
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg. , 13 October 2021
Sec. Pediatric Surgery
Volume 8 - 2021 | https://doi.org/10.3389/fsurg.2021.759937
Background: Major pediatric surgeries can cause severe intraoperative blood loss. This meta-analysis aims to evaluate the efficacy of tranexamic acid (TXA) in pediatric surgeries.
Methods: We searched PubMed, Embase, Web of Science, and Cochrane Library from the conception to March 31, 2021 to identify eligible randomized controlled trials (RCTs) that evaluated the efficacy of TXA in pediatric surgeries. Two reviewers choosed studies, evaluated quality, extracted data, and assessed the risk of bias independently. Mean difference (MD) was calculated as the summary statistic for continuous data. We used a random-effects model to measure mean effects. Data were generated from the corresponding 95% confidence interval (CI) using RevMan 5.3 software. Primary outcomes included intraoperative and postoperative blood loss, red blood cell (RBC) transfusion as well as fresh frozen plasma (FFP) transfusion.
Results: Fifteen studies enrolling 1,332 patients were included in this study. The pooled outcomes demonstrated that TXA was associated with a decreased intraoperative (MD = −1.57 mL/kg, 95% CI, −2.54 to −0.60, P = 0.002) and postoperative (MD = −7.85 mL/kg, 95% CI, −10.52 to −5.19, P < 0.001) blood loss, a decreased intraoperative (MD = −7.08 mL/kg, 95% CI, −8.01 to −6.16, P < 0.001) and postoperative (MD = −5.30 mL/kg, 95% CI, −6.89 to −3.70, P < 0.001) RBC transfusion, as well as a decreased intraoperative (MD = −2.74 mL/kg, 95% CI, −4.54 to −0.94, P = 0.003) and postoperative (MD = −6.09 mL/kg, 95% CI, −8.26 to −3.91, P < 0.001) FFP transfusion in pediatric surgeries. However, no significant difference was noted between two groups in duration of surgery (MD = −12.51 min, 95% CI −36.65 to 11.63, P = 0.31). Outcomes of intraoperative and postoperative blood loss and the duration of surgery in included studies were not pooled due to the high heterogeneity.
Conclusion: This meta-analysis demonstrated that TXA was beneficial for bleeding in pediatric surgeries.
Every year, millions of children are subjected to a variety of surgeries (1). The surgical operation is linked to significant blood loss in the perioperative period, which increases the risk of intraoperative hypotension, anemia, obstructed view of the surgical field, organ (particularly cardiac, renal, and pulmonary) damage, metabolic acidosis, infection, and other morbidities (2). Surgical blood loss and the requisite blood transfusions are a major cause of pediatric surgical mortality due to the complications of tissue hypoperfusion, electrolyte disorders, hemolytic reaction, and infectious diseases (3, 4). Therefore, it is necessary to prevent blood loss and minimize the need for blood transfusion in pediatric surgeries.
The pharmacological approach to reduce bleeding and the consequent need for transfusion have recently become an effective approach to preserve blood in adult patients. Tranexamic acid (TXA), a synthetic derivative of the amino acid lysine, acts by binding at lysine-binding sites and composes a convertible complex with both plasminogen and plasmin. By competitive blocking the transformation of plasminogen to plasmin, the proteolysis of plasmin on fibrin clots and platelets was inhibited, thus inhibiting fibrinolysis at the operative wound (5, 6). Many studies have shown that TXA can effectively reduce intraoperative bleeding in pediatric craniosynostosis, cardiac, scoliosis, adenotonsillectomy, and endoscopic sinus surgery (7–9). However, the results from individual studies of administering TXA in the pediatric population are not completely identical (10). Therefore, we conducted a systematic review and meta-analysis to assess the efficacy of TXA in reducing blood loss and blood transfusion in pediatric surgeries.
This systematic review and meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO; Registration NO. CRD42020198314) and was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement issued in 2009 (11). The four electronic databases PubMed, Embase (via Ovid), the Cochrane Library, and Web of Science were collected for studies to include in the present meta-analysis. Keywords included “tranexamic acid,” “pediatric,” “child,” “infant,” “toddler,” and “preschool.” The search included all articles published up to March 31, 2021. Any disagreements were resolved by discussion or by consulting the senior authors.
