
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 31 January 2022
Sec. Neurosurgery
Volume 8 - 2021 | https://doi.org/10.3389/fsurg.2021.761166
Background: Acute kidney injury (AKI) is a common complication in the clinical practice of managing patients with traumatic brain injury (TBI). Avoiding the development of AKI is beneficial for the prognosis of patients with TBI. We designed this study to testify whether serum lactate could be used as a predictive marker of AKI in patients with TBI.
Materials and Methods: In total, 243 patients with TBI admitted to our hospital were included in this study. Univariate and multivariate logistic regression analyses were utilized to analyze the association between lactate and AKI. The receiver operating characteristic (ROC) curves were drawn to verify the predictive value of lactate and the logistic model.
Results: Acute kidney injury group had higher age (p = 0.016), serum creatinine (p < 0.001), lactate (p < 0.001), and lower Glasgow Coma Scale (GCS; p = 0.021) than non-AKI group. Multivariate logistic regression showed that age [odds ratio (OR) = 1.026, p = 0.022], serum creatinine (OR = 1.020, p = 0.010), lactate (OR = 1.227, p = 0.031), fresh frozen plasma (FFP) transfusion (OR = 2.421, p = 0.045), and platelet transfusion (OR = 5.502, p = 0.044) were risk factors of AKI in patients with TBI. The area under the ROC curve (AUC) values of single lactate and predictive model were 0.740 and 0.807, respectively.
Conclusion: Serum lactate level in the early phase is associated with AKI in patients with TBI. Lactate is valuable for clinicians to evaluate the probability of AKI in patients with TBI.
Traumatic brain injury (TBI) is a public health issue, which has been paid widespread attention for the high incidence of mortality and disability. In addition to the initial brain injury severity, complications of the extracranial organ after injury also play a significant role in the poor prognosis of patients with TBI (1, 2). Among those organ dysfunctions, acute kidney injury (AKI) has received much attention for the high prevalence and close correlation with prognosis in patients with TBI. There are still no effective pharmaceuticals to treat and alleviate the unfavorable effects of AKI. Therefore, discovering risk factors of AKI in patients with TBI in the early phase and evaluating the possibility of developing AKI among them are essential to avoid the occurrence of AKI by reducing the usage of nephrotoxic drugs and adjusting treatment strategies. Recently, some research studies explored the value of neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and insulin-like growth factor-binding protein-7 (IGFBP-7) in evaluating the risk of AKI and even diagnosing AKI among critically ill patients, such as TBI (3–10). However, the results of these studies showed different values of these markers in predicting AKI. Additionally, high costs and no clear clinical interpretation of these markers make them still unable to be conveniently used in current clinical practice. While discovering some readily available markers and utilizing them to assess the risk of AKI are more available and practical for clinicians under conditions of limited resources.
As a commonly and conveniently used marker, the serum lactate level, which is usually considered as the reflect of tissue hypoperfusion, has been verified associated with mortality in many kinds of patients in previous studies (11–18). However, only a few studies confirmed the value of serum lactate in predicting AKI among specific patients (19–24). This study explores whether serum lactate level in the early phase after TBI is associated with AKI and verifies its ability for predicting AKI.
Patients admitted to the emergency department of West China hospital for TBI between September 2016 and June 2019 were eligible for this study. The diagnosis of TBI was confirmed by injury signs of CT or MRI of the head region after admission. Patients were excluded based on the following criteria: (1) patients transferred from other hospitals after suffering injury; (2) patients admitted to our hospital 6 h after suffering from injury; (3) patients lacked in relevant clinical information; (4) patients had developed AKI during the first day after admission. The flowchart of inclusion is shown in Figure 1. In total, 243 patients were finally included in this study. This study was approved by the ethics committee of West China hospital and performed abiding by the Declaration of Helsinki. Informed consent forms about joining the observational study of each patient were routinely signed by patients themselves or their legally authorized representatives in our hospital.
