- 1Department of General Surgery, Lanzhou University Second Hospital, The Second Clinical Medical College of Lanzhou University, Lanzhou, China
- 2Key Laboratory of Digestive System Tumors of Gansu, Lanzhou, China
Background: Small bowel adenocarcinoma (SBA) is a rare gastrointestinal tumor with high malignancy. The aim of this study was to comprehensively evaluate the distant metastasis pattern and establish nomograms predicting survival for SBA.
Methods: From 2010 to 2015, patients diagnosed with SBA were identified based on the Surveillance, Epidemiology, and End Results (SEER) database. Kaplan–Meier survival analysis was applied to compare survival differences between metastasis patterns. Then, univariate and multivariate cox analyses were applied to screened out independent prognostic factors of cancer-specific survival (CSS) and overall survival (OS), and identify the risk factors for metastasis of SBA. To assess the discrimination and calibration of nomograms, the concordance index (C-index), calibration curves, receiver-operating characteristic curve (ROC), and decision curve analysis (DCA) were calculated.
Results: Kaplan–Meier curves revealed that metastasis patterns were significantly correlated with CSS (p < 0.001) and OS (p < 0.001). Then, the metastasis pattern was showed to be an independent prognostic factor of OS and CSS in patients with SBA, as well as age, grade, T stage, N stage, surgery, retrieval of regional lymph nodes, and chemotherapy. Combining these factors, we constructed prognostic nomograms, which suggested that the metastasis pattern made the greatest contribution to the survival of patients with SBA. Nomograms for OS and CSS had a C-index of 0.787 and 0.793, respectively. Calibration curves showed an excellent agreement between probability and actual observation in the training and validation cohort. Decision curve analysis also exhibited its clinical value with an improved net benefit. In addition, the models we constructed had better prognostic accuracy and clinical utility than traditional TNM staging based on C-index and ROC. Further, Cox regression analysis showed that old age, poor differentiation, N2, and not receiving chemotherapy were the risk factors for prognosis in patients with metastatic SBA.
Conclusion: As an independent prognostic factor, the metastasis pattern exhibited the greatest predictive effect on OS and CSS for patients with SBA. Adjuvant chemotherapy had a positive effect on the survival of patients with SBA. Nomograms for predicting 3-and 5-year OS and CSS of patients with SBA were constructed, which could identify patients with higher risk and might be superior in predicting the survival of patients with SBA than TNM staging.
Introduction
Small bowel adenocarcinoma (SBA) often occurred in the glandular epithelium, accounting for about 36.9% in small intestinal cancer, and SBA, which mostly located in the duodenum, is the second most common histological type (1, 2). Due to the lack of specific symptoms and the narrow structure, it is difficult to diagnose SBA at the early stage (1). Moreover, the patients with SBA often need individual treatment, which depends on the original occurring position based on surgical resection, chemotherapy, radiation, and immunotherapy (3–5). Although complicated therapies were applied, the prognosis of patients with SBA with a median survival of 37% months remain poorly (6). Therefore, it is urgent to conduct an accurate prognostic analysis of patients with SBA to individualized treatment and monitoring.
Like other tumors, SBA can metastasize to the liver, lung, brain, and bone, with the liver being the dominant site of metastasis (7). Distant metastasis of SBA is one of the main causes of death. However, limited by the small sample size, few studies have analyzed and summarized the relationship between these metastasis sites and prognostic factors of multiple metastases. Therefore, it is important to identify SBA patients with early metastasis and take timely intervention measures. Patients undergoing radical surgery often die from distant metastases of the disease, suggesting the role of adjuvant chemotherapy (8).
Nomogram is a convenient and effective statistical prediction tool, widely used in cancer prognosis research (9, 10). However, the factors that affect tumor progression are complex and diverse. The nomogram can integrate and analyze various prognostic factors of individuals, simplify the classification of patients in clinical trials, and quantify the individualized outcome of patients (11, 12). Therefore, nomogram specially designed for predicting the survival of SBA is promising. This study aimed to establish the models for predicting cancer-specific survival (CSS) (P < 0.001) and overall survival (OS) of SBA and explore the effects of metastasis pattern on the prognosis of SBA. The Surveillance, Epidemiology, and End Results (SEER) database can provide us with corresponding data, which contains a wealth of large-scale information on tumor epidemiology, such as various clinical information and social information for specific populations (13–15).