We reviewed the titles and abstracts of all retrieved studies. Inclusion criteria: (a) only placebo-controlled randomized controlled trials (RCTs) that described the efficacy of TXA on reducing intraoperative and postoperative bleeding or blood transfusion or duration of surgery in children undergoing surgery were included; (b) studies enrolled only children aged 0–18 years. (c) studies contained sufficient raw data for weighed mean difference (WMD) with 95% confidence intervals (CIs). Reviews, conference abstracts, letters, retrospective or case series, and studies of adult surgery were excluded.
The following data were extracted: sample of patients, age, weight, surgery type, TXA dose, blood loss, transfusion of RBCs and FFP intraoperatively and 24 h postoperationly, as well as the duration of surgery.
Cochrane Collaboration tool (12) was used to assess the quality of included studies. Based on sequence generation, allocation concealment, blinding, data collection, and outcome reporting, we will assign the risk of bias into low risk, high risk, or unclear.
The statistical analysis was performed using Review Manager 5.3 software (The Cochrane Collaboration, Oxford, UK). Random-effects models were used to compute the WMD for continuous variables. Heterogeneity was assessed by I2 statistics, I2 > 50% and P < 0.01 were considered the existence of considerable heterogeneity. In studies with multiple dose groups, each dose group was defined as a separate study and compared with the control. A subgroup analysis was conducted to test the robustness of the pooled results.
The trial selection process is shown in Figure 1. Only 15 studies were eligible according to the inclusion criteria (7–10, 13–23). The characteristics of the included trials are summarized in Table 1. The trials involved cardiac (7 trials) (13–15, 17, 22, 23), craniosynostosis (4 trials) (7, 8, 18, 20), scoliosis (2 trials) (9, 21), adenotonsillectomy (1 trials) (10), and endoscopic sinus (1 trials) (19) surgery. All trials were placebo-controlled. For seven studies (7–9, 18, 20–22), the loading dose of TXA ranged from 10 to 100 mg/kg and maintenance dose ranged from 3 to 15 mg/kg/h; for four studies (10, 14, 19, 23), the loading dose of TXA ranged from 10 to 100 mg/kg and a maintenance dose was not administered; for other four cardiac studies (13, 15–17), TXA was administered thrice at a dosage of 10 mg/kg, before cardiopulmonary bypass (CPB), on CPB, and after CPB, respectively.
All included studies were double-blind RCTs. Except for three studies (15–17) that were deemed unclear for the method of random allocation, other studies had detailed description of randomization methods using a computer-generated allocation list. Two studies (13, 15) did not mention allocation concealment and were rated as unclear. Information about allocation concealment of two articles (16, 22) was insufficient to allow judgment, and these two articles were rated as unclear (Figure 2).
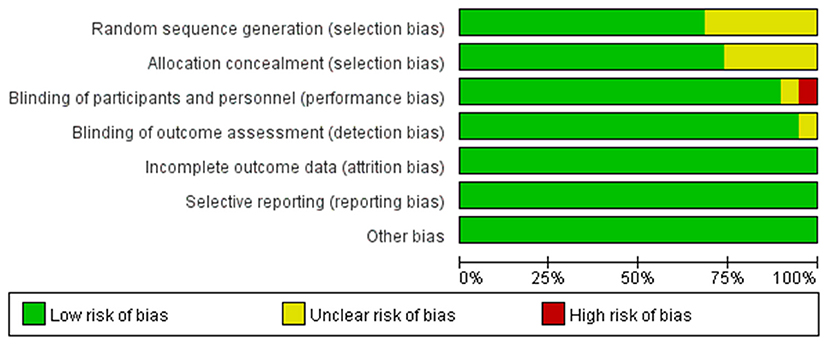
Figure 2. Quality of included studies. The overall quality of the selected studies was assessed by the Cochrane Collaboration tool.
