we opted for TT rather than palliative bracing to achieve functional restoration (18).
The use of TT increased the percentage of surgically treated patients, but it is possible that it justified the rates for those patients who were not treated on time due to referral issues. The use of TT in patients with failed recovery after neurolysis or nerve repair is advised as a salvage procedure, although in our study, no patients underwent this kind of augmentation (29).
Neurolysis is employed in the least extensive nerve injuries when nerve continuity is preserved and yields good results with a motor recovery ratio nearing 80% (30, 31). These results correspond to ours, with 88% of patients achieving M3+, and 72.2% recovering to the M4+.
Nerve repairs of PN lesions were previously reported to have a roughly half (50%) motor recovery rate with the use of grafts, while the direct repair carries a somewhat higher rate of 60%–80% (30–32), in our study there was a slight increase as 58.33% of patients achieved M3+ recovery, while 41.66% recovered to the useful functional state with M4 and M5. It should be mentioned that 10 of 12 patients who underwent nerve repair received sural nerve grafts for the repair, one patient's nerve was directly sutured and one patient received an artificial conduit. Since the patients with nerve repair achieve satisfactory outcomes in roughly half of cases, it was proposed that these patients may undergo TT as a salvage procedure (12), and some authors even proposed to perform the one-stage nerve repair and TT immediately (33).
TT have very good results when only motor strength recovery is observed with recovery rates over 80% (almost 85% when concurrent posterior tibial TT was employed in a systematic review, and up to 100% in single studies (16, 31). However, this procedure is indicated as salvage, for isolated PN palsy with good ankle mobility, good strength of the posterior tibial muscle and poor prognosis of spontaneous recovery in order to decrease dependence on brace for walking, and to improve hip and knee function with improved gait kinematics (16, 34). All our patients recovered to the M3+, while 66.7% recovered to M4, but there were no cases who recovered to M5 which corresponds to the results of previous studies.
Pain was not an indication for surgery in our patients, but the common pattern of pain decrease was noted regardless of the surgical strategy. Previously, the authors have reported performing (internal or external) neurolysis to treat neuropathic pain (29, 35), especially in patients with gunshot wounds (36). Based on our results, we can hypothesize, that the origin of pain in our patients was not neuropathic in the majority, but rather chronic foot pain due to the instability and the arch flattening.
The most dedicated foot drop inventories focus on the use of bracing as a main aim of the treatment, and the use of TT, rather than the functional recovery following nerve release or repair, and actual QoL (20, 21, 37). Although in a relatively small cohort, there were no statistically significant differences in the QoL scores between the three treatment options, suggesting that no surgical technique influences the QoL by itself, but rather the right approach allows to achieve a similar QoL. A previous study discovered that patients with chronic foot drop had a reduced QoL with significantly poorer scores in the physical and psychosocial domains (38). This was not the case in our study, as the majority of patients recovered satisfactorily, leading to better overall scores.
While results from the three questionnaires focused on the overall QoL are consistent, when employing the Stanmore system, and assessing purely motor outcomes and the need for prosthesis, we found an apparent advantage of neurolysis, over nerve repair, over TT. Probably, due to the different regeneration potential, but also nerve injury severity, leading to favorable outcomes, compared to the previously reported 69% of patients with chronic foot drop in the need for bracing (38).
The insufficient number of patients (for a more powerful statistical analysis) overall, and especially in the TT and nerve repair groups is a usual limitation of studies on peripheral nerve injuries, and it is similar or even advantageous to other studies on the topic (16, 30–32).
There is no specific tool for the evaluation of QoL in patients with PN injuries, but we have shown that readily available tools can capture the QoL well, and quite consistently.
Future studies should focus on the improvement of all three surgical procedures, and a unified guided surgical decision-making process, as every procedure has its place in specific patients. Larger cohorts of patients should be recruited in a multidisciplinary fashion and merged within prospective trials leading to high-quality recommendations and guidelines.
Conclusion
There is an apparent advantage of neurolysis, over nerve repair, over nerve transfer procedure, both in terms of useful functional recovery, and foot-drop-related QoL. However, when involving all aspects of QoL, these advantages diminish.
Individual approach to patients with severe PN injuries, involving all features and aspects of both injury and the patient, leads to the achievement of optimal results in all groups of patients, regardless of the regeneration potential and injury severity, but these should be considered as primary guides in choosing the surgical approach.
