- 1Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Biotherapy, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 3State Key Laboratory of Biotherapy, Department of Biotherapy, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 4West China School of Medicine, West China Hospital, Sichuan University, Chengdu, China
Myeloid-derived suppressor cells (MDSCs) are known to play an essential part in tumor progression under chronic stress settings through their manipulation of adaptive and innate immune systems. Previous researches mainly focus on MDSC's role in the chronic tumor immune environment. In addition, surgery can also serve as a form of acute stress within the patient's internal environment. Nevertheless, the part that MDSCs play in post-surgical tumor development has not gained enough attention yet. Although surgery is known to be an effective definite treatment for most localized solid tumors, there are still plenty of cancer patients who experience recurrence or metastasis after radical resection of the primary tumor. It is believed that surgery has the paradoxical capability to enhance tumor growth. Many possible mechanisms exist for explaining post-surgical metastasis. We hypothesize that surgical resection of the primary tumor can also facilitate the expansion of MDSCs and their pro-tumor role since these surgery-induced MDSCs can prepare the pre-metastatic niche (the “soil”) and at the same time interact with circulating tumor cells (the “seeds”). This vicious, reciprocal mechanism is a crucial point in the emergence of post-surgical metastasis. According to our hypothesis, MDSCs can be the precise target to prevent cancer patients from post-surgical recurrence and metastasis during the perioperative phase to break the wretched cycle and provide better long-term survival for these patients. Future studies are needed to validate this hypothesis.
Introduction
Myeloid-derived suppressor cells' (MDSCs) existence in pathologic conditions such as sepsis, stress, and trauma can be considered a reflection of emergency myelopoiesis. However, the tumor can utilize this phenomenon to create long-lasting abnormal myelopoiesis in favor of tumor growth and progression. Previous researches mainly focus on MDSC's role in chronic tumor environment: MDSCs can participate directly in both the adaptive and innate immune systems via a plethora of mechanisms, including the deprivation of arginine, the release of oxidizing molecules, the modulation of regulatory T cells (Tregs), and the interfere with T cell functions (1); and MDSC level correlates with primary tumor growth and poor prognosis (2–4).
MDSCs' function during trauma and sepsis processes has been reviewed in detail (5). In their review, Alex G Cuenca et al. believe that they may play a protective role in the host's acute stress reaction by suppressing the cytokine responses and inherent immunity. As in an acute inflammatory response process, there has been a question for quite a time: is the role of MDSC beneficial or detrimental, which has not been a satisfying answer yet. But at least the expansion in MDSCs could possibly either contributes to sepsis immune suppression or prevent it, depending on the conditions, illustrating its complexity. Ulteriorly, we are more interested in the role of MDSCs in the setting of an organism-environment where the tumor already exists.
Surgical resection is the mainstay for radically removing the primary tumor. Admittedly, surgical removal of the primary tumor is widely acknowledged as the best option in treating almost all localized solid tumors; surgery is still a significant disturbance to a living organism. Tumor recurrence and metastasis after complete resection of the primary tumor exists, resulting in a rather unsatisfactory long-term survival. Growing evidence indicates that surgery on the tumor mass can paradoxically promote post-surgical metastasis risk through complex processes that include multiple factors interplaying simultaneously (6).