Eight studies (7–10, 18–21) had sufficient data to analyze intraoperative blood loss. Using the random-effects model, the pooled MD showed a smaller total intraoperative blood loss in the TXA group vs. the control group (MD = −1.57 mL/kg, 95% CI, −2.54 to −0.60; I2 = 86%, P-heterogeneity < 0.00001) (Figure 3). The subgroup analyses showed that the effect of TXA on intraoperative blood loss varied by the type of surgery. For craniosynostosis surgery, the MD showed a smaller total intraoperative blood loss in the TXA group vs. the control group (MD = −18.64 mL/kg, 95% CI, −31.49 to −5.78; I2 = 63%, P-heterogeneity = 0.04) (Figure 3). For scoliosis surgery, the MD showed a smaller total intraoperative blood loss in the TXA group vs. the control group (MD = −1.90 mL/kg, 95% CI, −3.47 to −0.32; I2 = 92%, P-heterogeneity < 0.00001) (Figure 3). For endoscopic sinus and adenotonsillectomy surgery, there was no difference in intraoperative blood loss in the TXA group vs. the control group (MD = −0.46 mL/kg, 95% CI, −2.32 to 1.39; I2 = 87%, P-heterogeneity < 0.00001) (Figure 3).
Eleven studies (7, 8, 13–20, 22, 23) had sufficient data to analyze postoperative blood loss. Using the random-effects model, the pooled MD showed a smaller total postoperative blood loss in the TXA group vs. the control group (MD = −7.85 mL/kg, 95% CI, −10.52 to −5.19; I2 = 89%, P-heterogeneity < 0.00001) (Figure 4). The subgroup analyses showed that the effect of TXA on intraoperative blood loss varied by surgery types. For craniosynostosis surgery, there was no difference in postoperative blood loss in the TXA group vs. the control group (MD = −5.61 mL/kg, 95% CI, −13.36 to 2.14; I2 = 89%, P-heterogeneity < 0.00001) (Figure 4). For cardiac surgery, the MD showed a smaller total postoperative blood loss in the TXA group vs. the control group (MD = −9.14 mL/kg, 95% CI, −11.92 to −6.36; I2 = 83%, P-heterogeneity < 0.00001) (Figure 4).
Six studies (7, 8, 18, 20–22) had sufficient data to analyze intraoperative RBC transfusion. Using the random-effects model, the pooled MD showed a significantly smaller total intraoperative RBC transfusion in the TXA group vs. the control group (MD = −7.08 mL/kg, 95% CI, −8.01 to −6.16; I2 = 0%, P-heterogeneity = 0.51) (Figure 5).
Eight studies (7, 8, 14–18, 20) had sufficient data to analyze postoperative RBC transfusion. Using the random-effects model, the pooled MD showed a smaller total postoperative RBC transfusion in the TXA group vs. the control group (MD = −5.30 mL/kg, 95% CI, −6.89 to −3.70; I2 = 0%, P-heterogeneity = 0.48) (Figure 6).
Four studies (7, 8, 21, 22) had sufficient data to analyze intraoperative FFP transfusion. Using the random-effects model, the pooled MD showed a smaller total intraoperative FFP in the TXA group vs. the control group (MD = −2.74 mL/kg, 95% CI, −4.54 to −0.94; I2 = 38%, P-heterogeneity = 0.19) (Figure 7).
Six studies (7, 8, 14–17) had sufficient data to analyze postoperative FFP transfusion. Using the random-effects model, the pooled MD showed a significantly smaller total postoperative FFP in the TXA group vs. the control group (MD = −6.09 mL/kg, 95% CI, −8.26 to −3.91; I2 = 0%, P-heterogeneity = 0.72) (Figure 8).
Seven studies (7, 9, 18–21) had sufficient data to analyze duration of surgery. The random-effects model found no difference between the TXA and placebo groups (MD = −12.51 min, 95% CI, −36.65 to 11.63; I2 = 96%, P-heterogeneity < 0.00001) (Figure 9). The subgroup analyses showed that there was no difference in the duration of surgery for the craniosynostosis surgery (MD = 4.86 mL/kg, 95% CI −5.54 to 15.26; I2 = 0%, P-heterogeneity = 0.87) and scoliosis surgery (MD = −30.75 mL/kg, 95% CI, −82.61 to 21.10; I2 = 98%, P-heterogeneity < 0.00001) (Figure 9).
Bleeding and blood transfusion were commonly seen in children undergoing cardiac, craniosynostosis and scoliosis surgeries (24–26). The necessary RBC transfusion requirements heightens the risk of transmission of several infectious diseases (27), and seriously burdens hospitals and patients financially. TXA has been studied for its potential to reduce various surgical bleeding. Therefore, it is very meaningful to explore the effects of TXA on the blood loss and the need for blood transfusion in pediatric surgical procedures.