Materials and Methods
Patients
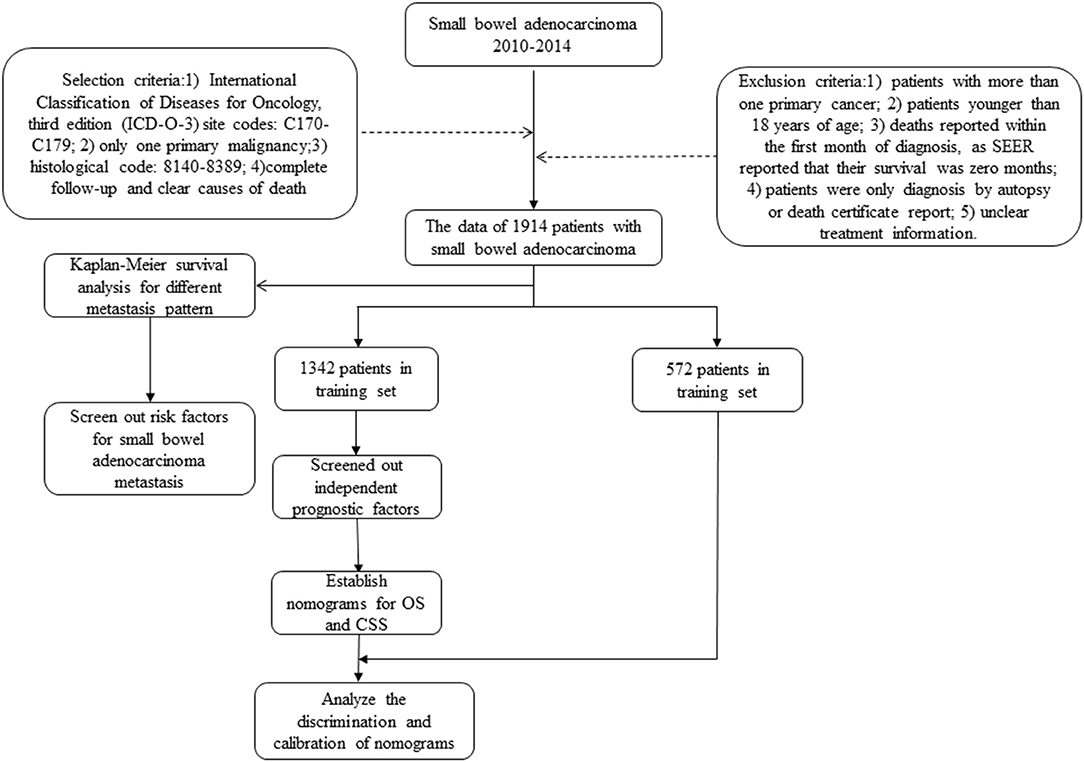
The cancer incidence information collected by SEER database includes data from 18 cancer registries, covering approximately 34.6% of the US population. Data on patients diagnosed with SBA were selected from 2010 to 2014, since the information on cancer metastasis sites was available from 2010 in SEER. The inclusion criteria for patients were based on International Classification of Diseases for Oncology, third edition (ICD-O-3) site codes: C170-C179 (C170: Duodenum, C171: Jejunum, C172: Ileum, C173: Meckels diverticulum, C178: Overlapping lesion of small intestine, C179: Small intestine), only one primary malignancy, histological code: 8140-8389, complete follow-up, and clear causes of death (Supplementary Table 1). Exclusion criteria were as follows: (1) patients with more than one primary cancer; (2) patients younger than 18 years of age; (3) deaths reported within the first month of diagnosis, as SEER reported that their survival was 0 months; (4) patients were only diagnosed by autopsy or death certificate report; and (5) unclear treatment information (Figure 1). Finally, a total of 1,914 patients were included.
