- 1Department of Orthopedics, Ningbo No.6 Hospital, Ningbo, China
- 2Department of Hepatobiliary Surgery, Shangyu People's Hospital of Shaoxing, Shaoxing, China
Objectives: Laminoplasty (LP) and laminectomy (LC) with or without fusion are recommended as treatment procedures for cervical spondylotic myelopathy (CSM). The purpose of this study is to conduct a meta-analysis to analyze the results of CSM patients undergoing LP or LC surgery.
Methods: We systematically and comprehensively searched Web of Science, Cochrane Library, PubMed, EMBASE, OVID, VIP database, Google Scholar, Chinese Bio-medicine Literature database, and China Scientific Journal Full-text database to July 2021 for randomized controlled trials (RCTs) and observational case series that compared LP and LC in patients with CSM. The main endpoints were the surgical process, radiographic outcomes, clinical outcomes, and surgical complications.
Results: A total of 19 were included the inclusion criteria in this meta-analysis (n = 4,348 patients). There was no significant difference in range of motion (ROM), sagittal vertical axis (SVA), Japanese Orthopedic Association (JOA), Cobb angle, visual analog scale (VAS), cervical curvature index (CCI), Nurick score, Neck Dysfunction Index (NDI), and complications. LP was found to be superior than LC in terms of complications of C5 radiculopathy and surperficial infection.
Conclusion: Our results indicate that LP can achieve better results in C5 radiculopathy and superficial infection in surgical treatment of CSM compared with LC. Further high-quality research is warranted to further verify our findings.
Systematic Review Registration: PRISMA: CRD42018107070.
Background
Cervical spondylotic myelopathy (CSM) refers to a clinical chronic disorder that is usually releated with degenerative disease of intervertebral disk (1). Cervical spondylotic myelopathy is the most common spinal cord degeneration in older sufferers, caused by the progressive spinal canal stenosis and subsequent nerve root compression (2). Surgical management is generally indicated for patients with CSM when conservative treatments are ineffective. Anterior cervical decompression and fusion for multilevel CSM is a complex procedure and may be associated with a long operative time, as well as complications, such as dysphagia, internal graft dislocation, and trigeminal nerve palsy (2). Laminoplasty (LP) and laminectomy (LC) with or without fusion are the primary posterior cervical surgical strategies for treating CSM to remove compressive elements, providing enough space for the cord, and decompressing the spinal cord (3).
Laminectomy, which is usually supplemented by additional fusion, was initially viewed as the gold standard practice for CSM (4). However, this technique is associated with many disadvantages, such as post-LC kyphosis, segmental instability, and subsequent neurological deterioration, which lead to a shorted indication. Laminoplasty was first reported by Tsuji et al. (5) in 1982, and is regarded as an effective way of maintaining anatomical cervical reduction. Laminoplasty retains a covering of the ligamentum flavum over the spinal cord and posterior laminar bone. Laminoplasty has the advantages of minimizing instability, limiting constriction of the dura from extradural scar formation, preserving motion, and avoiding complications related to fusion. However, LP is contraindicated in patients with CSM and >13° of kyphosis and severe neck pain (6). Although there are several disadvantages of LP, including vertebral canal reclosed problems, hinge fracture, higher technical requirements, and possible injuries to the cervical cord, LP has been gradually accepted by an increasing number of surgeons. At present, both surgical procedures decompress the spinal cord by enlarging the spinal canal and are regularly thought to be effective in treating CSM.
Although several meta-analyses or systematic reviews have been conducted to compare LC with LP in treating CSM, the results of these studies were not consistent. In a systematic review, Chen et al. (7) reported that LC with fusion had a higher rate of reoperation, non-union, and infection compared with LP. However, Fehlings et al. (8) suggested that LP and LC with fusion had similar effectiveness. Laminoplasty and LC with fusion may result in clinical recovery and a similar loss of lordosis. Similar to LC followed by fusion, expansive LP has a shorter operative time and less C5 palsy. In this study, we aimed to provide some references for clinical surgical treatment of CSM by systematically comparing the safety and efficacy of LP and LC regarding surgical outcomes, radiographic outcomes, clinical outcomes, and surgical complications.
Methods
Literature Search
International prospective register of systematic reviews (CRD42018107070) was prospectively registered in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (9) for this meta-analysis. Potentially relevant papers that were published in the Web of Science, Cochrane Library, PubMed, EMBASE, OVID, VIP database, Google Scholar, Chinese Bio-medicine Literature database, and China Scientific Journal Full-text database to July 2021 were retrieved and read. Additionally, we also screened the list of references included in publications and related reviews. All included literature only considers English or Chinese language. The following MeSH terms associated with text words were used: cervical vertebrae, spinal cord compression, LP, and LC.
Selection Criteria
Two researchers independently judged the eligibility of all studies retrieved from the database. Any disagreements between two independent researchers are resolved through discussion or consultation with a third researcher. This meta-analysis is included in the study based on the following criteria: (1) randomized controlled trial (RCTs) or observational studies; (2) studies that compared clinical outcomes between LP and LC with or without fusion; (3) studies that included patients with CSM caused by spinal stenosis; and (4) studies that included outcome measurements, such as surgical outcomes, radiographic outcomes, clinical outcomes, and surgical complications. The exclusion criteria are as follows: (1) The randomized study did not conduct a control group study; (2) The data in the paper or research report was incomplete, resulting in unclear research results; (3) Incomplete papers, including abstracts, conference reports, case reports, and comments And expert opinions; (4) There is no data in the research results to estimate the relative risk (RR) and mean difference (MD).