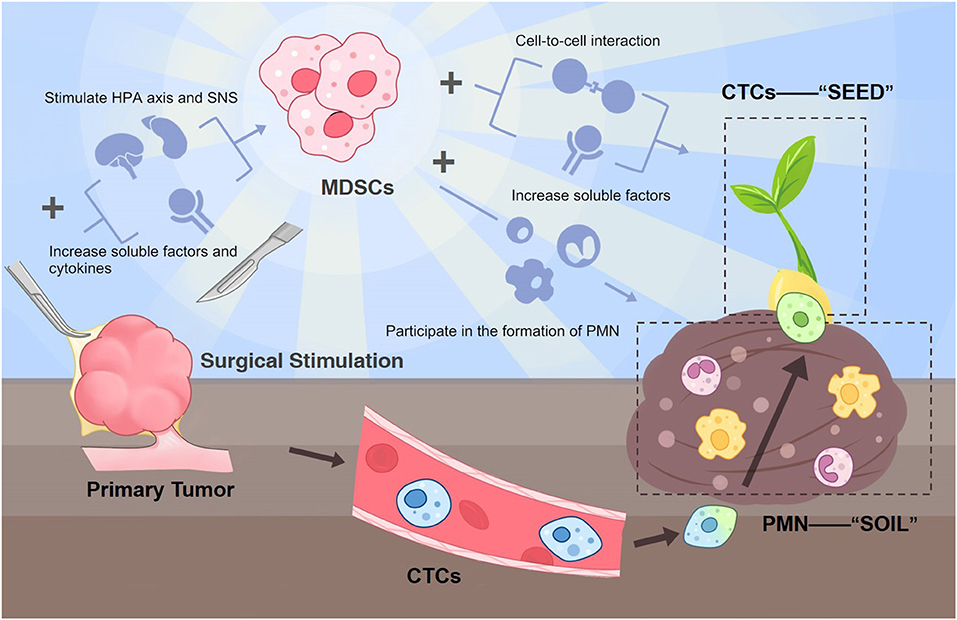
Researchers have been wondering about the possible mechanism for post-surgical metastasis. MDSCs in the tumor microenvironment (TME) play a significant role in tumor metastasis (7, 8). Studies show acute stress-like surgery is likely to stimulate MDSCs growth in the TME, which then regulate the immune suppression and participate in the formation of the pre-metastatic niche (PMN) (the “soil”) (7, 9). Not only can MDSCs be induced by surgical stress, being the most obviously increased immune-related cells immediately before and after the resection of tumor lesions, post-operatively induced MDSCs are also a very potent contributor to metastases. In addition, the combination of primary tumor resection and low-dose adjuvant epigenetic modifiers or gemcitabine (which targets MDSCs) can restrain subsequent metastatic growth. This further reinforces the critical value of MDSCs in post-surgical metastasis development (8, 10). Besides their ability to forge fertile “soil” for metastasis lesions, MDSCs can also influence the fate of circulating tumor cells (CTCs) (the “seeds”).
The reasons behind post-surgical metastasis are very complicated, with metabolic, inflammatory, neural, endocrine, and immunologic factors all inseparably intertwined. We hypothesize that surgical-induced MDSCs are potent causes of post-surgical metastasis by interacting with CTCs and augmenting the PMN for CTCs to colonize and grow. In other words, MDSCs can fertilize the “soil” as well as the “seeds” at the same time. Therefore, targeting this pivotal factor and the leading source of the following cascade from surgical insult to metastasis during the perioperative period can significantly improve cancer patients' prognosis after tumor resection surgery.
Evaluation of the Hypothesis
Surgery Can Induce the Expansion of MDSCs
Surgery has the paradoxical capability to enhance tumor growth (11–13). Early in 1982, Uchida A has reported the possibility that circulating “suppressor monocytes” might have contributed to the inhibition of NK activity in post-operative tumor patients (14); these cells, later, were believed to be MDSCs actually. Recent endeavors have been abundant but fragmentary, spanning from inflammation, tumor cell shedding, and tumor immunity. Studies using the acute infection and sepsis model show that MDSCs increase through the expansion and activation of immature myeloid cells through the acute inflammatory process (15, 16). Surgery can also be perceived as a kind of acute stress. Evidence validates that it can induce the expansion and accumulation of MDSCs in a tumor-host, as in numerous studies in mice (17, 18) and humans (8, 19–21).
Also, the MDSCs concentration seems to correlate with the surgical procedure intensity (22, 23). In a study within breast cancer patients, research has reported that targeting the overall tumor burden through resection of the primary tumor lesions contributed to the inhibition of MDSCs, therefore promoting survival benefits (24). At the same time, there are also studies showing no significant difference in MDSC levels in different operative types, id est the surgical stress intensity (25). We have several possible explanations for this phenomenon. Firstly, the surgery itself may have reached the ceiling level of surgical stress; thus, more aggressive procedures do not necessarily result in higher MDSC-related cytokines. On the other hand, carbon dioxide (CO2) pneumoperitoneum could be an important factor in enhancing the metastasis-promoting ability of laparoscopic surgery (26). We suppose that besides causing peritoneal damage, CO2 could also facilitate tumor metastases through increasing MDSC in the local environment, as MDSC percentage increases along with the growth of arterial CO2 pressure (27).
