
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 04 November 2022
Sec. Neurosurgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1000238
Objective: Nonsteroidal anti-inflammatory drugs (NSAID) are essential in surgeons' armamentarium for pain relief and antiphlogistic effects. However, spine surgeons are concerned about the drugs' impact on coagulation, fearing hemodynamic instability due to blood loss and neurological complications due to postoperative hematoma. Furthermore, there are no clear guidelines for the use of these drugs.
Materials and methods: In this retrospective subgroup analysis of a prospective observational study, we investigated 181 patients who underwent minimally invasive spinal fusions in degenerative lumbar spine pathologies. 83 patients were given NSAID perioperatively, 54 of which were female and 29 male. Of these patients who took NSAID, 39 were on NSAID until at least one day before surgery or perioperatively, whilst the others discontinued their NSAID medication at least three days before surgery. Differences in perioperative blood loss, as well as complication rates between patients with and without NSAID treatment, were investigated.
Results: A significantly higher amount of blood loss during surgery and the monitoring period was encountered in patients whose spine was fused in more than one level, regardless of whether NSAID medication was taken or not and up until what point. Furthermore, it was found that taking NSAID medication had no effect on the incidence of postoperative epidural hematomas.
Conclusion: Perioperatively taking NSAID medication does not increase blood loss or the incidence of postoperative hematoma in patients undergoing minimally invasive lumbar spinal fusion surgery.
Minimally invasive surgery (MIS) is thought to create a smaller corridor to the spine, resulting in less tissue injury. Furthermore, MIS is associated with reduced blood loss, faster recovery, and lower perioperative morbidity rates whilst yielding similar results to open procedures (1–5). Our study discusses the controversial subject of a possible elevated risk of bleeding associated with perioperative nonsteroidal anti-inflammatory drugs (NSAID) which are prescribed for their analgesic and antiphlogistic effects. The aim of this retrospective subgroup analysis of a prospective observational study, which is based on data from 187 patients, is to examine whether patients who undergo minimally invasive surgery (MIS) while taking NSAID are at risk of increased blood loss and incidence of postoperative hematoma compared to patients who do not receive NSAID treatment.
We obtained approval from the ethics committee of the Federal State of Lower Austria and registered the study at ClinicalTrials.gov (NCT01259960). Written consent of all patients was obtained to carry out the study. Of the 187 patients included in this research, 115 were female and 72 male. All patients were treated with one, two, three, or four level minimal invasive fusion. In 146 patients, additional decompression of the spinal canal was performed. Blood loss was defined as the primary endpoint. We recorded the amount of blood loss during surgery as well as during the monitoring period in the recovery unit and the postoperative period, the latter until the removal of the drainage. Volumes were measured and recorded in milliliters. As a secondary endpoint, we defined postoperative epidural hematomas. In the case of clinical suspicion of the presence of epidural hematomas, an MRI was performed. If the radiological findings described a postoperative epidural hematoma, we accordingly recorded this. We enrolled only patients in this study who regularly took NSAID as analgesics or antiphlogistics up until one day before surgery or perioperatively. Not all 187 patients were included in the analysis of this study. No information on NSAID intake was available for four patients, and for two patients, the information on blood loss (perioperative and monitoring) or drainage volume was missing. Thus, 181 relevant patients (111 female and 70 male) remained in this study.
After identifying the correct facet joint under fluoroscopy control, an incision was made 1.5 cm off the midline. Using a tubular retractor system, muscle tissue was sequentially dilated. After visualization of the facet joint and yellow ligament, percutaneous fusion was performed. In cases of spinal stenosis, a laminotomy and decompression were performed. For interbody fusion, a TLIF procedure (transforaminal interbody fusion) was followed. In nine patients, we did not implant an interbody device at every level because of the narrow disc space and the associated risk of fracturing the corresponding endplates. In four two-level fusion cases, we fused only one level with a TLIF cage. In four three-level fusion cases, we implanted two TLIF cages, and in one four-level fusion case, we inserted three TLIF cages.