Study Variables
The factors identified in the analysis included age, race, sex, marital status, primary site, histologic grade, TNM stage, surgery, chemotherapy, radiation, retrieval of reginal lymph nodes, the causes of death, and follow-up information. The endpoints of the study were OS and CSS. Overall survival is defined as the time from diagnosis to death, and CSS is the time from diagnosis to death from SBA.
Statistical Analyses
All cases meeting the inclusion criteria were included in our study, which were divided into training cohort and validation cohort in a ratio of 7:3 by a random split method. Chi-square test was performed for the basic clinicopathological characteristics of the two cohorts. Kaplan–Meier survival analysis was used to calculate the correlation between different metastasis pattern and OS and CSS, and the log-rank test was performed to compare the significance of survival curves. All variables were evaluated by univariate and multivariate Cox regression analyses to assess the correlation between each characteristic and OS and CSS, and to identify the risk factors for metastasis of SBA. Based on the results of Cox regression analysis, nomograms were constructed for predicting 3- and 5-year OS and CSS. Concordance index (C-index) was used to evaluate the predictive ability of nomograms (16, 17). Concordance index of 0.5 indicates that the model has no predictive power, while a C-index of 1.0 indicates perfect. Receiver-operating characteristic curve (ROC) can also verify nomogram. In addition, calibration curves were drawn to assess the prognosis and actual outcome of survival. To measure the clinical application of nomogram, decision curve analysis (DCA) was applied (18, 19). SPSS software and R statistical software were used for the analysis. A P-value < 0.05 was considered statistically significant.
