- 1Department of Hand Surgery, China–Japan Union Hospital of Jilin University, Changchun, China
- 2Department of Nursing, China–Japan Union Hospital of Jilin University, Changchun, China
Objectives: The treatment for neurogenic thoracic outlet syndrome (NTOS) conventionally involves first-rib resection (FRR) surgery, which is quite challenging to perform, especially for novices, and is often associated with postoperative complications. Herein, we report a new segmental resection approach through piezo surgery that involves using a bone cutter, which can uniquely provide a soft tissue protective effect.
Methods: This retrospective study involved the examination of 26 NTOS patients who underwent piezo surgery and another group of 30 patients who underwent FRR using the conventional technique. In the patient group that underwent piezo surgery, the rib was first resected into two pieces using a piezoelectric device and subsequently removed. In the patient group that underwent conventional surgery, the first rib was removed as one piece using a rib cutter and rongeurs.
Results: The piezo surgery group had significantly shorter operative time (96.85 ± 14.66 vs. 143.33 ± 25.64 min, P < 0.001) and FRR duration (8.73 ± 2.11 vs. 22.23 ± 6.27 min, P < 0.001) than the conventional group. The posterior stump length of the residual rib was shorter in the piezo surgery group than in the conventional group (0.54 ± 0.19 vs. 0.65 ± 0.15 cm, P < 0.05). There were no significant differences in postoperative complications and scores of the Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire, the Cervical Brachial Symptom Questionnaire (CBSQ), and the visual analog scale (VAS). Even the TOS index (NTOS Index = [DASH + (0.83 × CBSQ) + (10 × VAS)]/3) and patient self-assessments of both the groups showed no significant differences. Univariate analyses indicated that the type of treatment affected operative time.
Conclusion: Our results suggest that piezo surgery is safe, effective, and simple for segmental FRR in NTOS patients. Piezo surgery provides a more thorough FRR without damaging adjacent soft tissues in a relatively short duration and achieves similar functional recovery as conventional techniques. Therefore, piezo surgery can be a promising alternative for FRR during the surgical treatment of NTOS.
Introduction
Neurogenic thoracic outlet syndrome (NTOS) is a compressive neuropathy caused by brachial plexus compression in the thoracic outlet region (1–3). The first rib can often cause the compression of the brachial plexus (4, 5), and its removal is considered effective for thoracic outlet decompression (6).
Previous studies defined first-rib resection (FRR) as the most demanding and potentially dangerous component of TOS surgery (6, 7). A close relationship between the first rib and soft tissue can be associated with soft tissue injury, including pneumothorax (with an incidence of 2.5–10%), lymph leakage (incidence of 2–9.3%), and nerve injury (incidence of 11%) (8–18). Therefore, several methods for the minimal invasive resection of the first rib have been reported including video assisted thoracic surgery (VATS), and robotic-assisted thoracic surgery (19, 20). Zehnder et al. (21) and colleagues have reported a transthoracic video-assisted robotic approach with modified 3-port for first rib resection, brings minimally invasive mode with the fewest number of incisions. In addition, rib resection with a sufficient length is vital for NTOS decompression. The rib should be severed as posteriorly as possible, preferably at the junction with the transverse process, and as anteriorly as possible, ideally at the costochondral junction (3, 22). Removing the first rib in one piece within this range is a unique challenge in decompression procedures for NTOS because during vascular thoracic outlet syndrome decompression, only resection of the anterior portion is required (23, 24). Moreover, the posterior part of the first rib is rather deep, and the brachial plexus should be significantly retracted for adequate exposure of this portion, which can cause intraoperative nerve traction injury. Current instruments used for FRR include the Schumacher bone cutter, the Raney Rongeur, and Kerrison and duckbill rongeurs. These are operated manually and require a large working space; this affects the operational field and increases the risk of soft tissue injury, making the conventional FRR technique more challenging, especially for novices (3, 22, 23). To overcome these issues, we developed a sectional removal technique using an ultrasonic bone cutter, i.e., piezo surgery. Piezo surgery is an innovative technique that implements a specific frequency to cut bone, rather than soft tissue, to avoid damaging the surrounding soft tissues (25, 26). The piezo surgery device has a small operating head that takes up less working space. The improved visualization negates the need for forceful nerve retraction (27–31). We resected the first rib via three cuts. The removal of two smaller pieces is much easier than that of one lengthy piece. Piezo surgery is simple and straightforward even for novices (26, 32).
In this study, we examined NTOS patients who underwent FRR either through this new method or through the conventional technique. We compared the operative time, FRR duration, length of the posterior rib stump, length of hospitalization, complications, and results of physical examinations and scores on functional questionnaires between the two groups. Aim to provide a viable alternative for FRR in terms of safety and convenience.