Statistical analyses were performed using the R package npmv. The nonpartest was used to test the null hypothesis that the underlying distributions in the groups under investigation coincided. Whenever portions were considered, the standard k-sample test for equality of proportions was used. Linear dependence of variables was determined by Pearson's correlation, while the correlation was quantified by Spearman's rank correlation. Statistical significance was assumed at a p-value of <0.05.
Of the 181 patients under investigation, 83 (45.86%) received NSAID, 54 of which were female (48.65%) and 29 male (41.43%). Within this subset of 83 patients who had taken NSAID, 39 patients had taken the medication until at least the day before surgery or perioperatively. The remaining 44 patients had stopped taking NSAID as recommended three days before surgery. In the following section, the expressions “NSAID intake” or “patients in the NSAID group” refer to those patients who had taken NSAID until at least the day before surgery or perioperatively. All other patients (n = 142), including 44 individuals who had discontinued NSAID at least three days before surgery, are referred to as the “non-NSAID” group. Because age and blood loss/drainage volume are weakly positively correlated, we compared the age distribution of patients in the NSAID and the non-NSAID groups and found that they did not differ significantly (p = 0.58) (Figure 1). We further divided the patients into subsets of those with one fused level (“one-level” group) and those with two, three, or four fused levels (“two-plus” group). Patients in the NSAID group tend to have fewer levels operated on than patients in the non-NSAID group (p = 0.12) (Figure 2). We could not observe a difference in the proportion of NSAID-taking patients who had one, two, three, or four levels fused (p = 0.38).

Figure 1. Number and percentage of patients in the NSAID and the non-NSAID group; age distributions in the two groups.
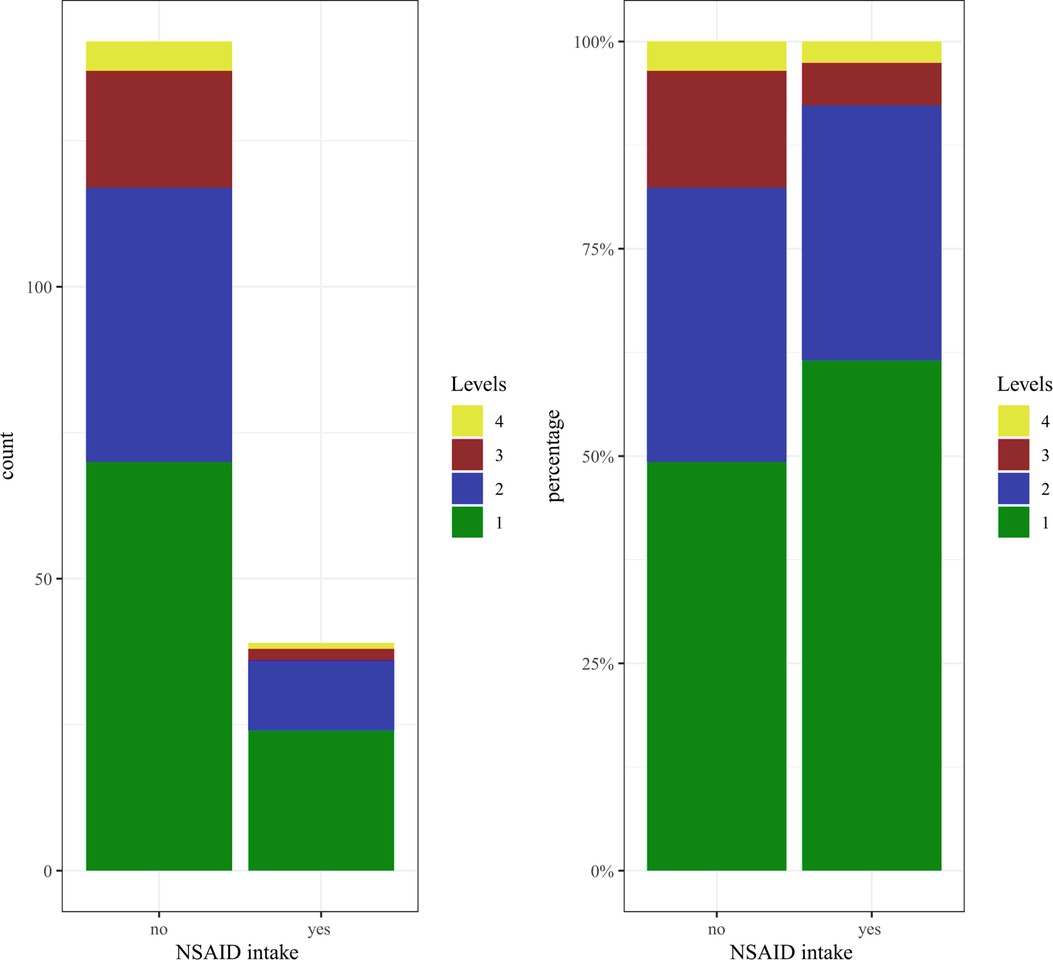
Figure 2. Distribution of the number of levels in the NSAID and the non-NSAID group (patients in the NSAID group tend to have fewer segments operated on than patients in the non-NSAID group).