Demographic information and clinical information, such as injury mechanism, vital signs on admission, Glasgow Coma Scale (GCS) on admission, injury severity score (ISS), laboratory tests, radiologic signs surgical options, records of transfusion during the first 24 h after admission, and abbreviated injury score (AIS) of head, chest, abdomen, and limbs, were collected. Results of laboratory tests were acquired by analyzing the first blood sample collected once patients were admitted to the emergency department of our hospital. The outcome of this study was the development of AKI on the second day after admission. Moreover, AKI was diagnosed according to the Kidney Disease Improving Global Outcome (KDIGO) criteria.
The normality of included variables was testified using the Kolmogorov-Smirnov test. Normally distributed and non-normally distributed variables were presented as mean ± SD and median (interquartile range), respectively. Categorical variables were shown in the form of numbers (percentage). We performed Independent Student's t-test and the Mann-Whitney U test, respectively, to analyze the difference between two groups of normally distributed and non-normally distributed variables. A χ2 test or Fisher test was utilized to compare the difference of categorical variables. Univariate logistic regression analysis was firstly performed to explore potential risk factors of AKI. Then, significant factors in univariate logistic regression with p < 0.05 were included in multivariate logistic regression analysis with the stepwise forward method. Finally, factors still significant in multivariate analysis were incorporated to construct a logistic model to predict the development of AKI. Nomogram was drawn to visually show the predictive model. The receiver operating characteristic (ROC) curves were drawn, and the area under the ROC curve (AUC) was calculated to assess the predictive value of single lactate and the logistic model.
A two-sided value of p < 0.05 was considered to be statistically significant. SPSS 22.0 Windows software (SPSS, Inc., Chicago, IL, USA) and R (version 3.6.1; R Foundation) were used for all statistical analyses.
Among overall included patients, 34 developed AKI with an incidence of 14.0% (Table 1). In the AKI group, the median of time from admission to AKI and AKI stage was 2 days and stage 1. AKI group had higher age (49 vs. 40, p = 0.016), serum creatinine (90 vs. 66, p < 0.001), lactate (3.9 vs. 2.1, p < 0.001), and lower GCS (5 vs. 6, p = 0.021) than non-AKI group. Compared with non-AKI group, AKI group was more likely to receive fresh frozen plasma (FFP) transfusion (55.9% vs. 23.4%, p < 0.001) and platelet transfusion (11.8% vs. 1.4%, p = 0.008). The mortality of the AKI group was significantly higher than that of the non-AKI group; while the length of ICU stay (4 vs. 12, p = 0.027) and the length of hospital stay (8 vs. 21, p = 0.001) were significantly shorter in the AKI group.
Results of univariate logistic regression showed that age (p = 0.008), GCS (p = 0.045), ISS (p = 0.043), serum creatinine (p < 0.001), lactate (p < 0.001), FFP transfusion (p < 0.001), and platelet transfusion (p = 0.005) were associated with AKI in patients with TBI (Table 2). After adjusting confounding effects, multivariate logistic regression presented that only age [odds ratio (OR) = 1.026, p = 0.022], serum creatinine (OR = 1.020, p = 0.010), lactate (OR = 1.227, p = 0.031), fresh frozen plasma transfusion (OR = 2.421, p = 0.045), and platelet transfusion (OR = 5.502, p = 0.044) were independent risk factors of AKI in included patients with TBI.