Materials and Methods
Preoperative Evaluation and Patient Selection
Following ethics approval from the Institutional Review Board of the China–Japan Union Hospital of Jilin University (code 2021-KYLL-060020, date June 2, 2021), we performed a retrospective chart review of patients with NTOS who had undergone FRR between March 1, 2010 and March 1, 2018. The study enrolled 56 patients (59 operations). The requirement for patient consent was waived due to the retrospective nature of the study. This research was conducted in accordance with the principles of the Declaration of Helsinki and its later amendments.
For all patients, surgery (conventional or piezo surgery) was performed after conservative management failed for at least 3 months. Cervical fluoroscopy and computed tomography were performed before surgery. Patients with a wide and vertical first rib were chosen to perform the FRR (33). All patients underwent FRR using a modified supraclavicular approach (34). Patients with a diagnosis of vascular TOS, cervical rib, distal nerve entrapment, cervical disc diseases, frozen shoulder, or neurogenic pectoralis minor syndrome were excluded from this study. Patients with symptoms suggestive of central nervous system diseases that might confound NTOS symptoms were also excluded. Patients who missed follow-up assessments or did not follow the postoperative physical therapy (PT) protocol were also excluded. The detailed descriptions of the NTOS diagnostic criteria implemented in our study are similar to those of previously published studies (34).
Patients were divided into two groups: the piezo surgery group, in which FRR was segmentally resected with piezo surgery, and the conventional instrument group, in which FRR was resected as one piece with a conventional rib cutter and rongeurs. Between March 1, 2010 and December 31, 2014, conventional instruments were used; the piezo surgery technique was introduced and applied from January 1, 2015 to March 1, 2018.
Surgical Technique
General endotracheal anesthesia was induced with the patient in the supine position, following which the patient's neck was extended and turned to the opposite side. A modified supraclavicular incision extending to the deltopectoral groove was made, as previously reported (34). The scalene fat pad was mobilized to expose the anterior scalene muscle, phrenic nerve, brachial plexus nerve roots, and middle scalene muscle. The lateral aspect of the first rib was palpated and visualized, and the long thoracic nerve was identified.
The anterior scalene muscle was divided and removed, the brachial plexus was separated, and complete external neurolysis was performed by removing all fibroinflammatory scar tissue around the nerve roots. With the brachial plexus retracted medially, the middle scalene muscle was partially excised and detached from the posterior surface of the first rib (Figure 1). The first rib evaluation was done with respect to the feature and the direction. Furthermore, the relationship between the first rib and brachial plexus was assessed statically and dynamically on movement of the upper extremity; if the nerve was in contact with the rib, the rib was removed (35). Finally, the wide first rib in vertical direction along with brachial plexus compression were indicated to be removed. A modified periosteal elevator (Figure 2) was used to separate the extra-pleural fascia and attachments of the intercostal muscles around the undersurface of the first rib. This provided a protective edge to help minimize the risk of injury to the neurovascular structures and pleura surrounding the first rib. In the conventional instrument group, the first rib was transected anteriorly near the costochondral junction and posteriorly close to the transverse process in one piece with a rib cutter and rongeurs. In the piezo surgery group, the first rib was transected anteriorly near the costochondral junction medial to the scalene tubercle and then transected in the middle, after which a posterior section was made as close to the transverse process as possible using the piezo surgery medical device (SMTP Technology®, Beijing, China) (Figure 2). The intercostal muscles along the posterolateral aspect of the first rib were divided using electrocautery, and the ribs were removed in sections (Figure 3; Supplementary Video). The rongeurs were occasionally used to trim stumps. Removing the first rib allowed the neurovascular bundle to descend into the pleural space; all its components were then identified. Hemostasis was performed, a suction drain was left in place, and layered closure was performed.

Figure 1. Image of the partial exposure of the first rib. The brachial plexus is retracted medially, and the middle scalene muscle is partially excised and detached to expose the first rib.

Figure 2. Image of the piezoelectric device and modified periosteal elevator used during the removal of the first rib. The piezo surgery medical device with a slender head (represented by an arrowhead) is shown here. The modified periosteal elevator (represented by a white arrow) was used for blunt dissections. This provides a protective edge to minimize injuries to neurovascular structures surrounding the first rib.

Figure 3. An image of removed rib sections. The first rib was transected into two pieces for easier removal. The recombinant first rib segments are shown here.
The same postoperative PT protocol was followed for each patient. Patients with poor compliance with PT, which was defined as completing less than 50% of the total required amount of PT (fewer than 3 days a week or less than one whole set per day) were excluded from the analysis, as previously described (34).
Outcome Measurements
Postoperative cervical and chest fluoroscopy and computed tomography were performed to evaluate the excision of the first rib, as well as diaphragmatic function.