Information on the number of operated levels, blood loss (perioperative and monitoring), and drainage volumes were available for 183 of the 187 patients. In order to be able to analyze the impact of NSAID on these measurements, we had to exclude other confounding factors at first. To do so, we split the data into two groups of approximately similar size: 94 patients (51.37%) were fused in only one level (“one-level” group), and 89 patients (48.64%) were fused in two to four levels (“two-plus” group). Patients in the “one-level” group experienced a significantly lower amount of blood loss than in the “two-plus” group (p = 0.019; Table 1). Interestingly, this difference was only detectable in male patients (p = 0.06), whereas no significant differences were seen in the female cohort (p = 0.36). Furthermore, on average, the patients in the “one-level” group experienced significantly lower levels of blood loss by drainage than the patients in the “two-plus” group (p < 0.001; Table 2). Surprisingly, no differences between the two groups were found in male patients (p = 0.19). However, a statistically significant difference was detectable in female patients (p < 0.001). This led to the assumption that the number of fused levels has a high impact on both blood loss and drainage volumes but that this effect differs by gender. Consequently, we not only analyzed the impact of NSAID on blood loss separately for the subgroup of patients in the “one-level” group and those in the “two-plus” group but also looked at possible gender differences.
In 181 patients (111 female and 70 male), the following variables were known: (I) number of fused levels, (II) blood loss (perioperative and monitoring), (III) drainage, and (IV) NSAID intake (Table 3). As mentioned previously, 39 of the 181 patients in this study were given NSAID medication until at least one day before surgery or perioperatively. However, there was no difference in perioperative blood loss or blood loss during the monitoring phase between patients who took NSAID and those in the “non-NSAID” group, neither in the “one level” group (p = 0.69) nor in the “two-plus” group (p = 0.74). Even when accounting for the impact of gender, we couldn't find any statistically significant differences concerning blood loss and NSAID intake between males and females in the “one level” and “two level” subgroups, although there was a slightly higher level of blood loss in women taking NSAID who were operated on two levels or more (Table 4; p = 0.06).

Table 3. Blood loss volume distributions and the possible impact of NSAID on mean and range of blood loss volume per subgroup.

Table 4. Resulting p-values when testing for equal blood loss distributions for NSAID and non-NSAID patients in the four different subgroups formed by sex and (aggregated) number of segments.