QoL system focusing on the peripheral nerve injuries is detrimental to a better understanding of the actual patient's state, recovery and satisfaction as present inventories lack specificity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Faculty of Medicine, University of Belgrade, Belgrade, Serbia. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. (1998) 45(1):116–22. doi: 10.1097/00005373-199807000-00025
2. Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. (2008) 87(5):381–5. doi: 10.1097/PHM.0b013e31815e6370
3. Rasulic L, Savic A, Zivkovic B, Vitosevic F, Micovic M, Bascarevic V, et al. Outcome after brachial plexus injury surgery and impact on quality of life. Acta Neurochir (Wien). (2017) 159(7):1257–64. doi: 10.1007/s00701-017-3205-1
4. Iizuka Y, Iizuka H, Tsutsumi S, Nakagawa Y, Nakajima T, Sorimachi Y, et al. Foot drop due to lumbar degenerative conditions: mechanism and prognostic factors in herniated nucleus pulposus and lumbar spinal stenosis. J Neurosurg Spine. (2009) 10(3):260–4. doi: 10.3171/2008.12.SPINE08500
5. Oosterbos C, Decramer T, Rummens S, Weyns F, Dubuisson A, Ceuppens J, et al. Evidence in peroneal nerve entrapment: a scoping review. Eur J Neurol. (2022) 29(2):665–79. doi: 10.1111/ene.15145
6. Kim DH, Kline DG. Management and results of peroneal nerve lesions. Neurosurgery. (1996) 39(2):312–9.; discussion 9–20. doi: 10.1097/00006123-199608000-00014
7. Liu Z, Yushan M, Liu Y, Yusufu A. Prognostic factors in patients who underwent surgery for common peroneal nerve injury: a nest case-control study. BMC Surg. (2021) 21(1):11. doi: 10.1186/s12893-020-01033-x
8. Carolus AE, Becker M, Cuny J, Smektala R, Schmieder K, Brenke C. The interdisciplinary management of foot drop. Dtsch Arztebl Int. (2019) 116(20):347–54. doi: 10.3238/arztebl.2019.0347
9. O'Brien M. Aids to the examination of the peripheral nervous system. Philadelphia, PA: Saunders (2010).
10. Buentjen L, Kupsch A, Galazky I, Frantsev R, Heinze HJ, Voges J, et al. Long-term outcomes of semi-implantable functional electrical stimulation for central drop foot. J Neuroeng Rehabil. (2019) 16(1):72. doi: 10.1186/s12984-019-0542-8
11. Ducic I, Felder JM 3rd. Minimally invasive peripheral nerve surgery: peroneal nerve neurolysis. Microsurgery. (2012) 32(1):26–30. doi: 10.1002/micr.20959
12. Gurbuz Y, Sugun TS, Ozaksar K, Kayalar M, Toros T, Ademoglu Y. Peroneal nerve injury surgical treatment results. Acta Orthop Traumatol Turc. (2012) 46(6):438–42. doi: 10.3944/AOTT.2012.2900
13. Oosterbos C, Rasulic L, Rummens S, Kiekens C, van Loon J, Lemmens R, et al. Controversies in treatment strategies in patients with foot drop due to peroneal nerve entrapment: results of a survey among specialists. Brain Spine. (2022) 2:100887. doi: 10.1016/j.bas.2022.100887
14. Head LK, Hicks K, Wolff G, Boyd KU. Clinical outcomes of nerve transfers in peroneal nerve palsy: a systematic review and meta-analysis. J Reconstr Microsurg. (2019) 35(1):57–65. doi: 10.1055/s-0038-1667047
15. Chen H, Meng D, Yin G, Hou C, Lin H. Translocation of the soleus muscular branch of the tibial nerve to repair high common peroneal nerve injury. Acta Neurochir (Wien). (2019) 161(2):271–7. doi: 10.1007/s00701-018-03797-x
16. Park JS, Casale MJ. Posterior tibial tendon transfer for common peroneal nerve injury. Clin Sports Med. (2020) 39(4):819–28. doi: 10.1016/j.csm.2020.07.003
17. Rodriguez-Argueta ME, Suarez-Ahedo C, Jimenez-Aroche CA, Rodriguez-Santamaria I, Perez-Jimenez FJ, Ibarra C, et al. Anterior tibial tendon side-to-side tenorrhaphy after posterior tibial tendon transfer: a technique to improve reliability in drop foot after common peroneal nerve injury. Arthrosc Tech. (2021) 10(5):e1361–8. doi: 10.1016/j.eats.2021.01.039
18. Poage C, Roth C, Scott B. Peroneal nerve palsy: evaluation and management. J Am Acad Orthop Surg. (2016) 24(1):1–10. doi: 10.5435/JAAOS-D-14-00420
19. Ho B, Khan Z, Switaj PJ, Ochenjele G, Fuchs D, Dahl W, et al. Treatment of peroneal nerve injuries with simultaneous tendon transfer and nerve exploration. J Orthop Surg Res. (2014) 9:67. doi: 10.1186/s13018-014-0067-6
20. Yeap JS, Singh D, Birch R. A method for evaluating the results of tendon transfers for foot drop. Clin Orthop Relat Res. (2001) 383:208–13. doi: 10.1097/00003086-200102000-00024
21. Stevoska S, Pisecky L, Stadler C, Gahleitner M, Klasan A, Klotz MC. Tendon transfer in foot drop: a systematic review. Arch Orthop Trauma Surg. (2021). doi: 10.1007/s00402-021-04162-x. [Epub ahead of print].