Postoperative outcomes were assessed with respect to operative time, FRR duration, length of the posterior rib stump, length of hospital stay, complications, the Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire, the Cervical Brachial Symptom Questionnaire (CBSQ), the visual analog scale (VAS), TOS index (NTOS Index = [DASH + (0.83 × CBSQ) + (10 × VAS)]/3) and each patient's self-assessment (reported as resolved, markedly improved, fair, or poor, depending on the degree of overall recovery; detailed criteria are presented in the Supplementary Material (Supplementary Table 1).
Statistical Analysis
The Shapiro–Wilk test was initially implemented to confirm the normal distribution of our data. A paired t-test was used to compare differences in group means for paired samples. Normally distributed data are expressed as means ± standard deviations and were compared using independent t-tests. Non-normally distributed data are presented as medians and ranges and compared using the Mann–Whitney U test. Categorical variables were compared using the chi-square test and Fisher's exact test. Univariate and multivariate analyses were conducted to identify factors independently associated with scale scores. The following parameters were examined: age, sex, trauma history before symptom onset, duration of the presence of symptoms prior to surgery, physical examinations, and functional scores. Statistical significance was set at P < 0.05 for all tests. Statistically significant (P < 0.05) or nearly statistically significant (P < 0.10) variables in the univariate analysis were entered into a multivariate analysis using stepwise multiple regression.
Results
Study Population and Pretreatment Characteristics
Among the initial cohort of 385 patients meeting the diagnostic criteria for NTOS, 96 (25%) obtained satisfactory symptom improvement with the initial PT trial and chose to continue conservative management. A total of 289 patients (75%) experienced insufficient improvement with PT and elected to undergo surgery. Among the 289 surgical cases, 63 included a modified supraclavicular approach; cases involving other surgical approaches were excluded from the study. Of the 63 patients, 35 underwent the conventional surgery (forming the conventional group) and the remaining 28 patients underwent surgery via the novel method (forming the piezo surgery group). Two patients from the conventional group and one from the piezo surgery group missed follow-up assessments; hence, they were excluded from the analysis. Further, three more patients from the conventional group and one patient from the piezo surgery group were excluded owing to discrepancies in following the PT protocol. Ultimately, 30 patients (31 surgeries including one bilateral) from the conventional group and 26 patients (28 surgeries including two bilateral) from the piezo surgery group were included in this analysis (Figure 4).

Figure 4. The flow diagram of patient selection of the study. TOS, thoracic outlet syndrome; NTOS, neurogenic thoracic outlet syndrome.
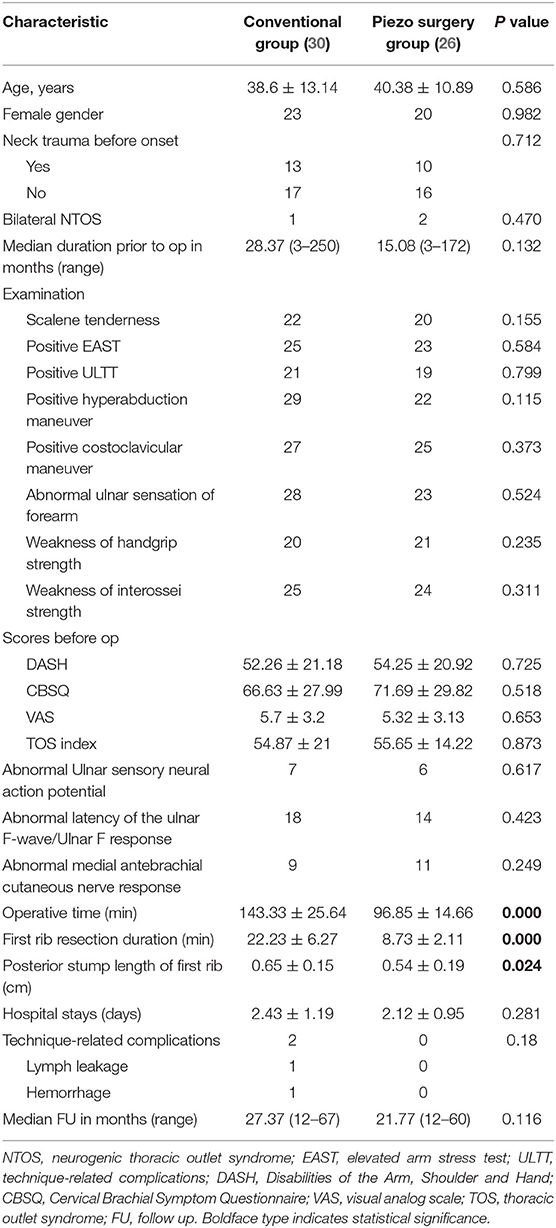
There were no significant differences in the presenting characteristics between the conventional and piezo surgery groups in terms of age, sex, previous injury, symptom duration, physical examination, pretreatment DASH scores, CBSQ scores, VAS scores, TOS index, hospital stay, or postoperative complications (Table 1).