Drainage volumes did not differ between NSAID and “non-NSAID” patients, neither in the “one level” group (p = 0.59), nor in the “two plus” group (p = 0.12). Furthermore, when taking into account differences in gender and the number of fused levels, no statistically significant differences were observed (Table 5). However, slightly higher drainage volumes were found among female patients who took NSAID and underwent fusion of two or more levels (p = 0.06).

Table 5. Resulting p-values when testing for equal drainage distributions in patients with and without NSAID in the four different subgroups formed by sex and (aggregated) number of segments.
Three of 181 patients encountered an intraspinal epidural hematoma (two females, one male). One further female patient, who suffered neurological disturbances postoperatively, was diagnosed with an extraforaminal hematoma. Thus, the incidence of an epidural hematoma was 2.2% in our series. All four patients with an epidural hematoma had to undergo revision surgery. However, only a single male patient was part of the NSAID group, whilst the females had discontinued NSAID medication ten days preoperatively, hadn't taken NSAID at all, or had not taken NSAID on a regular basis, respectively. The small subgroup size of patients with epidural hematomas doesn't allow for statistical analysis to be carried out. However, we assume that NSAID medication does not have a significant impact on the occurrence of epidural hematoma.
Prostaglandins are produced out of arachidonic acid, catalyzed by cyclooxygenase. NSAID works by blocking the synthesis of prostaglandins, thus mediating their analgesic, antipyretic and anti-inflammatory effects. Side effects of NSAID on the kidneys and stomach, or inhibition of thrombocyte aggregation, can be further consequences of this cascade (6). The detection of two different types of cyclooxygenase – COX 1 and COX 2 – helped to explain modes of action, which had, until then, seemed illogical (7). COX 1 produces prostaglandins which are responsible for the entire peripheral resistance, renal blood flow, and the renal elimination of sodium. COX 1 also catalyzes the production of protective prostaglandins in the stomach and the intestine. Moreover, it synthesizes thromboxane A2, which is responsible for the aggregation of thrombocytes and which makes it an interesting target for surgeons: blocking COX 1 leads to the suppression of thrombocyte aggregation, which in turn can result in greater bleeding. In contrast, COX 2 is primarily responsible for the production of prostaglandins during inflammatory reactions, which mostly occur during the course of pathophysiological processes mediated by Interleukin 1, Tumor necrosis factor-α, growth factor transformation, and others. To counteract only these effects whilst also reducing side effects, COX 2 selective inhibitors were introduced. Almost all NSAID which are used as painkillers or for antiphlogistic reasons block COX 1 or COX 2 in several dimensions (8, 9). Antiplatelet drugs such as acetylsalicylic acid (ASS) are widely used in primary and secondary prevention in atherosclerosis patients. This, in turn, caught the interest of surgeons due to possible bleeding complications. Korinth et al. presented the results of a survey of neurosurgeons on the topic of the discontinuation strategy of ASS (10). A broad range of days of discontinuation, seven days before surgery on average, was seen during the study. Two-thirds of the respondents felt that aspirin increased the risk of patients experiencing hemorrhagic complications, and more than half of the interviewed neurosurgeons reported having personally witnessed such problems during spinal operations. In a literature review, Gerstein et al. noted that the risk of perioperative bleeding associated with the continuation of aspirin medication is minimal in many operative procedures compared with the coincident thromboembolic risks associated with aspirin withdrawal. However, aspirin administration should be stopped in patients who are undergoing intracranial, middle ear, posterior eye, intramedullary spine, and possibly transurethral prostatectomy surgery (11). Soleman et al. investigated patients who underwent non-instrumented extradural lumbar spinal surgery (i.e., microscopic fenestration, recessotomy, foraminotomy, and sequestrectomy) under low-dose acetylsalicylic acid and without antiplatelet agents (12). They saw no statistical difference between the acetylsalicylic acid group and the control group and recommended the perioperative continuation of acetylsalicylic acid therapy, especially for the secondary prevention of perioperative complications in atherosclerotic patients. On the other hand, Park et al. (13) investigated the bleeding risk in patients undergoing one- or two-level lumbar spinal fusion surgery. They compared patients who discontinued aspirin medication more than seven days preoperatively, or between three and seven days preoperatively, with a group of patients who had not taken aspirin before surgery (control group). They found that if aspirin was discontinued more than seven days before surgery, there was no statistically significant difference in bleeding complications and blood loss compared with the control group. Cessation of aspirin medication three to seven days before surgery resulted in a significantly higher amount of drained blood and a longer duration of the indwelling of the drainage catheter than in the control group.