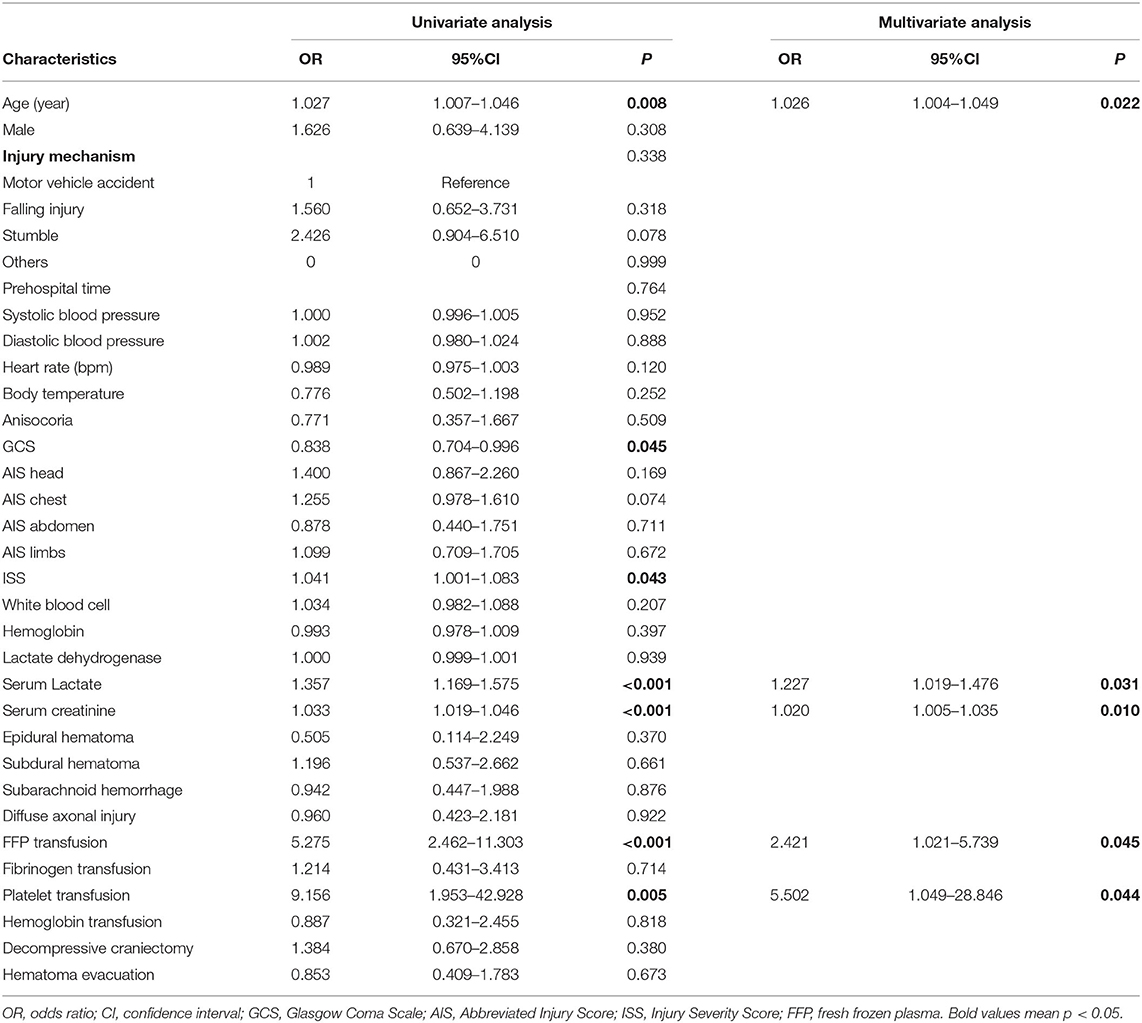
Table 2. Univariate and multivariate logistic regression analyses for risk factors of AKI in included TBI patients.
Five factors significant in multivariate logistic regression that include age, serum creatinine, lactate, fresh frozen plasma transfusion, and platelet transfusion were incorporated to construct a logistic model to predict the development of AKI in patients with TBI. For convenient clinical use, a visual nomogram of this model is drawn and is presented in Figure 2. Additionally, the AUC values of single lactate and predictive model were 0.740 and 0.807, respectively (Table 3). The ROC curves of single lactate and predictive model are presented as Figure 3.
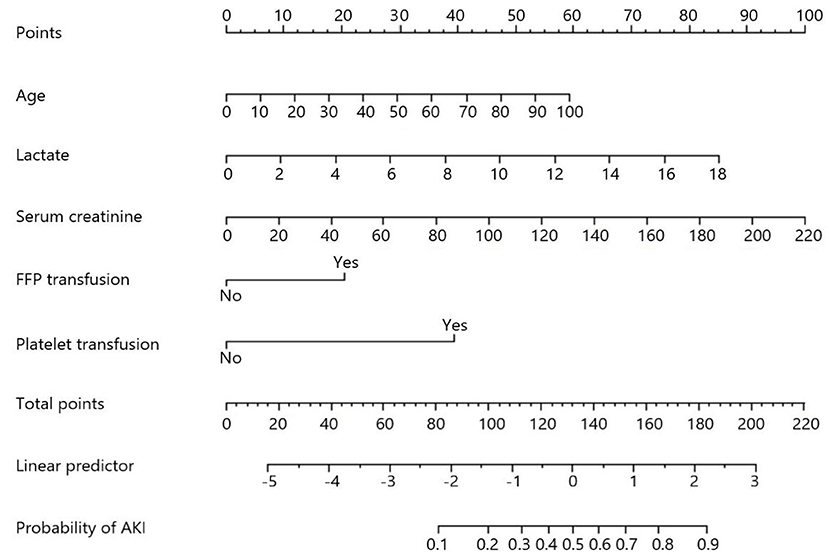
Figure 2. Nomogram of constructed a model for predicting acute kidney injury in included in patients with TBI.