Piezo Surgery and Operation Efficacy
We compared the differences between the two groups in mean operative time, FRR duration, and posterior stump length of the first rib. The mean operative time of the piezo surgery group was 96.85 ± 14.66 min, whereas that of the conventional group was 143.33 ± 25.64 min (P < 0.001). The FRR duration of the piezo surgery group was 8.73 ± 2.11 min, whereas that of the conventional group was 22.23 ± 6.27 min (P < 0.001). The posterior stump length of the first rib was shorter in the piezo surgery group (0.54 ± 0.19 cm) than that in the conventional group (0.65 ± 0.15 cm; P = 0.024) (Table 1; Figure 5). Univariate analyses demonstrated significant correlations between the operative time and the type of treatment (r = 0.818; P < 0.001).

Figure 5. Images of cervical fluoroscopy and a computed tomography scan of the first rib. (A) A representative image of preoperative cervical fluoroscopy of the involved first rib at the T1 level. (B) Postoperative cervical fluoroscopy image showing the bilateral removal of the proximal part of the first ribs (nearly up to their articulation) with transverse processes (represented by dashed rectangles). (C) A flat panel computed tomography 3D image showing a preoperative image of the first rib. (D) The posterior stump of the first rib (with an irregular surface) in the conventional surgery group (represented by a dashed square). (E) The posterior stump of the first rib (with a smooth surface) in the piezo surgery group (represented by a dashed square).
Follow-Up and Treatment Outcomes
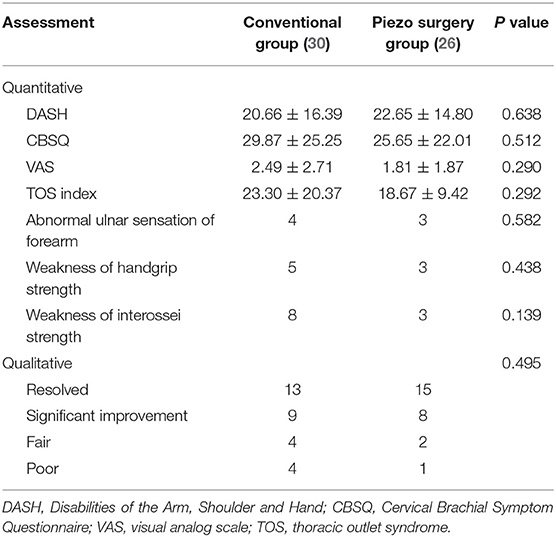
The mean postoperative follow-up period was 27.2 (12–67) months for the conventional group and 24.5 (12–60) months for the piezo surgery group. Although significant improvement was seen in both groups, postoperative DASH scores, CBSQ scores, VAS scores, TOS indices, and patient self-assessments were not significantly different between the two groups. Two patients of the conventional group presented with complications (one with lymph leakage and the other with hemorrhage), both of which were resolved through reoperation. No patients in the piezo surgery group developed technique-related complications (Table 2).
Discussion
In this study, we compared the segmental resection of the first rib through piezo surgery and one-piece resection using a conventional instrument. Our results indicated that piezo surgery is a safe alternative for FRR, with a shorter operative time and sufficient length resection. Patients in the two groups demonstrated similar surgical outcomes, as reflected by functional outcomes and patients' self-assessments; none of the patients in the piezo surgery group presented with technique-related postoperative complications; the operative time and FRR duration were significantly less (8.73 ± 2.11 vs. 22.23 ± 6.27) in the piezo surgery group than in the conventional group. Postoperative radiological examination demonstrated that the posterior stump length of the first rib in the piezo surgery group was shorter than that in the conventional group.
Same as the traditional supraclavicular approach, our modified supraclavicular approach has a relatively good access to the brachial plexus (21). Meanwhile, this modified approach can expose both supra- and infraclavicular areas, allowing us to visualize the subclavius muscle and resect it if it also compresses the brachial plexus in the infraclavicular region (34). However, even with this modified approach, safe exposure of the entire rib is still limited. Besides, intense traction of the neurovascular bundles is often required for adequate exposure in operation. With the help of piezo surgery, first rib can be managed easily in a safe mode with sufficient length.