According to the Cochrane risk-of-bias criteria, the risk of bias and methodological quality of the involved included studies was evaluated by two researchers independently. The methodological quality of each studies was assessed as unclear risk, low risk, or high risk.
Data Extraction
All revelant data were extracted independently by two researchers from article texts, tables, and figures. The extracted data includes: the first author, the time of publication, the origin country of the study, the type of experimental design, the sample size of the study, demographics, methods, length of post-operative follow-up and clinical results. The clinical results included length of operation, loss of blood, length of hospital stay, range of motion (ROM), sagittal vertical axis (SVA), Cobb angle, Japanese Orthopedic Association (JOA), mJOA, visual analog scale (VAS), cervical curvature index (CCI), SF-36 MCS, SF-36 PCS, Nurick score, NDI (neck disability index), and C5 radiculopathy.
Statistical Analysis
Relative risk with a 95% CI was computed for binary data, and the MD with a 95% CI was computed for continuous data. The heterogeneity between studies was assessed by the χ2 test and the I2 statistic. I2 ≤ 50% indicates acceptable heterogeneity, while xI2 > 50% means significant heterogeneity. A fixed effects model was applied, if P > 0.10 and I2 <50%. If not, a random effects model was chosen. Publication bias is evaluated by the symmetrical construction of the funnel chart. Review Manager (RevMan 5.3, Cochrane Collaboration, Nordic Cochrane Center, Copenhagen, Denmark) was performed for statistical analysis.
Results
Search Results
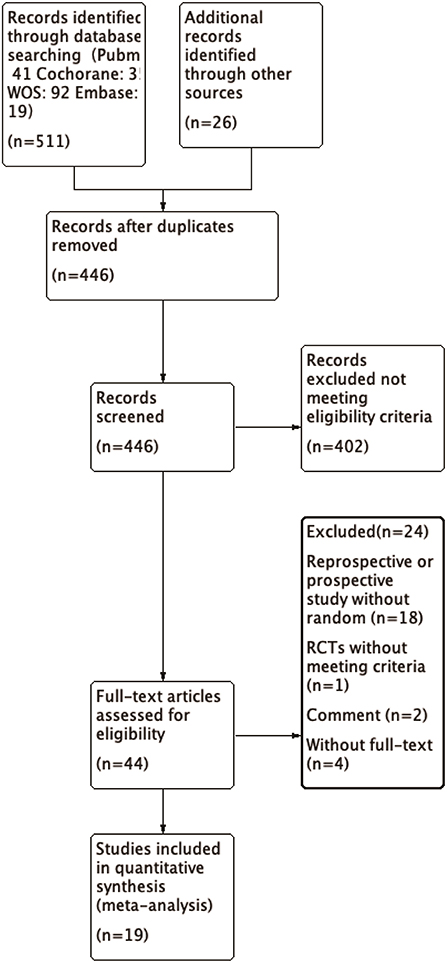
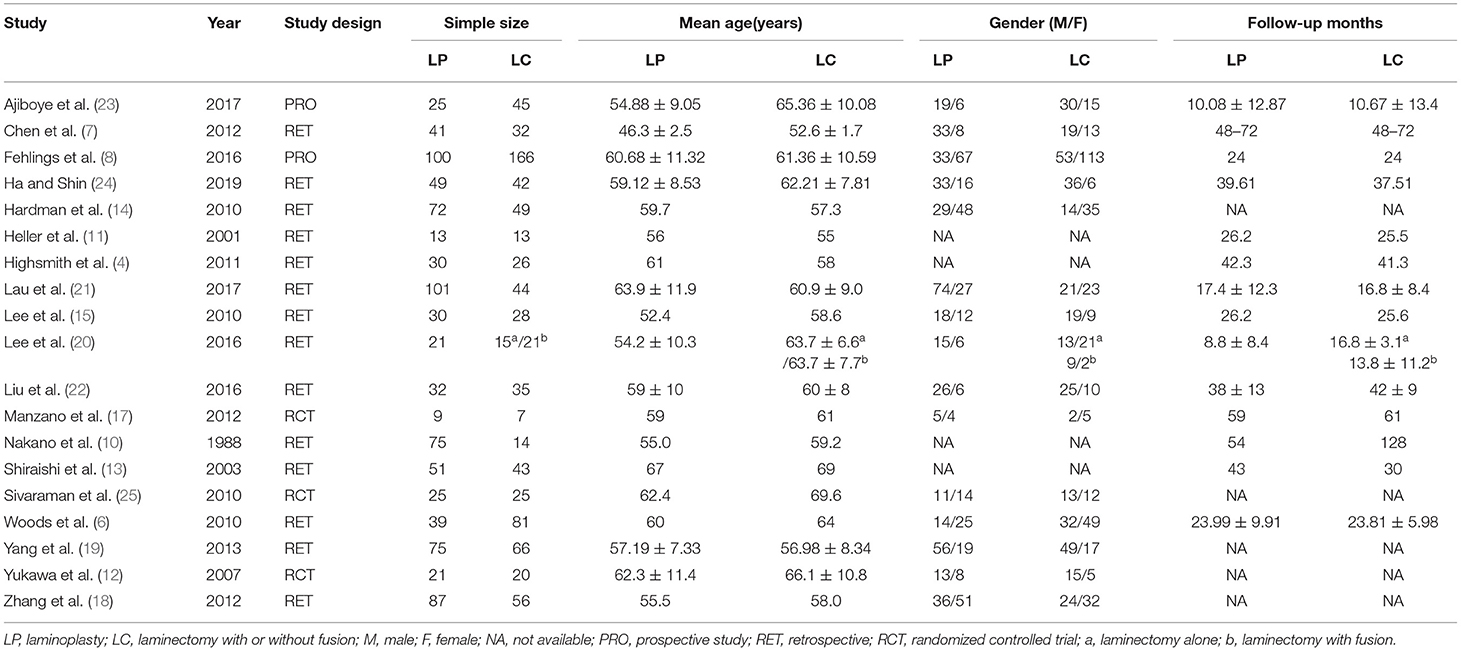
We identified a total of 511 probably related articles from the database. An additional 26 possible publications were identified through other sources, primarily through manual search of the reference list. Of the 537 articles, 443 articles were excluded due to duplication or the exclusion criteria. Finally, a total of 19 articles (4, 6–8, 10–24), which were published between 1988 and 2019, were considered for inclusion in this meta-analysis. The detailed search strategy based on database is provided in Figure 1. A total of 1,724 patients (896 treated with LP and 828 treated with LC), were included, and the follow-up period ranged between 8.8 and 72 months. As shown in Figures 2, 3, the results of methodological quality are presented in the risk of bias. Only three studies (12, 17, 25) were RCTs that compared LP with LC. The Adetailed overview of study characteristics can be consulted in Table 1.