This meta-analysis shows that TXA could reduce intraoperative and postoperative blood loss during all surgery compared with the placebo control. However, after subgroup analysis, we did not find a statistically significant reduction in intraoperative blood loss during pediatric scoliosis, endoscopic sinus, and adenotonsillectomy surgery, nor in postoperative blood loss during pediatric craniosynostosis surgery. Notably, TXA could reduce intraoperative blood loss during pediatric craniosynostosis surgery and reduce postoperative blood loss during pediatric cardiac surgery. The discrepancy is perhaps associated with the small number of included reports and with the differences in the measuring methods of blood loss.
However, it is not appropriate to aggregate these results to assess the effect of TXA in reducing bleeding and blood transfusion because the administered dose of TXA in these studies was different (loading doses from 10 to 100 mg/kg and infusion rates of 1–10 mg/kg per hour) and the experimental approaches were also varied. Moreover, the ages of the children and surgical procedures in these studies were also different.
Our results indicated a decrease of bleeding in the TXA group during surgery. However, there is a non-significant trend toward decrease in bleeding in the treated group compared with the placebo group during pediatric craniosynostosis surgery in postoperative period. Based on our data, it would be interesting to continue use of TXA in children treated for surgical correction of craniosynostosis.
The results of our meta-analysis indicate that TXA decreased postoperative blood loss and RBC and FFP transfusion compared with placebo in pediatric cardiac surgery. However, the outcomes regarding postoperative blood loss were too heterogeneously distributed. Many factors may contribute to the high degree of heterogeneity. The various dosage schemes used in included studies might be the major reasons. After anesthetic induction, some studies performed a single bolus with 10–100 mg/kg (14, 23). Several studies used multiple boluses at different time points: anesthetic induction, CPB priming, and after the administration of protamine (16, 17). While some used continuous infusion of TXA during and/or after CPB (13). One study (22) used 50 mg/kg of TXA as a bolus followed by a 15 mg/kg/h infusion and another 50 mg/kg into the bypass circuit. The TXA dosage used was empirical based on its effects on blood loss instead of based on pharmacodynamic data regarding the fibrinolytic inhibiting activity. Only one study performed by Chauhan and colleagues (15) compared different TXA dosage schemes in a pediatric population. They found that the maximum reduction in blood loss was acquired with a bolus of 10 mg/kg TXA after anesthetic induction and repeated at the initiation and after weaning from CPB, respectively.
The pharmacological data on TXA in the pediatric cardiac surgeries are not available. Pharmacokinetic data in adults indicated a loading dose of 12.5 mg/kg given more than 30 min, a maintenance dose of 6.5 mg/kg/h and a dose of 1 mg/kg for CPB priming is required to maintain TXA concentration higher than 345 μM in blood, which is considered to be the lowest concentration for complete inhibition of fibrinolysis. Through this scheme, the TXA plasma concentration was more stable compared with repeated bolus administration schemes (28). However, these pharmacokinetic data are not directly applicable to a pediatric population.
Adverse effects associated with TXA use were not adequately reported. Our study indicates that TXA reduces postoperative blood loss and blood transfusion need in pediatric cardiac surgery; further follow up studies to assess the effects of TXA on postoperative outcomes and to determine the optimal dosage of TXA are needed.
Two studies (9, 21) indicated that administration of TXA produces significant reduction of blood loss in children with scoliosis. However, there was a clear heterogeneity with I2 of 92%. One study (21) used 100 mg/kg of TXA as a bolus followed by 10 mg/kg/h infusion. Another (9) used two doses: the high dose was 50 mg/kg of TXA as a bolus followed by 20 mg/kg/h infusion, and the low dose was 10 mg/kg of TXA as a bolus followed by 1 mg/kg/h infusion. Interestingly, all these three doses were found to reduce intraoperative blood loss.
In one study with a dose of 25 mg/kg (19), there was a significant decrease in volume of intraoperative bleeding, and duration of surgery in TXA group as compared to placebo group in pediatric endoscopic sinus surgery. However, in another study with a dose of 10 mg/kg, there was no benefit in the use of TXA for reducing bleeding during the perioperative period of adenotonsillectomy in children (10).