The most outstanding advantage of piezo surgery is its soft tissue protective effect. Owing to the close relationship between the first rib and soft tissues (8), traditional FRR via the supraclavicular approach is usually associated with soft tissue injury, including pneumothorax (9, 10), neurovascular bundle injury (18, 36), sympathetic chain injury (9), and lymph leakage (11–16). In our study, there were no reports of soft tissue injury in the piezo surgery group. However, in the conventional group, one case of lymph fluid leakage and another of hemorrhage were reported, which required resolution by reoperation. In the patient with lymph fluid leakage, we found a small hole in the branch of the lymphatic vessel and repaired the damaged lymphatic vessel through microsurgery; for the patient with hemorrhage, we found blood oozing from the irregular posterior stump of the first rib and smoothened the fixation of the stump using rongeurs and smeared bone wax on the stump.
Since piezo surgery was first developed in 1988 by Italian oral surgeon Tomaso Vercelloti, it has been widely used in oral and maxillofacial surgery and otolaryngology-head and neck surgery as reported (37–39); moreover, to the authors' knowledge, the piezo surgery was not used previously in FRR. The piezoelectric ultrasound provides bone cutter ultrasonic micro-vibrations of 60–210 μm at 25–30 kHz, which allows for minimally invasive resection of the first rib without damaging soft tissues (28, 29). In addition, the stump of the first rib is relatively smooth, which facilitates the efficacy of smearing bone wax following piezo surgery to prevent bleeding.
Piezo surgery is a simple and user-friendly technique. Previous publications reported that the first rib is usually removed in one piece (6, 7, 23). The bottom of the first rib closely adheres to the pleura and requires long-distance detachment to be performed without direct vision. Segmental resection of the first rib is recommended to remove the first rib easily; however, this is difficult when using conventional instruments (3, 24, 34, 40). With the help of piezo surgery, the rib can be cut thrice into two pieces more easily, clearly, and without generating heat due to the cavitation effect followed by the divisions of the rib. This creates a trap-door configuration at two sites, thereby enlarging the operation field (26, 32, 41, 42). Additionally, the piezo surgery device has a slender head, which can be used in narrow and deep spaces with high dexterity and provides easier access to cut the first rib as close to the costotransverse joint as possible. These advantages reduce the operative time effectively, especially for patients with a wide first rib (43–46).
Because of the high dexterity of the piezo device, the surrounding tissue needs no excessive retraction during FRR. Various reports stated that remnant long posterior stumps of the first rib often led to NTOS recurrence (47–51). Moreover, FRR should be performed as posteriorly as possible (17, 18, 24, 47, 49). In our study, the posterior first-rib stump of patients in the piezo surgery group was dissected as closely as possible to its articulation with the transverse process and without intense nerve retraction, and the average length of the residual stump was shorter than that in the conventional group.
Our results showed that piezo surgery has many prominent advantages, including soft tissue protection, user-friendliness, and high dexterity. Nevertheless, our study also had some limitations. First, this was a nonrandomized retrospective study; thus, our ability to draw causal inferences is limited. Likewise, we were limited by the timeline of the implementation of different methods at our medical center, as piezo surgery was introduced at our institution much later than the conventional technique. All surgeries were done by the same surgeon, which may cause the bias of operative length. To exclude the bias caused by surgical experience, we choose the learning curve plateau period of the surgeon. Furthermore, we compared the FRR duration to exclude the impact of other procedures in the whole surgery. Future studies should aim to compare the conventional method and piezo surgery during the same period as this would help in inherently adjusting for time-dependent potential confounders. An additional limitation is that both groups had a small sample size. A unified operation approach may be necessary to compare the operation time effectively between the two groups.
In conclusion, our results show that piezo surgery is a safe, less damaging to soft tissue, effective, and easy-to-perform technique for FRR in patients with NTOS. Piezo requires a shorter operation duration, provides more thorough FRR without damaging adjacent soft tissues. Therefore, piezo surgery can be a promising alternative for FRR during the surgical treatment of NTOS.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
SC: conceptualization, supervision, funding acquisition, and writing—review and editing. ZZ and XG: methodology. YLi: software and writing—original draft preparation. SC, YLi, YLiu, XG, and ZZ: validation. YLi and YLiu: formal analysis and project administration. ZZ and SC: investigation. XG, ZZ, and SC: resources. YLiu: data curation. ZZ: visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jilin Scientific and Technological Development Program (Grant No. 20160101077JC to SC), the Jilin Scientific and Technological Development Program (Grant No. 20190905003SF to SC), the Jilin Provincial School Joint Construction Special Project (Grant No. SXGJQY2017-13 to SC), the National Natural Science Foundation of China (Grant No. 81671220 to SC), the Industrial Independent Innovation Capability Project of Jilin Development and Reform Commission (Grant No. 2019C005 to SC), and the National Key Research and Development Program of China (Grant No. 2016YFC1101602 to SC). The funding sources played no role in study conception and design, data analysis or interpretation, paper writing, or deciding to submit this paper for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.775403/full#supplementary-material
References
1. Hong J, Pisapia JM, Ali ZS, Heuer AJ, Alexander E, Heuer GG, et al. Zager: Long-term outcomes after surgical treatment of pediatric neurogenic thoracic outlet syndrome. J Neurosurg Pediatr. (2018) 21:54–64. doi: 10.3171/2017.7.Peds17257
2. Thompson RW, Dawkins C, Vemuri C, Mulholland MW, Hadzinsky TD, Pearl JG. Performance metrics in professional baseball pitchers before and after surgical treatment for neurogenic thoracic outlet syndrome. Ann Vasc Surg. (2017) 39:216–227. doi: 10.1016/j.avsg.2016.05.103
3. Sanders RJ, Hammond LS. Supraclavicular first rib resection and total scalenectomy: technique and results. Hand Clin. (2004) 20:61–70.