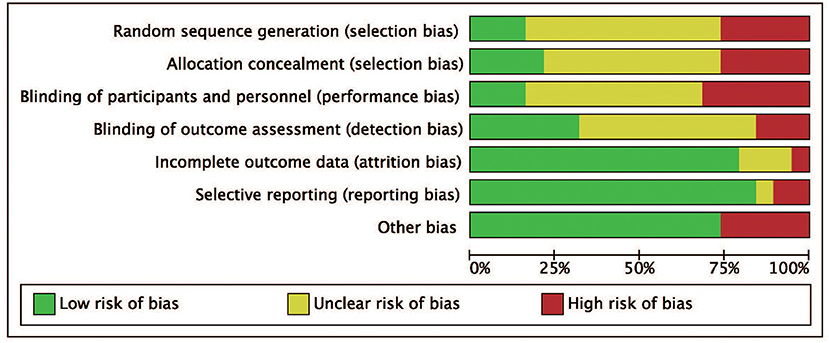
Figure 2. Risk of bias graph. Review authors' judgments for each risk of bias item are presented as percentages across all included studies.
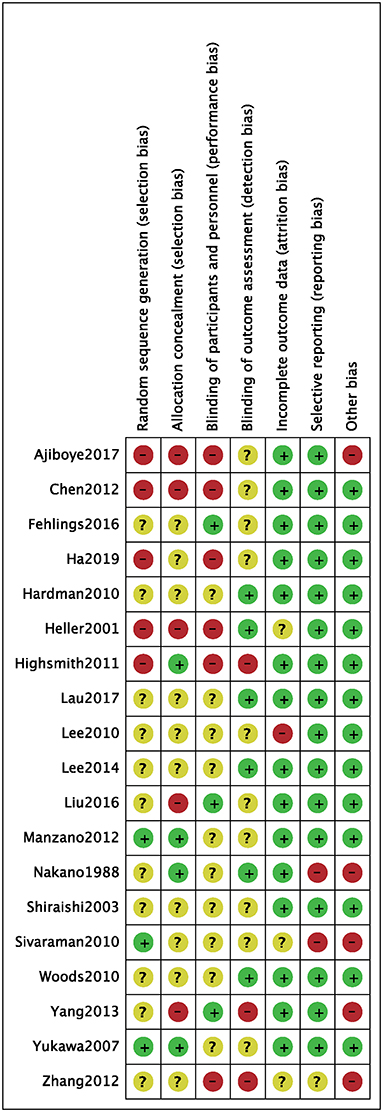
Figure 3. Risk of bias of included randomized controlled trials. +, no bias; –, bias; ?, bias unknown.
Clinical Outcomes
Length of Operation, Loss of Blood, Length of Hospital Stays
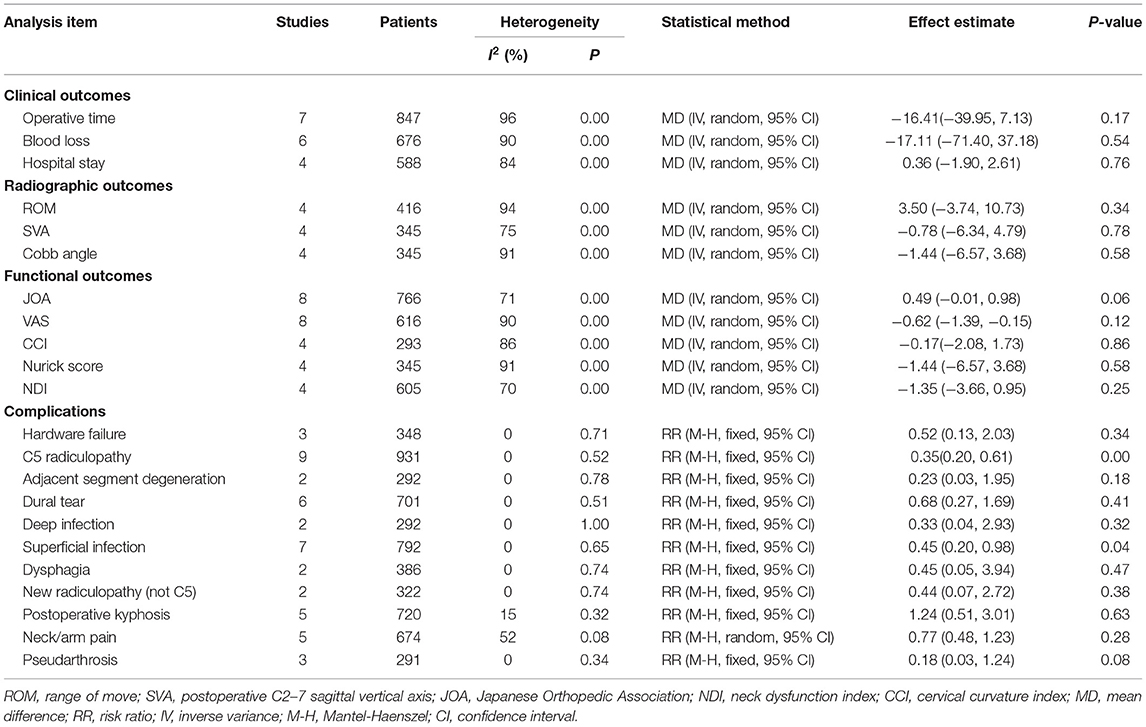
In seven studies (8, 10, 16, 18, 19, 22, 24) that reported the length of operation, there was no significant difference observed between the length of operation of the LP and LC groups (MD = −16.41, 95% CI: −39.95 to 7.13, I2 = 96%, P = 