Due to there are two reports in the literature that have described the length of hospital stay (8, 20) and one report in the literature that have described the complications (8), we did not compare with these two indicators in our meta-analysis. After a detailed literature research, no published studies concerning TXA used in the pediatric surgical oncology, pediatric emergency surgery and neonatal surgery, therefore, we did not analyze these surgery type, these issues merit further studies in the near future.
In conclusion, this meta-analysis suggests that TXA contributes to reduce the transfusion of RBC and FFP in children undergoing cardiac, craniosynostosis, scoliosis, and endoscopic sinus surgery. There is, However, controversy over the efficacy of TXA in reducing intraoperative and postoperative blood loss. As a consequence, new RCTs evaluating the effects of TXA in children with these surgeries should be performed.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zhao Z, Li B, Wu Y, Chen X, Guo Y, Shen Y, et al. Ketamine affects the integration of developmentally generated granule neurons in the adult stage. BMC Neurosci. (2019) 20:60. doi: 10.1186/s12868-019-0542-4
2. Allain J, Stramer S, Carneiro-Proietti A, Martins M, Lopes da Silva S, Ribeiro M, et al. Transfusion-transmitted infectious diseases. Biologicals. (2009) 37:71–7. doi: 10.1016/j.biologicals.2009.01.002
3. Vamvakas E. Long-term survival rate of pediatric patients after blood transfusion. Transfusion. (2008) 48:2478–80. doi: 10.1111/j.1537-2995.2008.01921.x
4. Vamvakas E, Blajchman M. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. (2009) 113:3406–17. doi: 10.1182/blood-2008-10-167643
5. McCormack P. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. (2012) 72:585–617. doi: 10.2165/11209070-000000000-00000
6. Wu G, Mazzitelli B, Quek A, Veldman M, Conroy P, Caradoc-Davies T, et al. Tranexamic acid is an active site inhibitor of urokinase plasminogen activator. Blood Adv. (2019) 3:729–33. doi: 10.1182/bloodadvances.2018025429
7. Fenger-Eriksen C, Lindholm AD, Norholt SE, von Oettingen G, Tarpgaard M, Krogh L, et al. Reduced perioperative blood loss in children undergoing craniosynostosis surgery using prolonged tranexamic acid infusion: a randomised trial. Br J Anaesth. (2019) 122:760–6. doi: 10.1016/j.bja.2019.02.017
8. Kim EJ, Kim YO, Shim KW, Ko BW, Lee JW, Koo BN. Effects of tranexamic acid based on its population pharmacokinetics in pediatric patients undergoing distraction osteogenesis for craniosynostosis: Rotational thromboelastometry (ROTEM™) analysis. Int J Med Sci. (2018) 15:788–95. doi: 10.7150/ijms.25008
9. Saleh AN, Mostafa RH. Increased nociception following administration of different doses of tranexamic acid in adolescent idiopathic scoliosis surgery. Open Anesthesiol J. (2018) 12:61–8. doi: 10.2174/2589645801812010061
10. Brum MR, Miura MS, Castro SF, Machado GM, Lima LH, Lubianca Neto JF. Tranexamic acid in adenotonsillectomy in children: a double-blind randomized clinical trial. Int J Pediatr Otorhinolaryngol. (2012) 76:1401–5. doi: 10.1016/j.ijporl.2012.04.028
11. Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
12. Higgins J, Altman D, Gøtzsche P, Jüni P, Moher D, Oxman A, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
13. Aggarwal V, Kapoor PM, Choudhury M, Kiran U, Chowdhury U. Utility of Sonoclot analysis and tranexamic acid in tetralogy of Fallot patients undergoing intracardiac repair. Ann Card Anaesth. (2012) 15:26–31. doi: 10.4103/0971-9784.91477
14. Bulutcu FS, Ozbek U, Polat B, Yalcin Y, Karaci AR, Bayindir O. Which may be effective to reduce blood loss after cardiac operations in cyanotic children: tranexamic acid, aprotinin or a combination? Pediatric Anesthesia. (2005) 15:41–6. doi: 10.1111/j.1460-9592.2004.01366.x