Surgery possibly promotes the numerical expansion of MDSCs via the stimulation of the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS), as well as their associated increased soluble factors and proinflammatory cytokines (IL-4, IL-10, TGF-β, and VEGF IL-6, IL-8, CXCR, CCL) (7, 28). These changes collectively create a favorable environment for the expansion and accumulation of MDSCs (29).
Surgery-Induced MDSCs Can Augment the PMN (Soil) and Interact With CTCs (Seeds)
The previously most accepted mechanism of metastases formation is CTC being disseminated into the blood during the procedure (30). However, this is controversial since reduced or nearly unaltered CTC counts following complete tumor resection are more often observed (31, 32). Also, some researchers claim that the CTC change is not related to patient prognosis (32). Thus, tumor resection surgery promotes post-surgical metastasis, which is yet to be debated, since surgery itself does not necessarily increase the CTC numbers. Regarding this question, there is evidence showing that MDSCs can enhance the survival and metastatic function of CTCs by soluble factors as well as direct contact (9, 33). This interaction between MDSCs and CTCs is mainly composed of two aspects: direct cell-to-cell interaction and soluble factors. Firstly, MDSCs can protect CTCs in circulation from a hostile environment and facilitate their extravasation through secreting reactive oxygen species (ROS) (34, 35). Furthermore, MDSCs can directly adhere to CTCs in vivo and in vitro, form a CTC/PMN-MDSC complex, and enhance their pro-tumorigenic differentiation (36).
In addition to the interaction with CTCs, which are disseminated during the surgical procedures or discharged into the circulation before, and promote their ability to colonize and survive in the PMN, MDSCs can renovate CTCs' living conditions (PMN) as well. Surgical trauma-inflicted MDSC expansion and host immunity suppression facilitate the development of PMN (37) through releasing various MDSC-derived factors, including TGF-β, VEGF, S100A8/9, HMGB1, MMP9, TIMP-1, Arg-1, ROS, and exosomes. These factors interact as a complex network to fertile the PMN for CTCs regarding many aspects such as the colonization of CTCs, ECM remodeling, inflammation, and immunosuppressive TME (38).
Although the interference of anesthesia could confound the possible mechanisms behind the relation of surgery and post-surgical metastasis, psychological stress, surgical eradication of surrounding nerves, etc. (39–44), we hypothesize that MDSCs inflicted by surgical stress are the key players connecting these complicated mechanisms for post-surgical metastasis. In other words, MDSCs can be perceived as an orchestration of the effects of circulating cancer cells, the suppressed antitumor immunity, and the PMN of the organisms with cancer who undergo surgical resection. Thus, MDSCs should be valued as a potential target for preventing metastases from happening during the perioperative period.
Consequences of the Hypothesis and Discussion
If the extent of surgery-induced immunosuppression manages to counteract the positive effect of primary tumor removal, surgery will fail to meet our expectations to prolong patient survival. These unwanted processes, such as MDSC expansion and its following cascade reactions, should be noted and avoided in the future. Currently, we have several methods to tackle MDSCs in cancer via targeting its expansion, infiltration, migration, activation, differentiation, Arg1 and iNOS induction, and so forth, which is reviewed detailedly in related reviews (45). Nevertheless, this crucial perioperative period is not given enough attention from the pharmacological intervention perspective. According to our hypothetical model, targeting MDSCs is very likely the key to preventing MDSCs induced/related post-surgical recurrence and metastasis.
Future studies are encouraged to first verify the change of MDSCs in various cancer types at a different time (before and after surgery), providing a concentration curve preferably to pinpoint a more accurate window phase for future intervention. The possible existence form and structure of the MDSC-CTC complex should also be measured. In vivo experiments testing whether precisely removing MDSCs can reverse their effects on CTC and PMN and the following prognosis difference is also needed. Also, researchers can use flow cytometry sorting to capture CTCs and co-culture them with MDSCs extracted after emergency surgical stimulation to verify MDSC's impact on CTCs and comparing to the blank control group. Under this circumstance, when the aforementioned tests proved true, we can promisingly move on to the time when surgeons can interrupt tumor progression during the perioperative phase. A schematic diagram of this whole hypothesis is shown in Figure 1.