0.17) (Figure 4A; Table 2). Intraoperative blood loss was evaluated in six studies (10, 19, 21, 22, 24). No significant differences were demonstrated between the volume of blood loss of the two groups (MD = −17.11, 95% CI: −71.40 to 37.18, I2 = 90%, P = 0.54) (Figure 4B; Table 2). The length of hospital stay was evaluated in four studies (4, 8, 14, 21), and there was no significant difference observed between the LP and LC groups (MD = 0.36, 95% CI −1.90 to 2.61, I2 = 84%, P = 0.76) (Figure 4C; Table 2).
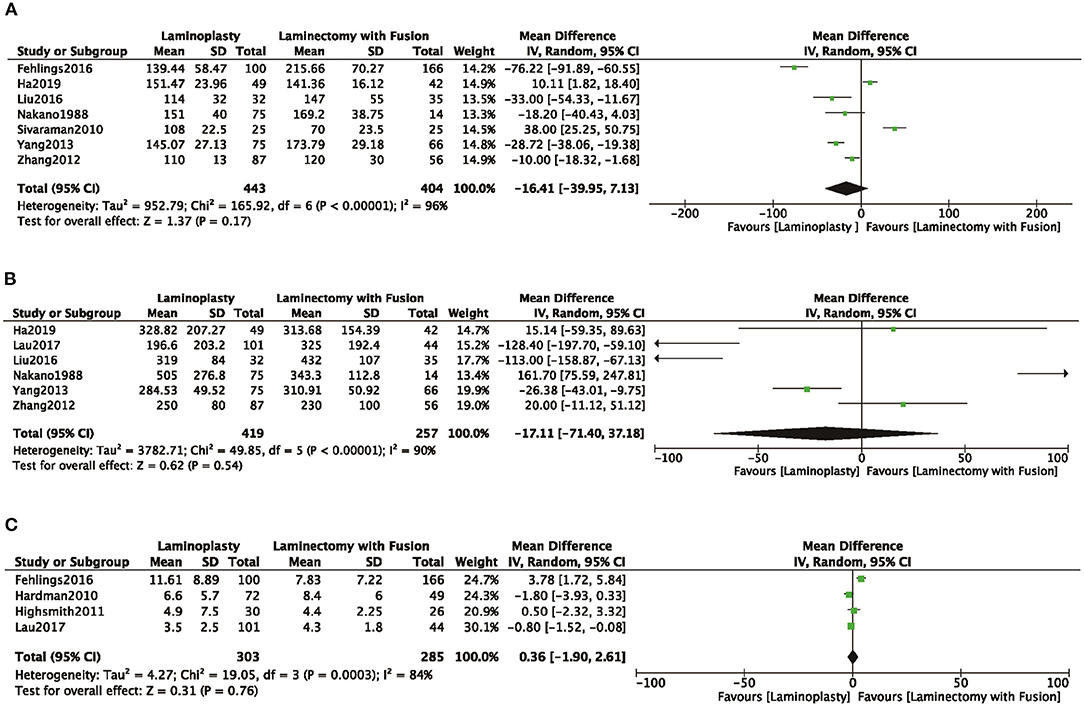
Figure 4. Comparison of (A) operative time; (B) intraoperative blood loss; (C) the time of hospital stay; between the LP group and the LC group.