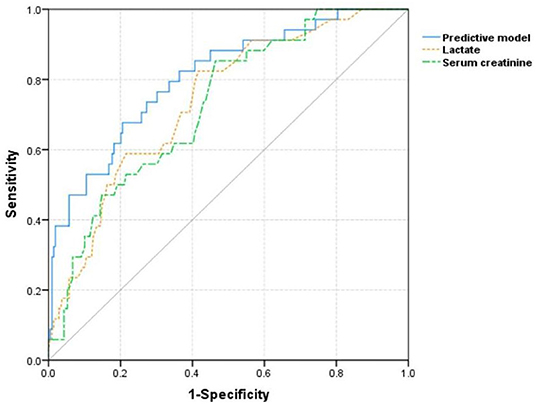
Figure 3. The receiver operating characteristic curves of single lactate and constructed model for predicting acute kidney injury in included patients with TBI.
Acute kidney injury is a common complication during the clinical management of TBI patients with incidence ranging from 7.6 to 24% (10, 25–28). In our study, 15 patients had developed AKI during the first day after admission and were consequently excluded from this study for no much need to predict the AKI development. In addition to these 15 patients, 34 patients developed AKI 1 day after admission with the incidence of 14.0%. Results of our study showed that age, serum creatinine, serum lactate, transfusion of FFP, and platelet were risk factors of AKI in included patients with TBI.
Widely recognized as a biomarker of tissue hypoperfusion, the serum lactate would increase in various kinds of critically ill patients. Actually, many studies have indicated the increased level of serum lactate after suffering from TBI and explored the correlation between increased lactate and prognosis of patients with TBI (29–31). The increased level of serum lactate after TBI may mainly be attributable to the systemic tissue hypoperfusion caused by massive blood loss and impaired cardiopulmonary function. Massive blood loss could lead to a decreased level of hemoglobin, which means reduced oxygen-carrying capacity and subsequent systemic hypoperfusion in patients with TBI, though the threshold of red blood cell transfusion specific for patients with TBI has not reached a consensus. Complications of the respiratory and cardiovascular system after an injury, such as neurogenic pulmonary edema, acute lung injury, and neurogenic myocardial dysfunction, are prevalent in patients with TBI. One previous study reported that the incidence of cardiovascular and respiratory failure reached up to 56 and 43% in severe patients with TBI, respectively (32). These cardiopulmonary dysfunctions could lead to the inadequate gas exchange and unstable cardiac output which may subsequently cause insufficient oxygen supplement in systemic organs and increased anaerobic metabolism. Additionally, the activated renin angiotensin system (RAS) and increased oxidative stress activity in systemic organs after TBI may also result in renal tissue hypoxia through vasoconstriction induced decreased perfusion and increasing oxygen consumption caused by hyperactive mitochondrial function (33–35). The hypoxia has been verified associated with the development of AKI in clinical research studies (36, 37). Moreover, animal experiments also indicated renal hypoxia played a pivotal role in the pathophysiological process of AKI and progression from AKI to chronic kidney disease (38, 39). In summary, hypoperfusion and ischemia of systemic tissues reflected by increased lactate level after TBI could result in the development of an impaired renal function.