4. Balderman J, Holzem K, Field BJ, Bottros MM, Abuirqeba AA, Vemuri C, et al. Thompson: associations between clinical diagnostic criteria and pretreatment patient-reported outcomes measures in a prospective observational cohort of patients with neurogenic thoracic outlet syndrome. J Vasc Surg. (2017) 66:533–544. doi: 10.1016/j.jvs.2017.03.419
5. Casey RG, Richards S M. O'Donohoe: Exercise induced critical ischaemia of the upper limb secondary to a cervical rib. Br J Sports Med. (2003) 37:455–6. doi: 10.1136/bjsm.37.5.455
6. DB. Roos: Experience with first rib resection for thoracic outlet syndrome. Ann Surg. (1971) 173:429–42. doi: 10.1097/00000658-197103000-00015
7. Cheng SW, Stoney JR. Supraclavicular reoperation for neurogenic thoracic outlet syndrome. J Vasc Surg. (1994) 19:565–72. doi: 10.1016/s0741-5214(94)70027-3
8. Jones FW. On the relation of the limb plexuses to the ribs and vertebral column. J Anat Physiol. (1910) 44:377–93.
9. Weiss JS, Coletta JM, Hall LD, Murray DJ. Vascular thoracic outlet syndrome. Curr Treat Options Cardiovasc Med. (2002) 4:195–206.
10. Peek J, Vos CG, Unlu C, Schreve MA, van de Mortel RHW, de Vries JPM. Long-term functional outcome of surgical treatment for thoracic outlet syndrome. Diagnostics. (2018) 8:10007. doi: 10.3390/diagnostics8010007
11. Dua A, Rothenberg KA, Gologorsky RC, Deslarzes-Dubuis C, Lee TJ. Long-term quality of life comparison between supraclavicular and infraclavicular rib resection in patients with vTOS. Ann Vasc Surg. (2020) 62:128–32. doi: 10.1016/j.avsg.2019.08.071
12. Melby SJ, Vedantham S, Narra VR, Paletta GA, Khoo-Summers L, Driskill M, et al. Thompson: comprehensive surgical management of the competitive athlete with effort thrombosis of the subclavian vein (Paget-Schroetter syndrome). J Vasc Surg. (2008) 47:809–20. doi: 10.1016/j.jvs.2007.10.057
13. Ohman JW, Abuirqeba AA, Jayarajan SN, Balderman J, Thompson WR. Influence of body weight on surgical treatment for neurogenic thoracic outlet syndrome. Ann Vasc Surg. (2018) 49:80–90. doi: 10.1016/j.avsg.2017.10.031
14. lllig K. “Surgical techniques: operative decompression using the supraclavicular approach for neurogenic thoracic outlet syndrome,” In: Illig KA, et al. editors. Thoracic Outlet Syndrome. London: Springer-Verlag. (2013), 209–216.
15. Sharp WJ, Nowak LR, Zamani T, Kresowik TF, Hoballah JJ, Ballinger BA, et al. Corson: long-term follow-up and patient satisfaction after surgery for thoracic outlet syndrome. Ann Vasc Surg. (2001) 15:32–6. doi: 10.1007/s100160010018
16. Thompson RW, Driskill M. “Thoracic outlet syndrome: neurogenic,” In: Cronenwett JL, Johnston KW, editors Rutherford's Vascular Surgery. 7th ed. Philadelphia, PA: Elsevier; 2010:1878–1898. Abdel Ghany (2016).