Radiographic Outcomes
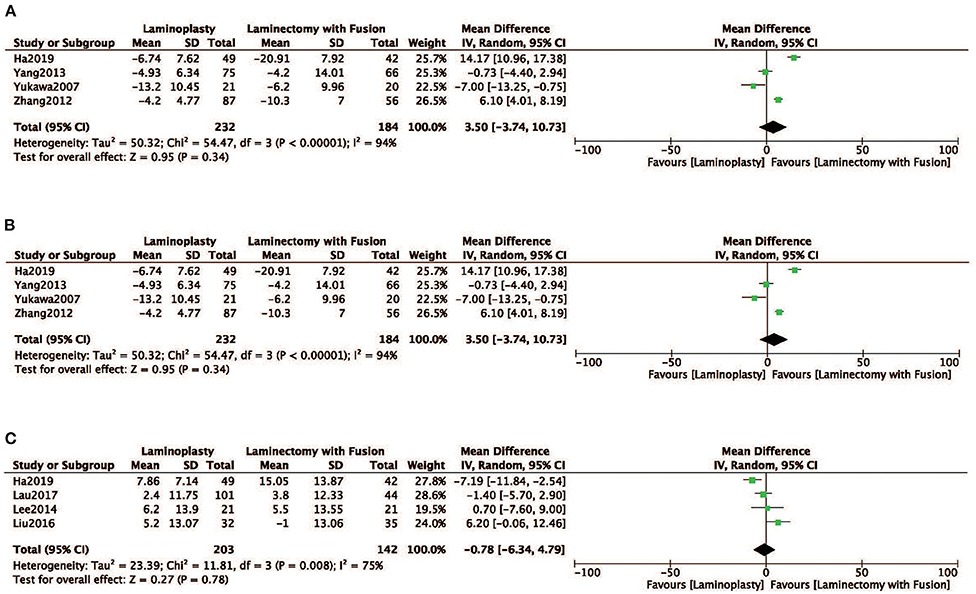
Radiographic parameters of the ROM, SVA, and the Cobb angle were evaluated in four studies (12, 18, 19, 24). No obvious difference was observed in ROM between the two groups the LP group compared with the LC (MD = 3.50, 95% CI: −3.74 to10.73, I2 = 94%, P = 0.34) (Figure 5A; Table 2). There was no significant difference observed in SVA between the two groups (MD = −0.78, 95% CI: −6.34 to 4.79, I2 = 75%, P = 0.78) (Figure 5B; Table 2). There was also no significant difference in the Cobb angle observed between the LP and LC (MD = −1.44, 95% CI: −6.57 to 3.68, I2 = 91%, P = 0.58) (Figure 5C; Table 2).
Functional Outcomes
Eight studies (4, 7, 8, 15, 17, 19, 22, 24) used the JOA to assess the clinical outcome. No significant difference was observed of JOA score in the LP group compared with the LC group (MD = 0.49, 95% CI: −0.01 to 0.98, I2 = 71%, P = 0.06) (Figure 6A; Table 2). There were no significant differences in the VAS, CCI, Nurick, and NDI scores between the two groups (Figures 6B–E; Table 2).
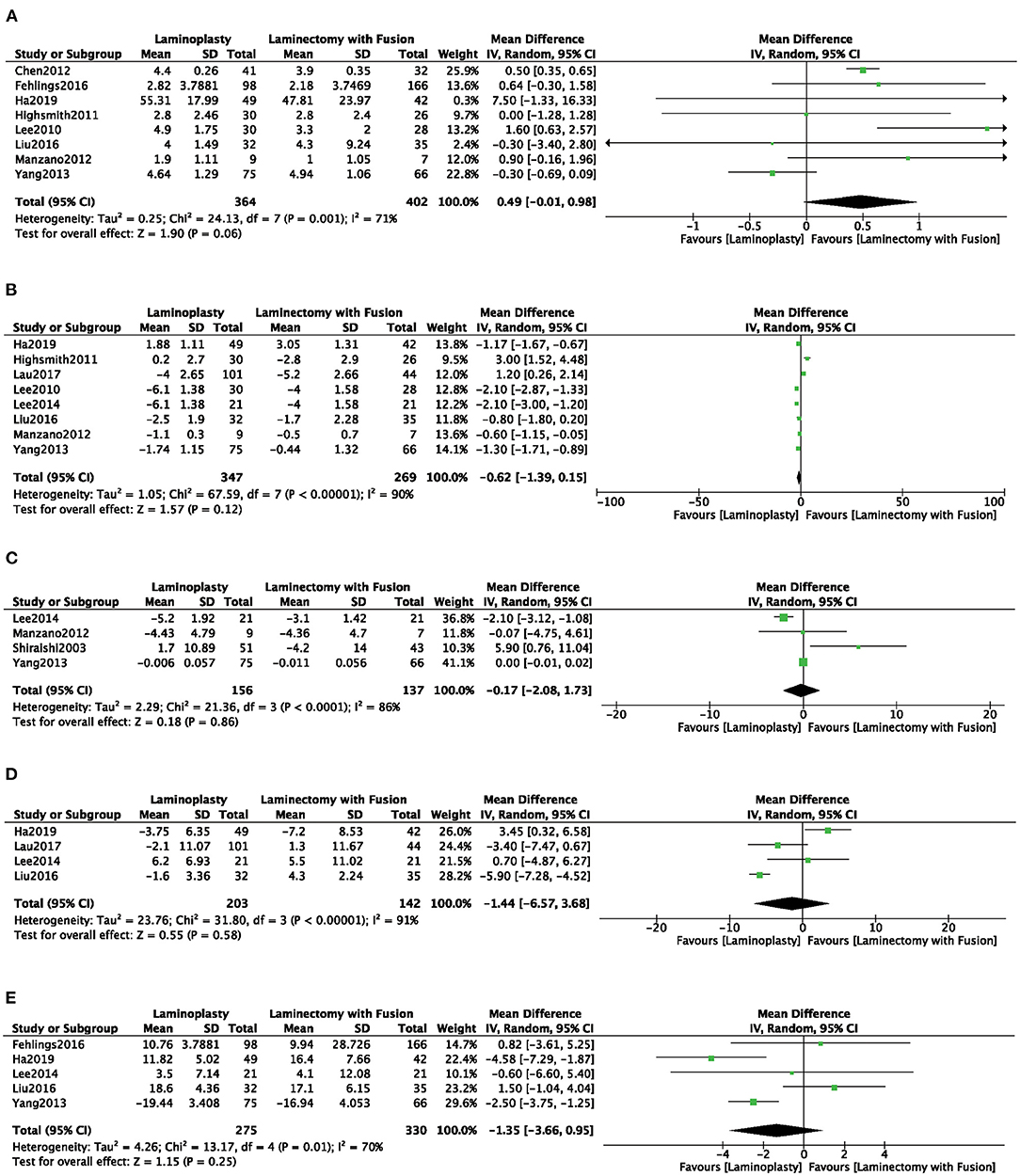
Figure 6. Comparison of (A) JOA; (B) VAS; (C) CCI; (D) Nurick; (E) NDI; between the LP group and the LC group.