In addition to serum lactate, age, initial serum creatinine, FFP transfusion, and platelet transfusion were also confirmed as risk factors of AKI. Some previous studies also confirmed that older age was positively associated with AKI (40, 41). The relation between increased age and AKI development in patients with TBI may be attributable to physiological changes in renal function and multiple comorbidities with age. In some studies, transfusion of FFP or platelet was confirmed as independent risk factor of AKI (42–46). Massive FFP transfusion may aggravate the impaired renal function through activating inflammatory and immunologic reactions (47, 48). The detailed mechanism of platelet transfusion contributing to AKI development has not been fully understood. Some research studies found platelet concentrates contained bioactive CD40 ligands, which could promote the pro-inflammatory markers release (49, 50). Therefore, platelet transfusion may impair renal function by promoting an unfavorable inflammatory state. Another research indicated platelet transfusion could result in the formation of microthrombi existing in the renal microvascular circulation (50). Combining abovementioned five factors, our constructed logistic model was more efficient in predicting AKI with a higher AUC value than single lactate.
This study had several limitations. Firstly, this study was conducted in a single medical center with a relatively inadequate sample size, so that selection bias may not be avoided. The predictive value of our constructed logistic model should be verified in other medical centers. Secondly, the detailed doses of blood products transfusion were not recorded so that we could not accurately analyze the dose relationship between transfusion and AKI. Thirdly, a history of underlying diseases was not included because of the rare incidence of comorbidities in our included patients. Future research studies could be conducted to adjust the confounding effects of comorbidities and investigate whether incorporating comorbidities into our model could improve predictive stability and accuracy. Finally, the serum lactate level was only recorded from analyzing the first blood sample collected on admission. Therefore, we could not analyze the association between AKI and the continuous and average level of serum lactate during a specific time period. Future studies collecting multiple measurements of lactate level within a specific duration are worthy to be designed and conducted to achieve this aim.
Higher serum lactate level in the early phase is positively associated with the development of AKI in patients with TBI. Measuring lactate is beneficial for clinicians to evaluate the probability of AKI occurrence in patients with TBI.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of West China Hospital, Sichuan University. Written informed consent to participate in this study was provided by the participants or their legal guardian/next of kin.
RW: contributed to the manuscript conception, design, and writing. MH and JZ: contributed to the collection and assembly of data. RW and SW: contributed to the data analysis and interpretation. JX: contributed to the manuscript proofreading and revision. All the authors contributed to the approval of the final manuscript and its publication.
This work was supported by the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18007) and Key Research and Development project of the Science and Technology Department of Sichuan Province (2019YFS0392).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hanna K, Hamidi M, Vartanyan P, Henry M, Castanon L, Tang A, et al. Non-neurologic organ dysfunction plays a major role in predicting outcomes in pediatric traumatic brain injury. J Pediatr Surg. (2020). doi: 10.1016/j.jpedsurg.2020.01.051
2. Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ. Non-neurologic organ dysfunction in severe traumatic brain injury. Crit Care Med. (2005) 3:654–60. doi: 10.1097/01.CCM.0000155911.01844.54