The use of NSAID as a painkiller or preoperative antiphlogistic therapy remains controversial. NSAID used as antiphlogistic or analgesic therapy are mostly COX 2 inhibitors. Nevertheless, they also show a limited amount of COX 1 inhibition. Consequently, the extent of the risk of bleeding associated with this medication remains subject to discussion. There is little literature examining this issue in spinal surgery. Park et al. looked at the possible increased blood loss in 106 patients who underwent at least two or more levels of lumbar fusion and who took NSAID (14). They found an increased level of blood loss in patients who took NSAID continuously before surgery compared with the non-NSAID group. In our patient group, blood loss during surgery, postoperative monitoring, and via drainage differed significantly between patients who had had one level fusion surgery and those who had had two and more level fusions. However, this effect differed by gender. In the literature, no sex difference has been described for lumbar spine surgery considering blood loss or complications like epidural hematomas (15, 16). While multi-level surgery is a known and logical appearing factor in an increase in blood loss (17), knowledge of the influence of sex seems to be lesser investigated. To our knowledge, no study focused specifically on this topic. In the cervical spine, a recent study by Wen et al. found a sex difference in blood loss in anterior cervical spine fusion (18), while a similar study by the same authors did not find any sex difference in blood loss in posterior lumbar fusion surgery (19). Due to our retrospective study design, no further conclusions or assumptions can be made on the reason for our findings. Further prospective studies are necessary to determine if there is a significant and clinically relevant sex difference in blood loss in posterior lumbar spine surgery.
We did not see a statistically significant difference between NSAID users (up until the day of or the day before surgery) and non-NSAID patients in any of the investigated subgroups.
We encountered three epidural hematomas which had to be revised, and one extraforaminal hematoma. Again, we found no statistically significant impact of NSAID medication on hematoma occurrence. We had previously investigated 33 patients aged 65 years or older who underwent minimally invasive spinal fusion surgery in another study (20). Interestingly, in this investigation, patients who preoperatively used NSAID as painkillers experienced greater levels of blood loss. We believe this contradiction to be the consequence of the low number of cases in the earlier study. The considerably higher number of cases investigated here gives this new study more weight.
Considering the usage of NSAID in spinal fusion, it also must be mentioned that NSAIDs are discussed as a factor responsible for the impairment of the fusion process. A review from 2017 found that the effect of NSAID to reduce fusion rates might only be present when using NSAID for a course of 2 weeks postoperative and more (21). Using NSAID in a short-term period postoperatively, this disadvantageous effect seems to be improbable. Also, other studies found a dose-dependent effect of NSAIDs on reduced fusion rates (22, 23). While we did not investigate these effects in our study, spine surgeons should consider not only possible effects on blood loss but also the fusion rates, especially when using NSAID for a long-term period postoperative.
Our study was limited due to the retrospective study design, and prospective, randomized controlled trials with a focus on NSAID and sex differences in blood loss after lumbar fusion surgery should be performed.
MISS techniques minimize soft tissue damage, reduce blood loss and show less postoperative pain and result in a shorter hospital stay (1, 2, 4, 5, 24). We consider NSAID medication to have no counter-productive effects in minimally invasive fusion procedures up to four-level fusion with regard to blood loss levels or postoperative hematoma occurrence. Nevertheless, we recommend further prospective studies to confirm our results.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics committee of the Federal State of Lower Austria. The patients/participants provided their written informed consent to participate in this study.