Limitations
Here we propose a general model to explain what happens in the perioperative period may pre-dispose impacts on the long-time prognosis of the tumor resection procedures, mainly discussing the change and consequences of surgery-induced MDSCs. However, different primary solid tumors are likely to differ in the peripheral responses after surgery slightly, It is still needed to explore further this model in well-designed basic and clinical researches in different cancers.
Conclusions
We hypothesize that surgical resection of the primary tumor can also facilitate the expansion of MDSCs and their pro-tumor role since these surgery-induced MDSCs can prepare the pre-metastatic niche (the “soil”) and at the same time interact with circulating tumor cells (the “seeds”). This vicious, reciprocal mechanism is a crucial point in the emergence of post-surgical metastasis. According to our hypothesis, MDSCs have the potential to be the precise target to prevent cancer patients from post-surgical recurrence and metastasis during the perioperative phase in order to break the wretched cycle and provide better long-term survival for these patients. Future studies are needed to validate this hypothesis.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
SZ wrote this manuscript. YZ created the figure. XM was involved in the idea formation and manuscript revision. YQ contributed to the reviewing and manuscript editing process. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of China (NSFC 81902685).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.783218/full#supplementary-material
References
1. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. (2012) 12:253–68. doi: 10.1038/nri3175
2. Porembka MR, Mitchem JB, Belt BA, Hsieh C-S, Lee H-M, Herndon J, et al. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. (2012) 61:1373–85. doi: 10.1007/s00262-011-1178-0
3. Wang L, Chang EWY, Wong SC, Ong S-M, Chong DQY, Ling KL. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol. (2013) 190:794–804. doi: 10.4049/jimmunol.1202088
4. Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. (2009) 58:49–59. doi: 10.1007/s00262-008-0523-4
5. Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med (Cambridge, Mass). (2011) 17:281–92. doi: 10.2119/molmed.2010.00178
6. Benish M, Ben-Eliyahu S. Surgery as a double-edged sword: a clinically feasible approach to overcome the metastasis-promoting effects of surgery by blunting stress and prostaglandin responses. Cancers (Basel). (2010) 2:1929–51. doi: 10.3390/cancers2041929
7. Wang J, Su X, Yang L, Qiao F, Fang Y, Yu L, et al. The influence of myeloid-derived suppressor cells on angiogenesis and tumor growth after cancer surgery. Int J Cancer. (2016) 138:2688–99. doi: 10.1002/ijc.29998
8. Lu Z, Zou J, Li S, Topper MJ, Tao Y, Zhang H, et al. Epigenetic therapy inhibits metastases by disrupting premetastatic niches. Nature. (2020) 579:284–90. doi: 10.1038/s41586-020-2054-x
9. Trovato R, Canè S, Petrova V, Sartoris S, Ugel S, De Sanctis F. The engagement between MDSCs and metastases: partners in crime. Front Oncol. (2020) 10:165. doi: 10.3389/fonc.2020.00165
10. Bosiljcic M, Cederberg RA, Hamilton MJ, LePard NE, Harbourne BT, Collier JL, et al. Targeting myeloid-derived suppressor cells in combination with primary mammary tumor resection reduces metastatic growth in the lungs. Breast Cancer Res. (2019) 21:103. doi: 10.1186/s13058-019-1189-x
11. Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. (2008) 19:1821–8. doi: 10.1093/annonc/mdn386
12. Murthy SM, Goldschmidt RA, Rao LN, Ammirati M, Buchmann T, Scanlon EF. The influence of surgical trauma on experimental metastasis. Cancer. (1989). 64:2035–44. doi: 10.1002/1097-0142(19891115)64:10<2035::aid-cncr2820641012>3.0.co;2-l
13. van der Bij GJ, Oosterling SJ, Beelen RH, Meijer S, Coffey JC, van Egmond M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg. (2009) 249:727–34. doi: 10.1097/SLA.0b013e3181a3ddbd
14. Uchida A, Kolb R, Micksche M. Generation of suppressor cells for natural killer activity in cancer patients after surgery. J Natl Cancer Inst. (1982) 68:735–41.