3. Wen Y, Parikh CR. Current concepts and advances in biomarkers of acute kidney injury. Crit Rev Clin Lab Sci. (2021) 58:1-24. doi: 10.1080/10408363.2021.1879000
4. Kulvichit W, Kellum JA, Srisawat N. Biomarkers in acute kidney injury. Crit Care Clin. (2021) 2:385–98. doi: 10.1016/j.ccc.2020.11.012
5. Wajda J, Dumnicka P, Kolber W, Sporek M, Maziarz B, Ceranowicz P, et al. The marker of tubular injury, kidney injury molecule-1 (KIM-1), in acute kidney injury complicating acute pancreatitis: a preliminary study. J Clin Med. 2020 9:5. doi: 10.3390/jcm9051463
6. Vogel MJ, Mustroph J, Staudner ST, Leininger SB, Hubauer U, Wallner S, et al. Kidney injury molecule-1: potential biomarker of acute kidney injury and disease severity in patients with COVID-19. J Nephrol. (2021) 34:1007–18. doi: 10.1007/s40620-021-01079-x
7. Waskowski J, Pfortmueller CA, Schenk N, Buehlmann R, Schmidli J, Erdoes G, et al. (TIMP2) x (IGFBP7) as early renal biomarker for the prediction of acute kidney injury in aortic surgery (TIGER). A single center observational study. PloS ONE. (2021) 1:e0244658. doi: 10.1371/journal.pone.0244658
8. Hatton GE, Wang YW, Isbell KD, Finkel KW, Kao LS, Wade CE. Urinary cell cycle arrest proteins urinary tissue inhibitor of metalloprotease 2 and insulin-like growth factor binding protein 7 predict acute kidney injury after severe trauma: a prospective observational study. J Trauma Acute Care Surg. (2020) 4:761–7. doi: 10.1097/TA.0000000000002864
9. Gocze I, Koch M, Renner P, Zeman F, Graf BM, Dahlke MH, et al. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS ONE. (2015) 3:e0120863. doi: 10.1371/journal.pone.0120863
10. Li N, Zhao WG, Xu FL, Zhang WF, Gu WT. Neutrophil gelatinase-associated lipocalin as an early marker of acute kidney injury in patients with traumatic brain injury. J Nephrol. (2013) 6:1083–8. doi: 10.5301/jn.5000282
11. Ryoo SM, Lee J, Lee Y-S, Lee JH, Lim KS, Huh JW, et al. Lactate level versus lactate clearance for predicting mortality in patients with septic shock defined by sepsis-3. Crit Care Med. (2018) 6:e489–e95. doi: 10.1097/CCM.0000000000003030
12. Saad S, Mohamed N, Moghazy A, Ellabban G, El-Kamash S. Venous glucose, serum lactate and base deficit as biochemical predictors of mortality in patients with polytrauma. Turk J Trauma Emer Surg. (2016) 1:29–33. doi: 10.5505/tjtes.2015.96832
13. Haas SA, Lange T, Saugel B, Petzoldt M, Fuhrmann V, Metschke M, et al. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med. (2016) 2:202–10. doi: 10.1007/s00134-015-4127-0
14. Gwak MH, Jo S, Jeong T, Lee JB, Jin YH, Yoon J, et al. Initial serum lactate level is associated with inpatient mortality in patients with community-acquired pneumonia. Am J Emerg Med. (2015) 5:685–90. doi: 10.1016/j.ajem.2015.03.002
15. Bai Z, Zhu X, Li M, Hua J, Li Y, Pan J, et al. Effectiveness of predicting in-hospital mortality in critically ill children by assessing blood lactate levels at admission. BMC Pediatr. (2014) 14:83. doi: 10.1186/1471-2431-14-83
16. Sammour T, Kahokehr A, Caldwell S, Hill AG. Venous glucose and arterial lactate as biochemical predictors of mortality in clinically severely injured trauma patients–a comparison with ISS and TRISS. Injury. (2009) 1:104–8. doi: 10.1016/j.injury.2008.07.032
17. Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. (2009) 5:1670–7. doi: 10.1097/CCM.0b013e31819fcf68
18. Jansen TC, van Bommel J, Woodward R, Mulder PGH, Bakker J. Association between blood lactate levels, Sequential Organ Failure Assessment subscores, and 28-day mortality during early and late intensive care unit stay: a retrospective observational study. Crit Care Med. (2009) 8:2369–74. doi: 10.1097/CCM.0b013e3181a0f919