Preparation of the study: WS, WT, JF; Data collection and analysis: WS, HS, WT, JF; Preparation of the manuscript: WS, HS, SA, WT; Editing of the manuscript: HS, JF, SA, AG. All authors contributed to the article and approved the submitted version.
Open access publication was funded by the Johannes Kepler University Linz.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ntoukas V, Müller A. Minimally invasive approach versus traditional open approach for one level posterior lumbar interbody fusion. Minim Invasive Neurosurg. (2010) 53(1):21–4. doi: 10.1055/s-0030-1247560
2. Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine (Phila Pa 1976). (2007) 32(5):537–43. doi: 10.1097/01.brs.0000256473.49791.f4
3. Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine (Phila Pa 1976). (2003) 28(15 Suppl):S26–35. doi: 10.1097/01.Brs.0000076895.52418.5e
4. Franke J, Manson N, Buzek D, Kosmala A, Hubbe U, Rosenberg W, et al. Masters-D study: a prospective, multicenter, pragmatic, observational, data-monitored trial of minimally invasive fusion to treat degenerative lumbar disorders, one-year follow-up. Cureus. (2016) 8(6):e640. doi: 10.7759/cureus.640
5. Kim KT, Lee SH, Suk KS, Bae SC. The quantitative analysis of tissue injury markers after Mini-open lumbar fusion. Spine (Phila Pa 1976). (2006) 31(6):712–6. doi: 10.1097/01.brs.0000202533.05906.ea
6. Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. (1971) 231(25):232–5. doi: 10.1038/newbio231232a0
7. Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. Tis10, a phorbol ester tumor promoter-inducible mrna from Swiss 3t3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. (1991) 266(20):12866–72. doi: 10.1016/S0021-9258(18)98774-0
8. Klein T, Nusing RM, Pfeilschifter J, Ullrich V. Selective inhibition of cyclooxygenase 2. Biochem Pharmacol. (1994) 48(8):1605–10. doi: 10.1016/0006-2952(94)90205-4
9. Laneuville O, Breuer DK, Dewitt DL, Hla T, Funk CD, Smith WL. Differential inhibition of human prostaglandin endoperoxide H synthases-1 and -2 by nonsteroidal anti-inflammatory drugs. J Pharmacol Exp Ther. (1994) 271(2):927–34. Available at: https://jpet.aspetjournals.org/content/271/2/9277965814
10. Korinth MC, Gilsbach JM, Weinzierl MR. Low-Dose aspirin before spinal surgery: results of a survey among neurosurgeons in Germany. Eur Spine J. (2007) 16(3):365–72. doi: 10.1007/s00586-006-0216-7
11. Gerstein NS, Schulman PM, Gerstein WH, Petersen TR, Tawil I. Should more patients continue aspirin therapy perioperatively?: clinical impact of aspirin withdrawal syndrome. Ann Surg. (2012) 255(5):811–9. doi: 10.1097/SLA.0b013e318250504e
12. Soleman J, Baumgarten P, Perrig WN, Fandino J, Fathi AR. Non-Instrumented extradural lumbar spine surgery under low-dose acetylsalicylic acid: a comparative risk analysis study. Eur Spine J. (2016) 25(3):732–9. doi: 10.1007/s00586-015-3864-7
13. Park JH, Ahn Y, Choi BS, Choi KT, Lee K, Kim SH, et al. Antithrombotic effects of aspirin on 1- or 2-level lumbar spinal fusion surgery: a comparison between 2 groups discontinuing aspirin use before and after 7 days prior to surgery. Spine (Phila Pa 1976). (2013) 38(18):1561–5. doi: 10.1097/BRS.0b013e31829a84d2
14. Park HJ, Kwon KY, Woo JH. Comparison of blood loss according to use of aspirin in lumbar fusion patients. Eur Spine J. (2014) 23(8):1777–82. doi: 10.1007/s00586-014-3294-y