17. Mingoli A, Feldhaus RJ, Farina C, Cavallari NP, Sapienza, di Marzo L, et al. Long-term outcome after transaxillary approach for thoracic outlet syndrome. Surgery. (1995) 118:840–4. doi: 10.1016/s0039-6060(05)80274-0
18. Urschel HC, Razzuk MA. The failed operation for thoracic outlet syndrome: the difficulty of diagnosis and management. Ann Thorac Surg. (1986) 42:523–8. doi: 10.1016/s0003-4975(10)60574-7
19. Gharagozloo F, Meyer M, Tempesta B, Strother E, Margolis M R. Neville: proposed pathogenesis of paget-schroetter disease: impingement of the subclavian vein by a congenitally malformed bony tubercle on the first rib. J Clin Pathol. (2012) 65:262–6. doi: 10.1136/jclinpath-2011-200479
20. George RS, Milton R, Chaudhuri N, Kefaloyannis EK. Papagiannopoulos: totally endoscopic (VATS) first rib resection for thoracic outlet syndrome. Ann Thorac Surg. (2017) 103:241–5. doi: 10.1016/j.athoracsur.2016.06.075
21. Zehnder A, Dorn P, Lutz J, Minervini F, Kestenholz P, Gelpke H, et al. Kocher: completely thoracoscopic 3-port robotic first rib resection for thoracic outlet syndrome. Ann Thorac Surg. (2021) 8:53. doi: 10.1016/j.athoracsur.2021.08.053
22. Sanders RJ, Raymer S. The supraclavicular approach to scalenectomy and first rib resection: description of technique. J Vasc Surg. (1985) 2:751–6.
23. Sanders RJ, Annest JS. Technique of supraclavicular decompression for neurogenic thoracic outlet syndrome. J Vasc Surg. (2015) 61:821–5. doi: 10.1016/j.jvs.2014.11.047
24. Roos DB. Transaxillary approach for first rib resection to relieve thoracic outlet syndrome. Ann Surg. (1966) 163: 354–8. doi: 10.1097/00000658-196603000-00005
25. Zhang Q, Chang C, Meng Z, Huang J, Guo J Ge Z. Novel treatment of revision malarplasty with piezosurgery: a case report. Medicine. (2020) 99:e22529. doi: 10.1097/md.0000000000022529
26. Silberman JJ, Moldauer BI, Torres J, Gallardo CD. Palatal root surgery of a maxillary molar using a piezosurgery transantral approach with simultaneous sinus lift grafting: a case report. Int Endod J. (2021) 54:464–475. doi: 10.1111/iej.13423
27. Robiony M, Toro C, Costa F, Sembronio S, Polini F, Politi M. Piezosurgery: a new method for osteotomies in rhinoplasty. J Craniofac Surg. (2007) 18:1098–100. doi: 10.1097/scs.0b013e3180de6489
28. Grauvogel J, Scheiwe C, Masalha W, Jarc N, Grauvogel T, Beringer A. Piezosurgery in modified pterional orbital decompression surgery in graves disease. World Neurosurg. (2017) 106:422–9. doi: 10.1016/j.wneu.2017.06.180
29. Labanca M, Azzola F, Vinci R, Rodella FL. Piezoelectric surgery: twenty years of use. Br J Oral Maxillofac Surg. (2008) 46:265–9. doi: 10.1016/j.bjoms.2007.12.007
30. Kocak I, Dogan R, Gokler O. A comparison of piezosurgery with conventional techniques for internal osteotomy. Eur Arch Otorhinolaryngol. (2017) 274:2483–91. doi: 10.1007/s00405-017-4514-y
31. Gerbault O, Daniel RK, Kosins MA. The role of piezoelectric instrumentation in rhinoplasty surgery. Aesthet Surg J. (2016) 36:21–34. doi: 10.1093/asj/sjv167
32. Abella F, de Ribot J, Doria G, Duran-Sindreu F, Roig M. Applications of piezoelectric surgery in endodontic surgery: a literature review. J Endod. (2014) 40:325–32. doi: 10.1016/j.joen.2013.11.014
33. Sanders RJ. “Thoracic outlet syndrome: general considerations,: In: Cronenwett JL, Johnston KW, editors. Rutherford's vascular surgery. 7th ed. Philadelphia: Saunders, p. 1867 (2010).