Surgical Complications
Eight studies (4, 7, 8, 13, 18, 19, 22, 24) evaluated the rate of C5 radiculopathy. A significantly higher complication rate of C5 radiculopathy was observed in the LC gcompared with the LP (RR = 0.35, 95% CI: 0.20 to 0.61, I2 = 0%, P < 0.01) (Figure 7A). C5 radiculopathy occurred in 17 (3.51%) of 485 patients who were treated with LP and in 40 (8.20%) of 488 patients who were treated with LC. Moreover, the complication of superficial infection occurred significantly less in the LP compared with the LP frequently (RR = 0.65, 95% CI: 0.20 to 0.98, I2 = 0%, P = 0.04) (Figure 7B). Additionally, other complications, such as hardware failure, adjacent segment degeneration, dural tear, deep infection, dysphagia, non-C5 radiculopathy, postoperative kyphosis, neck/arm pain, and pseudarthrosis, were not significantly different between the two groups (Figures 7C–K).

Figure 7. Comparison of (A) complication rate of C5 radiculopathy; (B) superficial infection; (C) hardware failure; (D) adjacent segment degeneration; (E) dural tear; (F) deep infection; (G) dysphagia; (H) new radiculopathy (not C5); (I) postoperative kyphosis; (J) neck/arm pain; (K) pseudarthrosis; between the LP group and the LC group.
Discussion
Our study showed that patients receiving LP for CSM had less frequent occurrence of C5 radiculopathy and superficial infection than those who underwent LP. Length of operation, loss of blood, length of hospital stays, Cobb angle, SVA, VAS score, CCI score, Nurick score, NDI score, and other surgical complications were not different observed between these two groups. These results suggest that LP is a useful therapeutic procedure promoting management of CSM.
Several previous systematic reviews and meta-analyses that analyzed LP and LC with or without fusion for CSM have been published and showed different results compared with our study (26, 27). A meta-analysis by Lee et al. (28) compared LP with LC for treating CSM. In this article, a total of seven studies were included in the meta-analysis (six English papers and one Chinese paper). The authors focused on the clinical and radiological outcomes between these two different methods. The two groups did not show significant differences in JOA grade, VAS score, and CCI at the baseline state. The authors suggest that both methods may obtain clinical improvement and lead to a similar loss of lordosis, but definitive conclusion could not be reached regarding which surgical approach is more effective for the treatment of CSM. Liu et al. (29) presented a meta-analysis of 23 studies comparing LP with LC for treating CSM. They focused on clinical outcome (JOA, CCI, VAS, and cervical lordosis), complication (C5 palsy and axial pain), blood loss, and operation time. The LP group showed shorter operation time and fewer C5 palsy. Others had may achieve clinical improvement and a similar result. Phan and Scherman et al. (30) compared LP with LC for treating CSM in 10 studies when treating patients with CSM. They focused on the Postoperative JOA, postoperative VAS neck pain, postoperative CCI, postoperative Nurich grade, complication (reoperation rate and nerve palsy), operative time, and intraoperative blood loss. They found that there was no difference in terms of clinical improvement. However, a higher complication of nerve palsy was found in the LC than in the LC. Otherwise, results of others studies showed that LP and LC fusion methods were similarly useful (31).
Patients were matched and both groups had a similar length of operation, blood loss, and hospital stay. These characteristics were not examined in other relevant meta-analyses. Heterogeneity in our meta-analysis was high (I2 > 75%), those personal difference and surgical processes had multivariate analyses and reports for them are speculative. Pooled results from three studies (21, 22) showed that the Cobb angle was more acceptable in the LP than in the LC. The reason why the Cobb angle was more acceptable in the LP group may be that the muscles and ligamentous structures of the cervical spine are dissected minimally and restored maximally in the LP group. In our study, clinical measurements, such as VAS, CCI, SF-36 MCS, SF-36 PCS, Nurick, and neck disability index scores, were not significantly different in two groups. However, the LP procedure was superior to the LC procedure in evaluation of the JOA score for patients with CSM. These findings indicated that the effect of LP was more favorable than that for LC, which suggested that LP could be considered as the method of surgery for patients with CSM. Most importantly, these results are supportive of a significant improvement in the management of CSM. Specifically, LP as a treatment strategy for CSM can obtain better results in the surgical process, radiographic outcomes, and clinical outcomes (Cobb angle, JOA score, and risk of C5 radiculopathy) than LC.