Results
Patient Characteristics
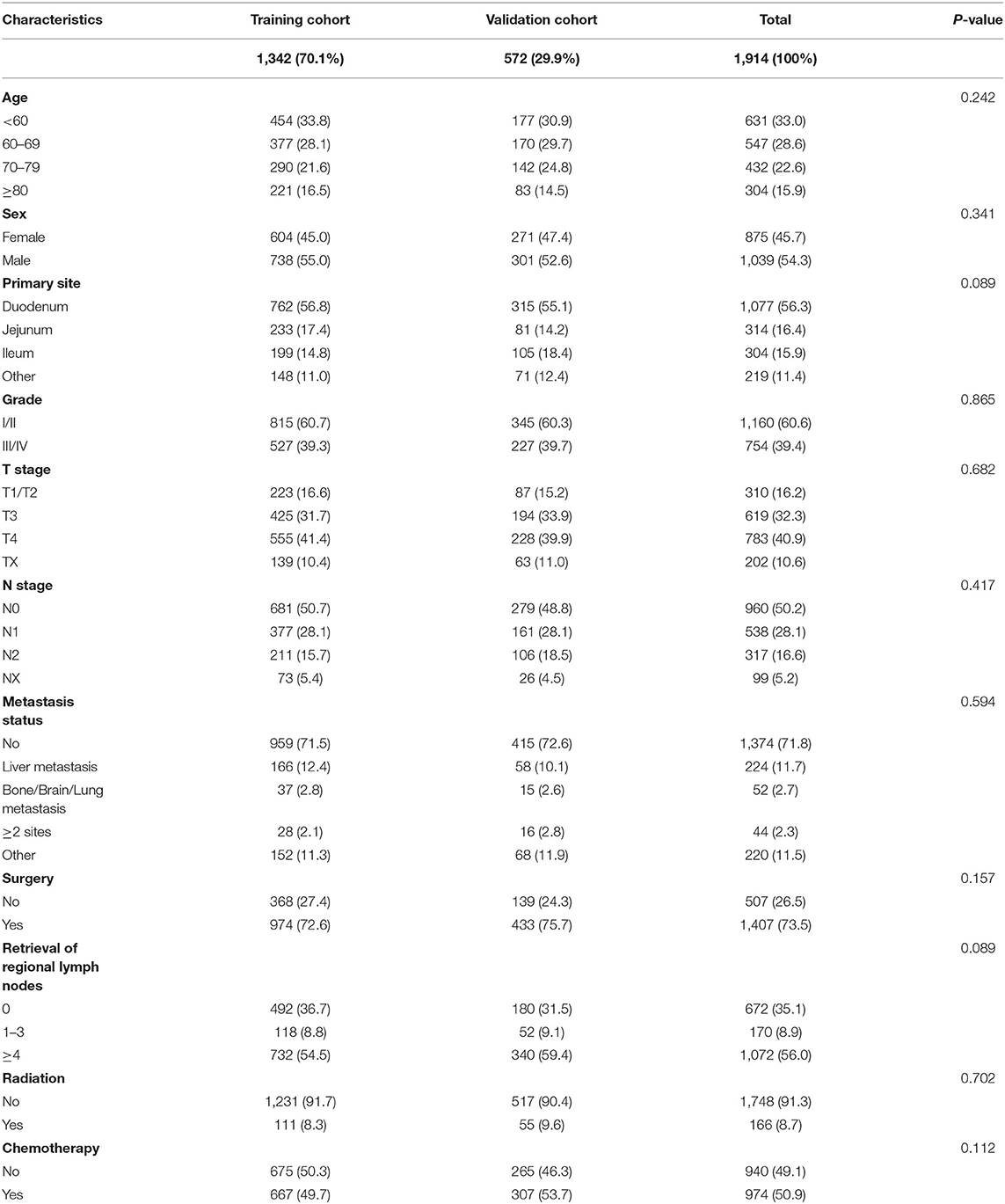
This study included 1,914 patients with SBA in SEER database from 2010 to 2015. Random split-sample method was used to randomly divide patients into training cohort (n = 1,342) and validation cohort (n = 572). Training cohort information was used to create nomogram, and validation cohort was applied to build external verification of nomogram. As shown in Table 1, age (p = 0.242), sex (p = 0.341), primary site (p = 0.089), grade (p = 0.865), T stage (p = 0.682), N stage (p = 0.417), metastasis status (p = 0.594), surgery (p = 0.157), retrieval of reginal lymph nodes (p = 0.089), radiation (p = 0.702), and chemotherapy (p = 0.112) were all factors that were similar between two groups.
Survival Analysis for Different Metastasis Pattern
In order to evaluate the impact of different metastasis patterns on OS and CSS in patients with SBA, Kaplan–Meier survival analysis was performed on all patients. As shown in Figure 2A, the difference in OS among different metastasis pattern was statistically significant (p < 0.001).The survival was highest for SBA patients without metastasis, followed by patients with liver and bone/brain/lung metastasis, and the worst in patients with multiple metastases. Furthermore, the Kaplan–Meier survival analysis on CSS in patients with SBA was consistent with the results of OS (Figure 2B).
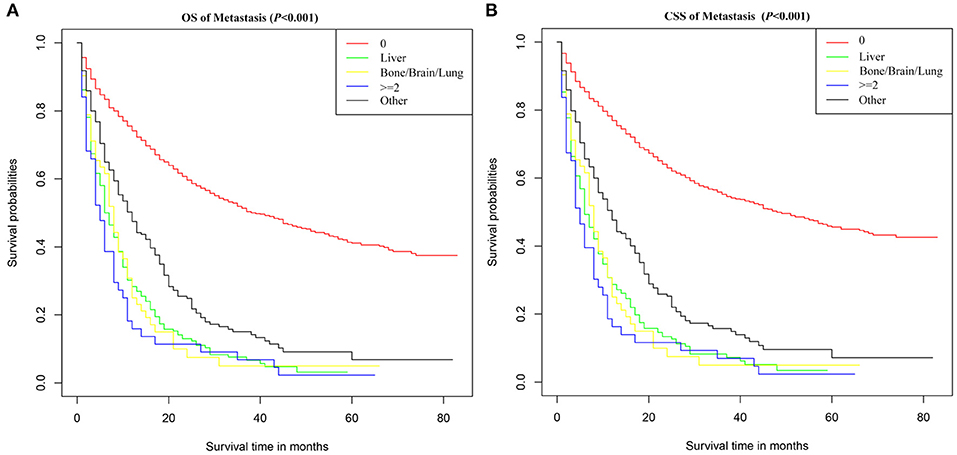
Figure 2. Survival curves of (A) OS and (B) CSS for SBA according to metastasis pattern. 0, no metastasis; ≥2, metastasis to at least two sites; Other, metastasis occurred, but the SEER database did not provide the site of metastasis.