34. Liu Y, Zhang Z, Wang J, Wu G, Yu W, Cui S. Improved functional outcome in NTOS patients following resection of the subclavius muscle with radiological signs of nerve impingement: indication of participation of the subclavius in brachial plexus compression. J Neurosurg. (2018) 18:1–11. doi: 10.3171/2018.5.Jns18429
35. GT. Martin: First rib resection for the thoracic outlet syndrome. Br J Neurosurg. (1993) 7:35–8. doi: 10.3109/02688699308995053
36. Archie MM, Rollo JC, Gelabert AH. Surgical missteps in the management of venous thoracic outlet syndrome which lead to reoperation. Ann Vasc Surg. (2018) 49:261–7. doi: 10.1016/j.avsg.2018.01.067
37. Kirpalani TH. Dym: role of piezo surgery and lasers in the oral surgery office. Dent Clin North Am. (2020) 64:351–63. doi: 10.1016/j.cden.2019.12.007
38. Puttasiddaiah PM, Browning TS. Removal of external ear canal exostoses by piezo surgery: a novel technique. J Laryngol Otol. (2018) 132:840–1. doi: 10.1017/s0022215118001263
39. Robiony M, Lazzarotto A, Nocini R, Costa F, Sembronio S, Franz L. Piezosurgery: ten years' experience of percutaneous osteotomies in rhinoplasty. J Oral Maxillofac Surg. (2019) 77:1237–44. doi: 10.1016/j.joms.2019.01.035
40. Jing X, Zhou Y, Ding J, Wang Y, Qin Z, Wang Y. The learning curve for thermal ablation of liver cancers: 4,363-session experience for a single central in 18 years. Front Oncol. (2020) 10:540239. doi: 10.3389/fonc.2020.540239
41. Crosetti E, Battiston B, Succo G. Piezosurgery in head and neck oncological and reconstructive surgery: personal experience on 127 cases. Acta Otorhinolaryngol Ital. (2009) 29:1–9.
42. Otake Y, Nakamura M, Henmi A, Takahashi T, Sasano Y. Experimental comparison of the performance of cutting bone and soft tissue between piezosurgery and conventional rotary instruments. Sci Rep. (2018) 8:17154. doi: 10.1038/s41598-018-35295-6
43. Cerfolio RJ, Smood B, Ghanim A, Townsley MM, Downing M. Decreasing time to place and teach double-lumen endotracheal intubation: engaging anesthesia in lean. Ann Thorac Surg. (2018) 106:1512–8. doi: 10.1016/j.athoracsur.2018.06.023
44. Cerfolio RJ, Steenwyk BL, Watson C, Sparrow J, Belopolsky V, Townsley M, et al. Gayeski: decreasing the preincision time for pulmonary lobectomy: the process of lean and value stream mapping. Ann Thorac Surg. (2016) 101:1110–5. doi: 10.1016/j.athoracsur.2015.09.004
45. Iwasaki Y, Shimada J, Kato D, Nishimura M, Ito K, Terauchi K, et al. Improvements in thoracic surgery outcomes: a multi-institutional collaboration study. J Cardiothoracic Surg. (2015) 10:30. doi: 10.1186/s13019-015-0228-7
46. Hoefsmit PC, Cerfolio RJ, de Vries R, Dahele M, Zandbergen RH. Systematic review of interventions to reduce operating time in lung cancer surgery. Clin Med Insights Oncol. (2021) 15:1179554920987105. doi: 10.1177/1179554920987105
47. Geven LI, Smit AJ, Ebels T. Vascular thoracic outlet syndrome. Longer posterior rib stump causes poor outcome. Eur J Cardiothorac Surg. (2006) 30:232–6. doi: 10.1016/j.ejcts.2006.04.018
48. Thompson JF, Webster HJ. First rib resection for vascular complications of thoracic outlet syndrome. Br J Surg. (1990) 77:555–7. doi: 10.1002/bjs.1800770529
49. Gelabert HA, Jabori S, Barleben A, Kiang S, O'Connell J, Jimenez JC, et al. Regrown first rib in patients with recurrent thoracic outlet syndrome. Ann Vasc Surg. (2014) 28:933–8. doi: 10.1016/j.avsg.2014.01.004
50. Sanders RJ, Haug CE, Pearce HW. Recurrent thoracic outlet syndrome. J Vasc Surg. (1990) 12:390–8.
Keywords: neurogenic thoracic outlet syndrome, piezo surgery, rib resection, complications, Disabilities of the Arm, Shoulder and Hand (DASH)
Citation: Li Y, Liu Y, Zhang Z, Gao X and Cui S (2021) A Novel Approach to First-Rib Resection in Neurogenic Thoracic Outlet Syndrome. Front. Surg. 8:775403. doi: 10.3389/fsurg.2021.775403
Received: 15 September 2021; Accepted: 26 October 2021;
Published: 12 November 2021.
Edited by:
Luca Bertolaccini, European Institute of Oncology (IEO), ItalyReviewed by:
Jon Andri Lutz, Fribourg Cantonal Hospital, SwitzerlandDania Nachira, Catholic University of the Sacred Heart, Italy
Francesco Zaraca, Ospedale di Bolzano, Italy
Antonio Mazzella, European Institute of Oncology (IEO), Italy
Copyright © 2021 Li, Liu, Zhang, Gao and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shusen Cui, Y3Vpc3NAamx1LmVkdS5jbg==
†These authors have contributed equally to this work
 Yueying Li1†
Yueying Li1† Zhan Zhang
Zhan Zhang Shusen Cui
Shusen Cui