Several limitations must be considered when interpreting the results. First, the quality of the studies included 3 RCTs and 16 observational studies was low. Most of the RCTs did not provide any insufficient information on the exact methods of randomization to determine if credibility analysis was present. Allocation concealment was performed in three studied using sealed envelopes. Based on the selection criteria, we could not make further deletions or additions to the included papers. Second, the number of individuals in these studies were relatively small and therefore statistical power might be limited. Third, we guess that the research heterogeneity is mainly caused by the low quality of the included articles, which is mainly reflected in the high heterogeneity of the articles. The heterogeneity among the indicators included in our meta-analysis was mainly reflected in the time of operative, intraoperative blood loss, the time of hospital stay, ROM, SVA, Cobb angle, JOA, VAS, CCI, Nurick NDI, and Pseudarthrosis, and for the above heterogeneity, we used a random effects model for treatment statistics, while rate of C5 radiculopathy, Superficial infection, Hardware failure, Adjacent segment degeneration, Dural tea, Deep infection, Dysphagia, New radiculopathy (not C5), Postoperative kyphosis, and Neck/arm pain had lower heterogeneity and we used a fixed effects model for the treatment statistics. In addition, this general clinical heterogeneity may be caused by individual differences in patients, technical differences in the surgeon team, differences in medical equipment, and follow-up time. We considered whether our results might be affected by confounding factors. Therefore, high-quality RCTs are required to examine the long-term effects of these two surgical procedures on patients with CSM.
Conclusion
Our study shows that LP can achieve better results in C5 radiculopathy and superficial infection in surgical treatment of CSM compared with LC. These results suggest that LP is a therapeutic procedure for promoting management of CSM. Furthermore, high-quality research, adequately powered randomized studies are required to provide more evidence for the optimal surgical treatment of CSM for definite conclusions.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
ZZ and YZ: protocol and project development. LP: data collection or management. WM and SX: data analysis. HZ and RR: manuscript writing and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
References
1. Sampath P, Bendebba M, Davis JD, Ducker TB. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine. (2000) 25:670–6. doi: 10.1097/00007632-200003150-00004
2. Liu Y, Hou Y, Yang L, Chen H, Wang X, Wu X, et al. Comparison of 3 reconstructive techniques in the surgical management of multilevel cervical spondylotic myelopathy. Spine. (2012) 37:E1450–8. doi: 10.1097/BRS.0b013e31826c72b4
3. Mehdi SK, Alentado VJ, Lee BS, Mroz TE, Benzel EC, Steinmetz MP. Comparison of clinical outcomes in decompression and fusion versus decompression only in patients with ossification of the posterior longitudinal ligament: a meta-analysis. Neurosurg Focus. (2016) 40:E9. doi: 10.3171/2016.3.FOCUS1630
4. Highsmith JM, Dhall SS, Haid RW Jr, Rodts GE Jr, Mummaneni PV. Treatment of cervical stenotic myelopathy: a cost and outcome comparison of laminoplasty versus laminectomy and lateral mass fusion. J Neurosurg Spine. (2011) 14:619–25. doi: 10.3171/2011.1.SPINE10206
5. Tsuji H. Laminoplasty for patients with compressive myelopathy due to so-called spinal canal stenosis in cervical and thoracic regions. Spine. (1982) 7:28–34. doi: 10.1097/00007632-198200710-00002
6. Woods BI, Hohl J, Lee J, Donaldson W III, Kang J. Laminoplasty versus laminectomy and fusion for multilevel cervical spondylotic myelopathy. Clin Orthop Relat Res. (2010) 469:688–95. doi: 10.1007/s11999-010-1653-5
7. Chen Y, Liu X, Chen D, Wang X, Yuan W. Surgical strategy for ossification of the posterior longitudinal ligament in the cervical spine. Orthopedics. (2012) 35:e1231–7. doi: 10.3928/01477447-20120725-25
8. Fehlings MG, Santaguida C, Tetreault L, Arnold P, Barbagallo G, Defino H, et al. Laminectomy and fusion versus laminoplasty for the treatment of degenerative cervical myelopathy: results from the AOSpine North America and International prospective multicenter studies. Spine J. (2017) 17:102–8. doi: 10.1016/j.spinee.2016.08.019
9. Borderud SP, Li Y, Burkhalter JE, Sheffer CE, Ostroff JS. Erratum: Electronic cigarette use among patients with cancer: characteristics of electronic cigarette users and their smoking cessation outcomes. Cancer. (2015) 121:800. doi: 10.1002/cncr.29118
10. Nakano N, Nakano T, Nakano K. Comparison of the results of laminectomy and open-door laminoplasty for cervical spondylotic myeloradiculopathy and ossification of the posterior longitudinal ligament. Spine. (1988). 13:792–4. doi: 10.1097/00007632-198807000-00014
11. Heller JG, Edwards CC II, Murakami H, Rodts GE. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: an independent matched cohort analysis. Spine. (2001) 26:1330–6. doi: 10.1097/00007632-200106150-00013
12. Yukawa Y, Kato F, Ito K, Horie Y, Hida T, Ito Z, et al. Laminoplasty and skip laminectomy for cervical compressive myelopathy: range of motion, postoperative neck pain, and surgical outcomes in a randomized prospective study. Spine. (2007) 32:1980–5. doi: 10.1097/BRS.0b013e318133fbce
13. Shiraishi T, Fukuda K, Yato Y, Nakamura M, Ikegami T. Results of skip laminectomy-minimum 2-year follow-up study compared with open-door laminoplasty. Spine. (2003) 28:2667–72. doi: 10.1097/01.BRS.0000103340.78418.B2
14. Hardman J, Graf O, Kouloumberis PE, Gao WH, Chan M, Roitberg BZ. Clinical and functional outcomes of laminoplasty and laminectomy. Neurol Res. (2010) 32:416–20. doi: 10.1179/174313209X459084
15. Lee CW, Kang JH, Lee KY, Sung HW. Laminoplasty versus laminectomy and fusion for multilevel cervical spondylosis. J Kor Soc Spine Surg. (2010). 17:147–53. doi: 10.4184/jkss.2010.17.3.147
16. Sivaraman AAKB, Altaf F, Singh A, Casey AT, Crawford RJ. Skip laminectomy—a new treatment for cervical spondylotic myelopathy, preserving bilateral muscular attachments to the spinous processes. Spine J. (2010) 2:108–15. doi: 10.1016/S1529-9430(01)00118-8
17. Manzano GR, Casella G, Wang MY, Vanni S, Levi AD. A prospective, randomized trial comparing expansile cervical laminoplasty and cervical laminectomy and fusion for multilevel cervical myelopathy. Neurosurgery. (2012) 70:264–77. doi: 10.1227/NEU.0b013e3182305669
18. Zhang H, Sun T, Lu S, Li Q, Yadav SK. [Comparison of effectiveness between laminoplasty and laminectomy decompression and fusion with internal fixation for cervical spondylotic myelopathy]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. (2012) 26:1191–6.