15. Arocena AR, Onofrio LI, Pellegrini AV, Carrera Silva AE, Paroli A, Cano RC, et al. Myeloid-derived suppressor cells are key players in the resolution of inflammation during a model of acute infection. Eur J Immunol. (2014) 44:184–94. doi: 10.1002/eji.201343606
16. Schrijver IT, Théroude C, Roger T. Myeloid-derived suppressor cells in sepsis. Front Immunol. (2019) 10:327. doi: 10.3389/fimmu.2019.00327
17. Pyter LM, Suarez-Kelly LP, Carson WE, III, Kaur J, Bellisario J, Bever SR. Novel rodent model of breast cancer survival with persistent anxiety-like behavior and inflammation. Behav Brain Res. (2017) 330:108–17. doi: 10.1016/j.bbr.2017.05.011
18. Ananth AA, Tai LH, Lansdell C, Alkayyal AA, Baxter KE, Angka L, et al. Surgical stress abrogates pre-existing protective T cell mediated anti-tumor immunity leading to postoperative cancer recurrence. PLoS ONE. (2016) 11:e0155947. doi: 10.1371/journal.pone.0155947
19. Li W, Wu K, Zhao E, Shi L, Li R, Zhang P, et al. HMGB1 recruits myeloid derived suppressor cells to promote peritoneal dissemination of colon cancer after resection. Biochem Biophys Res Commun. (2013) 436:156–61. doi: 10.1016/j.bbrc.2013.04.109
20. Ma X, Wang M, Yin T, Zhao Y, Wei X. Myeloid-derived suppressor cells promote metastasis in breast cancer after the stress of operative removal of the primary cancer. Front Oncol. (2019) 9:855. doi: 10.3389/fonc.2019.00855
21. Xu P, He H, Gu Y, Wang Y, Sun Z, Yang L, et al. Surgical trauma contributes to progression of colon cancer by downregulating CXCL4 and recruiting MDSCs. Exp Cell Res. (2018) 370:692–8. doi: 10.1016/j.yexcr.2018.07.035
22. Coffey JC, Wang JH, Smith MJ, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. (2003) 4:760–8. doi: 10.1016/S1470-2045(03)01282-8
23. Da Costa ML, Redmond P, Bouchier-Hayes DJ. The effect of laparotomy and laparoscopy on the establishment of spontaneous tumor metastases. Surgery. (1998) 124:516–25. doi: 10.1016/S0039-6060(98)70098-4
24. Rashid OM, Nagahashi M, Ramachandran S, Graham L, Yamada A, Spiegel S, et al. Resection of the primary tumor improves survival in metastatic breast cancer by reducing overall tumor burden. Surgery. (2013) 153:771–8. doi: 10.1016/j.surg.2013.02.002
25. Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. (2009) 10:44–52. doi: 10.1016/S1470-2045(08)70310-3
26. Gupta A, Watson DI, Ellis T, Jamieson GG. Tumour implantation following laparoscopy using different insufflation gases. ANZ J Surg. (2002) 72:254–7. doi: 10.1046/j.1445-2197.2002.02385.x
27. Brajer-Luftmann B, Nowicka A, Kaczmarek M, Grabicki M, Kuznar-Kamińska B, Bromińska B, et al. Myeloid-derived suppressor cells in bronchoalveolar lavage fluid in patients with chronic obstructive pulmonary disease. Pol Arch Med Wewn. (2016) 126:980–8. doi: 10.20452/pamw.3718
28. Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med. (2018) 16:8. doi: 10.1186/s12967-018-1389-7
29. Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. (2018) 19:108–19. doi: 10.1038/s41590-017-0022-x
30. Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg. (2000) 232:58–65. doi: 10.1097/00000658-200007000-00009
31. Juratli MA, Siegel ER, Nedosekin DA, Sarimollaoglu M, Jamshidi-Parsian A, Cai C, et al. In vivo long-term monitoring of circulating tumor cells fluctuation during medical interventions. PLoS ONE. (2015) 10:e0137613. doi: 10.1371/journal.pone.0137613
32. van Dalum G, van der Stam GJ, Tibbe AGJ, Franken B, Mastboom WJB, Vermes I, et al. Circulating tumor cells before and during follow-up after breast cancer surgery. Int J Oncol. (2015) 46:407–13. doi: 10.3892/ijo.2014.2694
33. Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. (2019) 566:553–7. doi: 10.1038/s41586-019-0915-y
34. Ohl K, Tenbrock K. Reactive oxygen species as regulators of MDSC-mediated immune suppression. Front Immunol. (2018) 9:2499. doi: 10.3389/fimmu.2018.02499
35. Wang L, Leite de Oliveira R, Huijberts S, Bosdriesz E, Pencheva N, Brunen D, et al. An acquired vulnerability of drug-resistant melanoma with therapeutic potential. Cell. (2018) 173:1413–25.e14. doi: 10.1016/j.cell.2018.04.012
36. Sprouse ML, Welte T, Boral D, Liu HN, Yin W, Vishnoi M, et al. PMN-MDSCs enhance CTC metastatic properties through reciprocal interactions via ROS/Notch/Nodal signaling. Int J Mol Sci. (2019) 20:1916. doi: 10.3390/ijms20081916
37. Ceelen W, Pattyn P, Mareel M. Surgery, wound healing, and metastasis: recent insights and clinical implications. Crit Rev Oncol Hematol. (2014) 89:16–26. doi: 10.1016/j.critrevonc.2013.07.008
38. Wang Y, Ding Y, Guo N, Wang S. MDSCs: key criminals of tumor pre-metastatic niche formation. Front Immunol. (2019) 10:172. doi: 10.3389/fimmu.2019.00172
39. Weber M, Moebius P, Büttner-Herold M, Amann K, Preidl R, Neukam FW, et al. Macrophage polarisation changes within the time between diagnostic biopsy and tumour resection in oral squamous cell carcinomas–an immunohistochemical study. Br J Cancer. (2015) 113:510–9. doi: 10.1038/bjc.2015.212
40. Yan T, Zhang G-H, Cheng Y-Z, Wu L-X, Liu X-Y, Sun Y-L, et al. Effects of anesthetic technique and surgery on myeloid-derived suppressor cells and prognosis in women who underwent breast cancer surgery: a prospective study. Cancer Manag Res. (2019) 11:5513–22. doi: 10.2147/CMAR.S183519
41. Mundy-Bosse BL, Thornton LM, Yang H-C, Andersen BL, Carson WE. Psychological stress is associated with altered levels of myeloid-derived suppressor cells in breast cancer patients. Cell Immunol. (2011) 270:80–7. doi: 10.1016/j.cellimm.2011.04.003
42. Koo KC, Park SU, Kim KH, Rha KH, Hong SJ, Yang SC, et al. Prognostic impacts of metastatic site and pain on progression to castrate resistance and mortality in patients with metastatic prostate cancer. Yonsei Med J. (2015) 56:1206–12. doi: 10.3349/ymj.2015.56.5.1206
43. van den Beuken-van Everdingen MHJ, de Graeff A, Jongen JLM, Dijkstra D, Mostovaya I, Vissers KC. Pharmacological treatment of pain in cancer patients: the role of adjuvant analgesics, a systematic review. Pain Pract. (2017) 17:409–19. doi: 10.1111/papr.12459
44. Page GG. Surgery-induced immunosuppression and postoperative pain management. AACN Clin Issues. (2005) 16:302–9. doi: 10.1097/00044067-200507000-00004
Keywords: myeloid-derived suppressor cells, stress, surgery, tumor recurrence, metastasis
Citation: Zhu S, Zhao Y, Quan Y and Ma X (2021) Targeting Myeloid-Derived Suppressor Cells Derived From Surgical Stress: The Key to Prevent Post-surgical Metastasis. Front. Surg. 8:783218. doi: 10.3389/fsurg.2021.783218
Received: 25 September 2021; Accepted: 15 November 2021;
Published: 09 December 2021.
Edited by:
Lucillia Bezu, Gustave Roussy Cancer Campus, FranceReviewed by:
Beatrice Aramini, University Hospital of Modena, ItalyLorenzo Crepaz, Ospedale San Camillo, Italy
Copyright © 2021 Zhu, Zhao, Quan and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuelei Ma, ZHJtYXh1ZWxlaUBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Sha Zhu
Sha Zhu Yunuo Zhao
Yunuo Zhao Yuxin Quan4
Yuxin Quan4 Xuelei Ma
Xuelei Ma