22. Novak CB, Kelly L, Mackinnon SE. Sensory recovery after median nerve grafting. J Hand Surg Am. (1992) 17(1):59–68. doi: 10.1016/0363-5023(92)90114-5
23. Kretschmer T, Ihle S, Antoniadis G, Seidel JA, Heinen C, Borm W, et al. Patient satisfaction and disability after brachial plexus surgery. Neurosurgery. (2009) 65(4 Suppl):A189–96. doi: 10.1227/01.NEU.0000335646.31980.33
24. Ware JE Jr. SF-36 health survey update. Spine. (2000) 25(24):3130–9. doi: 10.1097/00007632-200012150-00008
25. Lingaiah P, Jaykumar K, Sural S, Dhal A. Functional evaluation of early tendon transfer for foot drop. J Orthop Surg. (2018) 26(3):2309499018799766. doi: 10.1177/2309499018799766
26. Agha RA, Sohrabi C, Mathew G, Franchi T, Kerwan A, O'Neill N, et al. The PROCESS 2020 guideline: updating consensus preferred reporting of CasE series in surgery (PROCESS) guidelines. Int J Surg. (2020) 84:231–5. doi: 10.1016/j.ijsu.2020.11.005
27. Rodríguez Aceves CA, Córdoba Mosqueda ME, García Velazco RA, González Ugalde H, Soriano Solís HA, Ortega Ponce FEE, et al. Traumatic injuries of the common peroneal nerve and current surgical strategies for improving foot drop. A clinical series and literature review. Arch Neurol. (2020) 1(1):16–26. sequentially, first page paper 2-sequentially, last page paper.
28. Fugleholm K. The surgery of peripheral nerves (including tumors). Handb Clin Neurol. (2013) 115:781–802. doi: 10.1016/B978-0-444-52902-2.00045-X
29. Seidel JA, Koenig R, Antoniadis G, Richter HP, Kretschmer T. Surgical treatment of traumatic peroneal nerve lesions. Neurosurgery. (2008) 62(3):664–73; discussion -73. doi: 10.1227/01.neu.0000317315.48612.b1
30. Horteur C, Forli A, Corcella D, Pailhe R, Lateur G, Saragaglia D. Short- and long-term results of common peroneal nerve injuries treated by neurolysis, direct suture or nerve graft. Eur J Orthop Surg Traumatol. (2019) 29(4):893–8. doi: 10.1007/s00590-018-2354-0
31. Mackay MJ, Ayres JM, Harmon IP, Tarakemeh A, Brubacher J, Vopat BG. Traumatic peroneal nerve injuries: a systematic review. JBJS Rev. (2022) 10(1). doi: 10.2106/JBJS.RVW.20.00256
32. Roganovic Z, Pavlicevic G. Difference in recovery potential of peripheral nerves after graft repairs. Neurosurgery. (2006) 59(3):621–33; discussion -33. doi: 10.1227/01.NEU.0000228869.48866.BD
33. Ferraresi S, Garozzo D, Buffatti P. Common peroneal nerve injuries: results with one-stage nerve repair and tendon transfer. Neurosurg Rev. (2003) 26(3):175–9. doi: 10.1007/s10143-002-0247-4
34. Olsen MH, Fugleholm K, Andersen GR. Tendon transfer as a treatment modality of peroneal nerve palsy. Ugeskr Laeger. (2020) 182(2).
35. Kim DH, Murovic JA, Tiel RL, Kline DG. Management and outcomes in 318 operative common peroneal nerve lesions at the Louisiana State University Health Sciences Center. Neurosurgery. (2004) 54(6):1421–8; discussion 8–9. doi: 10.1227/01.NEU.0000124752.40412.03
36. Roganovic Z, Mandic-Gajic G. Pain syndromes after missile-caused peripheral nerve lesions: part 2–treatment. Neurosurgery. (2006) 59(6):1238–49; discussion 49–51. doi: 10.1227/01.NEU.0000245618.16979.32
37. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. (1994) 15(7):349–53. doi: 10.1177/107110079401500701
Keywords: peroneal nerve, trauma, outcome, quality of life, surgery
Citation: Rasulić L, Nikolić Ž, Lepić M, Savić A, Vitošević F, Novaković N, Radojević S, Mićić A, Lepić S and Mandić-Rajčević S (2022) Useful functional recovery and quality of life after surgical treatment of peroneal nerve injuries. Front. Surg. 9:1005483. doi: 10.3389/fsurg.2022.1005483
Received: 28 July 2022; Accepted: 3 October 2022;
Published: 14 November 2022.
Edited by:
Shimon Rochkind, Tel Aviv University, IsraelReviewed by:
Rahul K. Nath, Texas Nerve and Paralysis Institute, United StatesRadek Kaiser, Charles University, Czechia
© 2022 Rasulić, Nikolić, Lepić, Savić, Vitošević, Novaković, Radojević, Mićić, Lepić and Mandić-Rajčević. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lukas Rasulić bHVrYXMucmFzdWxpY0BnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
 Lukas Rasulić
Lukas Rasulić Živan Nikolić
Živan Nikolić Milan Lepić
Milan Lepić Andrija Savić
Andrija Savić Filip Vitošević
Filip Vitošević Nenad Novaković
Nenad Novaković Stefan Radojević1
Stefan Radojević1 Aleksa Mićić
Aleksa Mićić Stefan Mandić-Rajčević
Stefan Mandić-Rajčević