19. Yan G, Wang D, Tang C, Ma G. The association of serum lactate level with the occurrence of contrast-induced acute kidney injury and long-term prognosis in patients undergoing emergency percutaneous coronary intervention. Int J Gen Med. (2021) 14:3087–97. doi: 10.2147/IJGM.S316036
20. Nasu T, Ueda K, Kawashima S, Okishio Y, Kunitatsu K, Iwasaki Y, et al. Prehospital blood pressure and lactate are early predictors of acute kidney injury after trauma. J Surg Res. (2021) 265:180–6. doi: 10.1016/j.jss.2021.03.037
21. Radovic M, Bojic S, Kotur-Stevuljevic J, Lezaic V, Milicic B, Velinovic M, et al. Serum lactate as reliable biomarker of acute kidney injury in low-risk cardiac surgery patients. J Med Biochem. (2019) 2:118–25. doi: 10.2478/jomb-2018-0018
22. Hsu YC, Hsu CW. Septic acute kidney injury patients in emergency department: The risk factors and its correlation to serum lactate. Am J Emerg Med. (2019) 2:204–8. doi: 10.1016/j.ajem.2018.05.012
23. Zhang Z, Ni H. Normalized lactate load is associated with development of acute kidney injury in patients who underwent cardiopulmonary bypass surgery. PLoS ONE. (2015) 3:e0120466. doi: 10.1371/journal.pone.0120466
24. Silva Junior GB, Daher EF, Barreto AG, Pereira ED. Acute kidney injury after liver transplantation is associated with viral hepatitis, prolonged warm ischemia, serum lactate and higher mortality. Ann Hepatol. (2015) 6:939–40. doi: 10.5604/16652681.1171790
25. Corral L, Javierre CF, Ventura JL, Marcos P, Herrero JI, Manez R. Impact of non-neurological complications in severe traumatic brain injury outcome. Critical Care. (2012) 2:R44. doi: 10.1186/cc11243
26. Ahmed M, Sriganesh K, Vinay B, Umamaheswara Rao GS. Acute kidney injury in survivors of surgery for severe traumatic brain injury: Incidence, risk factors, and outcome from a tertiary neuroscience center in India. Br J Neurosurg. (2015) 4:544–8. doi: 10.3109/02688697.2015.1016892
27. Robba C, Banzato E, Rebora P, Iaquaniello C, Huang CY, Wiegers EJA, et al. Acute Kidney Injury in Traumatic Brain Injury Patients: Results From the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury Study. Crit Care Med. (2021) 1:112–26. doi: 10.1097/CCM.0000000000004673
28. Moore EM, Bellomo R, Nichol A, Harley N, Macisaac C, Cooper DJ. The incidence of acute kidney injury in patients with traumatic brain injury. Ren Fail. (2010) 9:1060–5. doi: 10.3109/0886022X.2010.510234
29. Fu Y-Q, Bai K, Liu C-J. The impact of admission serum lactate on children with moderate to severe traumatic brain injury. PLoS One. (2019) 9:e0222591. doi: 10.1371/journal.pone.0222591
30. Dübendorfer C, Billeter AT, Seifert B, Keel M, Turina M. Serial lactate and admission SOFA scores in trauma: an analysis of predictive value in 724 patients with and without traumatic brain injury. Eur J Trauma Emerg Surg. (2013) 1:25–34. doi: 10.1007/s00068-012-0212-z
31. Svedung Wettervik T, Engquist H, Howells T, Rostami E, Hillered L, Enblad P, et al. Arterial lactate in traumatic brain injury - Relation to intracranial pressure dynamics, cerebral energy metabolism and clinical outcome. J Crit Care. (2020) 60:218–25. doi: 10.1016/j.jcrc.2020.08.014
32. Zygun D, Berthiaume L, Laupland K, Kortbeek J, Doig C. SOFA is superior to MOD score for the determination of non-neurologic organ dysfunction in patients with severe traumatic brain injury: a cohort study. Critical Care. (2006) 4:R115. doi: 10.1186/cc5007
33. Palm F, Nordquist L. Renal tubulointerstitial hypoxia: cause and consequence of kidney dysfunction. Clin Exp Pharmacol Physiol. (2011) 7:474–80. doi: 10.1111/j.1440-1681.2011.05532.x
34. Sharma N, Anders HJ, Gaikwad AB. Fiend and friend in the renin angiotensin system: An insight on acute kidney injury. Biomed Pharmacother. (2019) 110:764–74. doi: 10.1016/j.biopha.2018.12.018
35. Nangaku M, Fujita T. Activation of the renin-angiotensin system and chronic hypoxia of the kidney. Hypertens Res. (2008) 2:175–84. doi: 10.1291/hypres.31.175