15. Lei F, Li Z, He W, Tian X, Zheng L, Kang J, et al. Hidden blood loss and the risk factors after posterior lumbar fusion surgery: a retrospective study. Med (Baltimore). (2020) 99(19):e20103. doi: 10.1097/md.0000000000020103
16. Domenicucci M, Mancarella C, Santoro G, Dugoni DE, Ramieri A, Arezzo MF, et al. Spinal epidural hematomas: personal experience and literature review of more than 1000 cases. J Neurosurg Spine. (2017) 27(2):198–208. doi: 10.3171/2016.12.Spine15475
17. Huang YH, Ou CY. Significant blood loss in lumbar fusion surgery for degenerative spine. World Neurosurg. (2015) 84(3):780–5. doi: 10.1016/j.wneu.2015.05.007
18. Wen L, Jin D, Xie W, Li Y, Chen W, Zhang S, et al. Hidden blood loss in anterior cervical fusion surgery: an analysis of risk factors. World Neurosurg. (2018) 109:e625–e9. doi: 10.1016/j.wneu.2017.10.050
19. Wen L, Jin D, Xie W, Li Y, Chen W, Ding J, et al. Hidden blood loss in posterior lumbar fusion surgery: an analysis of risk factors. Clin Spine Surg. (2018) 31(4):180–4. doi: 10.1097/bsd.0000000000000626
20. Senker W, Meznik C, Avian Mag A. Perioperative complication rate using minimally invasive lumbar fusion techniques in elderly and obese patients with degenerative lumbar disease. J Spine. (2012) 01(03):2–3. doi: 10.4172/2165-7939.1000117
21. Sivaganesan A, Chotai S, White-Dzuro G, McGirt MJ, Devin CJ. The effect of nsaids on spinal fusion: a cross-disciplinary review of biochemical, animal, and human studies. Eur Spine J. (2017) 26(11):2719–28. doi: 10.1007/s00586-017-5021-y
22. Moussalem C, Ftouni L, Abou Mrad Z, Bsat S, Houshiemy M, Alomari S, et al. Negative pharmacological effect on spine fusion: a narrative review of the literature of evidence-based treatment. Clin Neurol Neurosurg. (2021) 207:106799. doi: 10.1016/j.clineuro.2021.106799
23. Lumawig JM, Yamazaki A, Watanabe K. Dose-Dependent inhibition of diclofenac sodium on posterior lumbar interbody fusion rates. Spine J. (2009) 9(5):343–9. doi: 10.1016/j.spinee.2008.06.455
24. Pereira P, Buzek D, Franke J, Senker W, Kosmala A, Hubbe U, et al. Surgical data and early postoperative outcomes after minimally invasive lumbar interbody fusion: results of a prospective, multicenter, observational data-monitored study. PLoS One. (2015) 10(3):e0122312. doi: 10.1371/journal.pone.0122312
Keywords: minimally invasive spine surgery, lumbar fusion, blood loss, hematoma, non-steroidal anti-inflammatory drugs, NSAID
Citation: Senker W, Aspalter S, Trutschnig W, Franke J, Gruber A and Stefanits H (2022) Nonsteroidal anti-inflammatory drugs (NSAID) do not increase blood loss or the incidence of postoperative epidural hematomas when using minimally invasive fusion techniques in the degenerative lumbar spine. Front. Surg. 9:1000238. doi: 10.3389/fsurg.2022.1000238
Received: 21 July 2022; Accepted: 18 October 2022;
Published: 4 November 2022.
Edited by:
Davide Croci, University of South Florida, United StatesReviewed by:
Michel Roethlisberger, University Hospital of Basel, Switzerland© 2022 Senker, Aspalter, Trutschnig, Franke, Gruber and Stefanits. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Aspalter c3RlZmFuLmFzcGFsdGVyQGprdS5hdA==
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.