15. Chauhan S, Bisoi A, Kumar N, Mittal D, Kale S, Kiran U, et al. Dose comparison of tranexamic acid in pediatric cardiac surgery. Asian Cardiovasc Thorac Ann. (2004) 12:121–4. doi: 10.1177/021849230401200208
16. Chauhan S, Bisoi A, Modi R, Gharde P, Rajesh MR. Tranexamic acid in paediatric cardiac surgery. Indian J Med Res. (2003) 118:86–9. doi: 10.1093/fampra/cmg432
17. Chauhan S, Das SN, Bisoi A, Kale S, Kiran U. Comparison of epsilon aminocaproic acid and tranexamic acid in pediatric cardiac surgery. J Cardiothorac Vasc Anesth. (2004) 18:141–3. doi: 10.1053/j.jvca.2004.01.016
18. Dadure C, Sauter M, Bringuier S, Bigorre M, Raux O, Rochette A, et al. Intraoperative tranexamic acid reduces blood transfusion in children undergoing craniosynostosis surgery a randomized double-blind study. Anesthesiology. (2011) 114:856–61. doi: 10.1097/ALN.0b013e318210f9e3
19. Eldaba AA, Amr YM, Albirmawy OA. Effects of tranexamic acid during endoscopic sinsus surgery in children. Saudi J Anaesth. (2013) 7:229–33. doi: 10.4103/1658-354X.115314
20. Goobie SM, Meier PM, Pereira LM, McGowan FX, Prescilla RP, Scharp LA, et al. Efficacy of tranexamic acid in pediatric craniosynostosis surgery: a double-blind, placebo-controlled trial. Anesthesiology. (2011) 114:862–71. doi: 10.1097/ALN.0b013e318210fd8f
21. Sethna NF, Zurakowski D, Brustowicz RM, Bacsik J, Sullivan LJ, Shapiro F. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology. (2005) 102:727–32. doi: 10.1097/00000542-200504000-00006
22. Shimizu K, Toda Y, Iwasaki T, Takeuchi M, Morimatsu H, Egi M, et al. Effect of tranexamic acid on blood loss in pediatric cardiac surgery: a randomized trial. J Anesth. (2011) 25:823–30. doi: 10.1007/s00540-011-1235-z
23. Zonis Z, Seear M, Reichert C, Sett S, Allen C. The effect of preoperative tranexamic acid on blood loss after cardiac operations in children. J Thoracic Cardiovasc Surg. (1996) 111:982–7. doi: 10.1016/S0022-5223(96)70374-4
24. Goobie S, Zurakowski D, Isaac K, Taicher B, Fernandez P, Derderian C, et al. Predictors of perioperative complications in paediatric cranial vault reconstruction surgery: a multicentre observational study from the Pediatric Craniofacial Collaborative Group. Br J Anaesth. (2019) 122:215–23. doi: 10.1016/j.bja.2018.10.061
25. Oetgen M, Litrenta J. Perioperative blood management in pediatric spine surgery. J Am Acad Orthop Surg. (2017) 25:480–8. doi: 10.5435/JAAOS-D-16-00035
26. Siemens K, Sangaran D, Hunt B, Murdoch I, Tibby S. Strategies for prevention and management of bleeding following pediatric cardiac surgery on cardiopulmonary bypass: a scoping review. Pediatric Crit Care Med. (2018) 19:40–7. doi: 10.1097/PCC.0000000000001387
27. Squires J. Risks of transfusion. South Med J. (2011) 104:762–9. doi: 10.1097/SMJ.0b013e31823213b6
Keywords: tranexamic acid, pediatric, surgery, blood loss, blood transfusion, meta-analysis
Citation: Wei Y, Zhang Y, Jin T, Wang H, Li J and Zhang D (2021) Effects of Tranexamic Acid on Bleeding in Pediatric Surgeries: A Systematic Review and Meta-Analysis. Front. Surg. 8:759937. doi: 10.3389/fsurg.2021.759937
Received: 17 August 2021; Accepted: 15 September 2021;
Published: 13 October 2021.
Edited by:
Alessandro Inserra, Bambino Gesù Children Hospital (IRCCS), ItalyReviewed by:
Simone Frediani, Bambino Gesù Children Hospital (IRCCS), ItalyCopyright © 2021 Wei, Zhang, Jin, Wang, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Li, NDk0ODg5MjI2QHFxLmNvbQ==; Donghang Zhang, emhhbmdkaHNjdUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.