36. Knight J, Hill A, Melnyk V, Doney L, D'Cunha J, Kenkre T, et al. Intraoperative Hypoxia Independently Associated With the Development of Acute Kidney Injury Following Bilateral Orthotopic Lung Transplantation. Transplantation. (2021). doi: 10.1097/TP.0000000000003814
37. Zhu MZL, Martin A, Cochrane AD, Smith JA, Thrift AG, Harrop GK, et al. Urinary hypoxia: an intraoperative marker of risk of cardiac surgery-associated acute kidney injury. Nephrol Dial Transplant. (2018) 12:2191–201. doi: 10.1093/ndt/gfy047
38. Chihanga T, Ruby HN, Ma Q, Bashir S, Devarajan P, Kennedy MA. NMR-based urine metabolic profiling and immunohistochemistry analysis of nephron changes in a mouse model of hypoxia-induced acute kidney injury. Am J Physiol Renal Physiol. (2018) 315:F1159-f73. doi: 10.1152/ajprenal.00500.2017
39. Ullah MM, Basile DP. Role of renal hypoxia in the progression from acute kidney injury to chronic kidney disease. Semin Nephrol. (2019) 6:567–80. doi: 10.1016/j.semnephrol.2019.10.006
40. Chen D, Yuan H, Cao C, Liu Z, Jiang L, Tan Y, et al. Impact of acute kidney injury on in-hospital outcomes in Chinese patients with community acquired pneumonia. BMC Pulm Med. (2021) 1:143. doi: 10.1186/s12890-021-01511-9
41. Chebotareva N, Berns S, Berns A, Androsova T, Lebedeva M, Moiseev S. Acute kidney injury and mortality in coronavirus disease 2019: results from a cohort study of 1,280 patients. Kidney Res Clin Pract. (2021) 2:241–9. doi: 10.23876/j.krcp.20.128
42. Tong J, Cao L, Liu L, Jin M. Impact of autologous platelet rich plasma use on postoperative acute kidney injury in type A acute aortic dissection repair: a retrospective cohort analysis. J Cardiothorac Surg. (2021) 1:9. doi: 10.1186/s13019-020-01383-w
43. Li CN, Ge YP, Liu H, Zhang CH, Zhong YL, Chen SW, et al. Blood Transfusion and acute kidney injury after total aortic arch replacement for acute stanford type A aortic dissection. Heart Lung Circ. (2021). doi: 10.1016/j.hlc.2021.05.087
44. Yu JH, Kwon Y, Kim J, Yang SM, Kim WH, Jung CW, et al. Influence of transfusion on the risk of acute kidney injury: ABO-compatible versus ABO-incompatible liver transplantation. J Clin Med. (2019) 8:11. doi: 10.3390/jcm8111785
45. Kalisvaart M, Schlegel A, Umbro I, de Haan JE, Polak WG JN IJ, et al. The AKI Prediction Score: a new prediction model for acute kidney injury after liver transplantation. HPB. (2019) 12:1707–17. doi: 10.1016/j.hpb.2019.04.008
46. Kim WH, Park MH, Kim HJ, Lim HY, Shim HS, Sohn JT, et al. Potentially modifiable risk factors for acute kidney injury after surgery on the thoracic aorta: a propensity score matched case-control study. Medicine. (2015) 2:e273. doi: 10.1097/MD.0000000000000273
47. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. (2012) 52 1:65s−79s. doi: 10.1111/j.1537-2995.2012.03663.x
48. Sachs UJ. Non-infectious serious hazards in plasma transfusion. Transfus Apher Sci. (2010) 3:381–6. doi: 10.1016/j.transci.2010.09.005
49. Blumberg N, Gettings KF, Turner C, Heal JM, Phipps RP. An association of soluble CD40 ligand (CD154) with adverse reactions to platelet transfusions. Transfusion. (2006) 10:1813–21. doi: 10.1111/j.1537-2995.2006.00979.x
50. Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. (2006) 108 7:2455–62. doi: 10.1182/blood-2006-04-017251
Keywords: serum lactate, acute kidney injury, traumatic brain injury, marker, predictive model
Citation: Wang R, Wang S, Zhang J, He M and Xu J (2022) Serum Lactate Level in Early Stage Is Associated With Acute Kidney Injury in Traumatic Brain Injury Patients. Front. Surg. 8:761166. doi: 10.3389/fsurg.2021.761166
Received: 19 August 2021; Accepted: 24 December 2021;
Published: 31 January 2022.
Edited by:
Thomas Lustenberger, Aarau Cantonal Hospital, SwitzerlandReviewed by:
Beat Schnüriger, University of Bern, SwitzerlandCopyright © 2022 Wang, Wang, Zhang, He and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min He, aGVtaW4xOTkxMDMwNkB3Y2hzY3UuY24=; Jianguo Xu, eHVqZ0BzY3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.