19. Yang L, Gu Y, Shi J, Gao R, Liu Y, Li J, et al. Modified plate-only open-door laminoplasty versus laminectomy and fusion for the treatment of cervical stenotic myelopathy. Orthopedics. (2013) 36:e79–87. doi: 10.3928/01477447-20121217-23
20. Lee C-H, Jahng T-A, Hyun S-J, Kim K-J, Kim H-J. Expansive laminoplasty versus laminectomy alone versus laminectomy and fusion for cervical ossification of the posterior longitudinal ligament. J Spin Disord Tech. (2016) 29:E9–15. doi: 10.1097/BSD.0000000000000058
21. Lau D, Winkler EA, Than KD, Chou D, Mummaneni PV. Laminoplasty versus laminectomy with posterior spinal fusion for multilevel cervical spondylotic myelopathy: influence of cervical alignment on outcomes. J Neurosurg Spine. (2017) 27:508–17. doi: 10.3171/2017.4.SPINE16831
22. Liu X, Chen Y, Yang H, Li T, Xu B, Chen D. Expansive open-door laminoplasty versus laminectomy and instrumented fusion for cases with cervical ossification of the posterior longitudinal ligament and straight lordosis. Eur Spine J. (2017) 26:1173–80. doi: 10.1007/s00586-016-4912-7
23. Ajiboye RM, Zoller SD, Ashana AA, Sharma A, Sheppard W, Holly LT. Regression of disc-osteophyte complexes following laminoplasty versus laminectomy with fusion for cervical spondylotic myelopathy. Int J Spine Surg. (2017) 11:17. doi: 10.14444/4017
24. Ha Y, Shin JJ. Comparison of clinical and radiological outcomes in cervical laminoplasty versus laminectomy with fusion in patients with ossification of the posterior longitudinal ligament. Neurosurg Rev. (2019). 43:1409–21. doi: 10.1007/s10143-019-01174-5
25. Sivaraman A, Bhadra AK, Altaf F, Singh A, Rai A, Casey AT, et al. Skip laminectomy and laminoplasty for cervical spondylotic myelopathy: a prospective study of clinical and radiologic outcomes. J Spinal Disord Tech. (2010) 23:96–100. doi: 10.1097/BSD.0b013e318198c92a
26. Lao L, Zhong G, Li X, Qian L, Liu Z. Laminoplasty versus laminectomy for multi-level cervical spondylotic myelopathy: a systematic review of the literature. J Orthop Surg Res. (2013) 8:45. doi: 10.1186/1749-799X-8-45
27. Sun S, Li Y, Wang X, Lu G, She L, Yan Z, et al. Safety and efficacy of laminoplasty versus laminectomy in the treatment of spinal cord tumors: a systematic review and meta-analysis. World Neurosurg. (2019) 125:136–45. doi: 10.1016/j.wneu.2018.12.033
28. Lee CH, Lee J, Kang JD, Hyun SJ, Kim KJ, Jahng TA, et al. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: a meta-analysis of clinical and radiological outcomes. J Neurosurg Spine. (2015) 22:589–95. doi: 10.3171/2014.10.SPINE1498
29. Liu FY, Yang SD, Huo LS, Wang T, Yang DL, Ding WY. Laminoplasty versus laminectomy and fusion for multilevel cervical compressive myelopathy: a meta-analysis. Medicine (Baltimore). (2016) 95:e3588. doi: 10.1097/MD.0000000000003588
30. Phan K, Scherman DB, Xu JS, Leung V, Virk S, Mobbs RJ. Laminectomy and fusion vs laminoplasty for multi-level cervical myelopathy: a systematic review and meta-analysis. Eur Spine J. (2017) 26:94–103. doi: 10.1007/s00586-016-4671-5
Keywords: cervical myelopathy, laminoplasty, laminectomy, meta-analysis, systematic review
Citation: Zhao H, Ren R, Ma W, Xu S, Peng L, Zhong Z and Zheng Y (2022) Comparison of Laminoplasty vs. Laminectomy for Cervical Spondylotic Myelopathy: A Systematic Review and Meta-Analysis. Front. Surg. 8:790593. doi: 10.3389/fsurg.2021.790593
Received: 07 October 2021; Accepted: 13 December 2021;
Published: 17 January 2022.
Edited by:
Jeremy Steinberger, Icahn School of Medicine at Mount Sinai, United StatesCopyright © 2022 Zhao, Ren, Ma, Xu, Peng, Zhong and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zheng, bmJ6aGVuZ3lhbjEwMjJAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Huaguo Zhao1†
Huaguo Zhao1† Weihu Ma
Weihu Ma Yan Zheng
Yan Zheng