Independent Prognostic Factors for OS and CSS
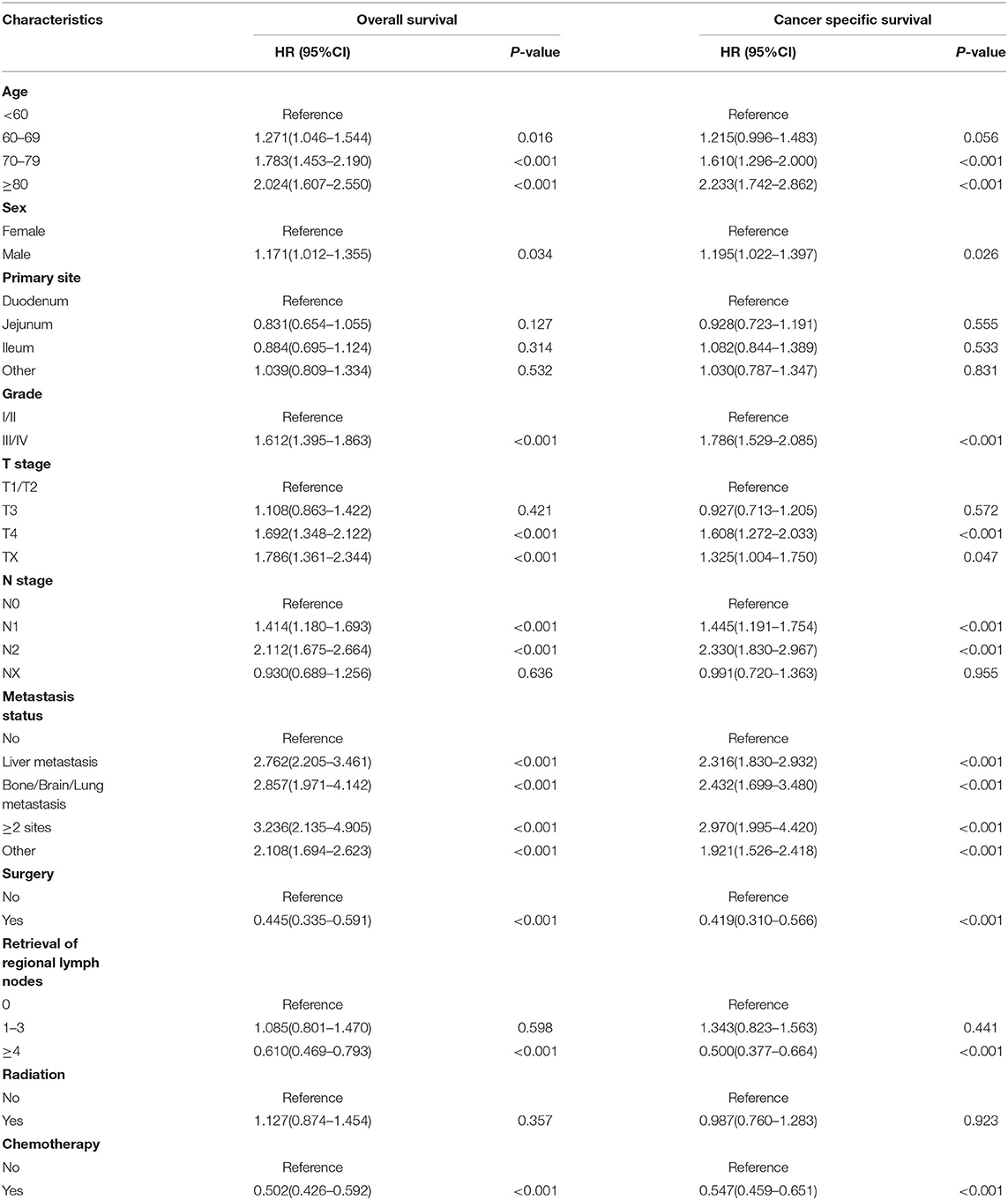
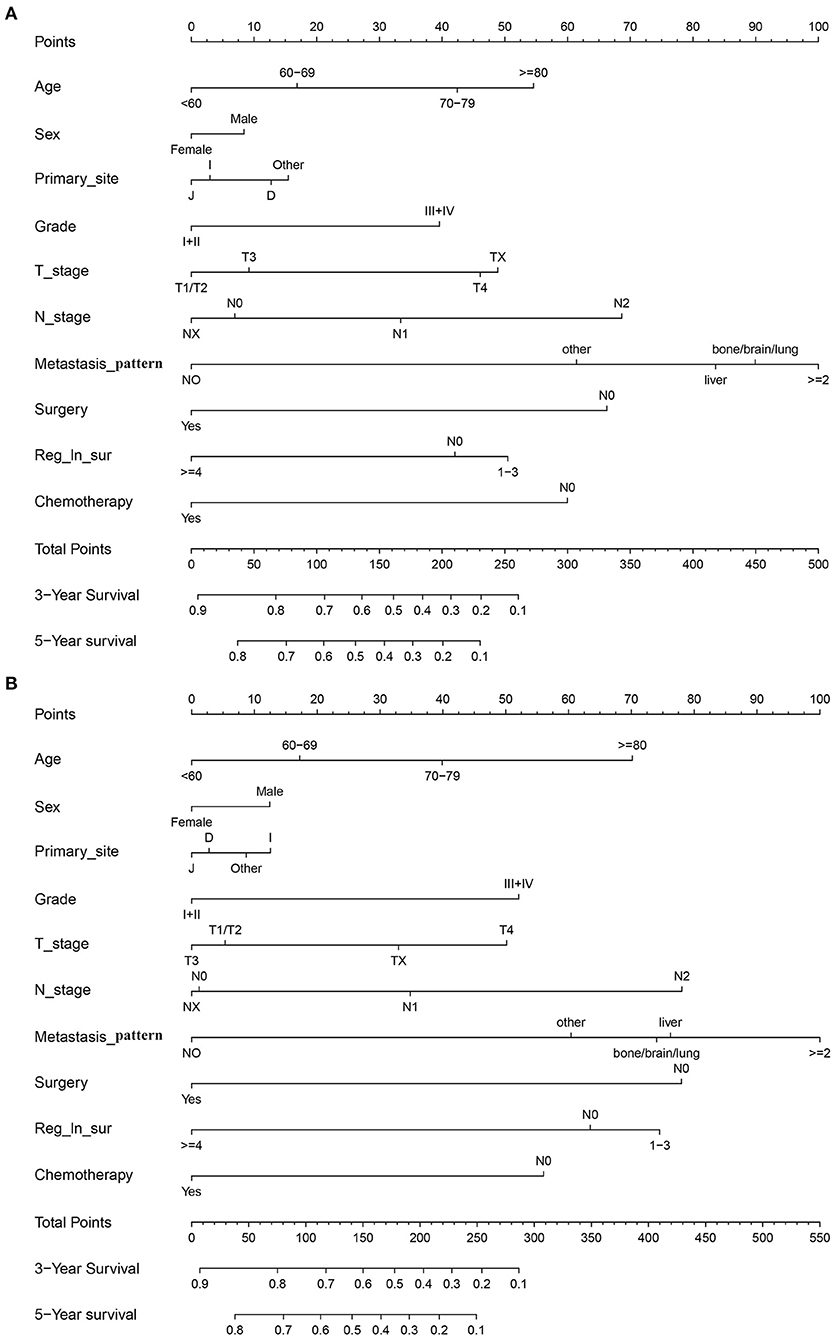
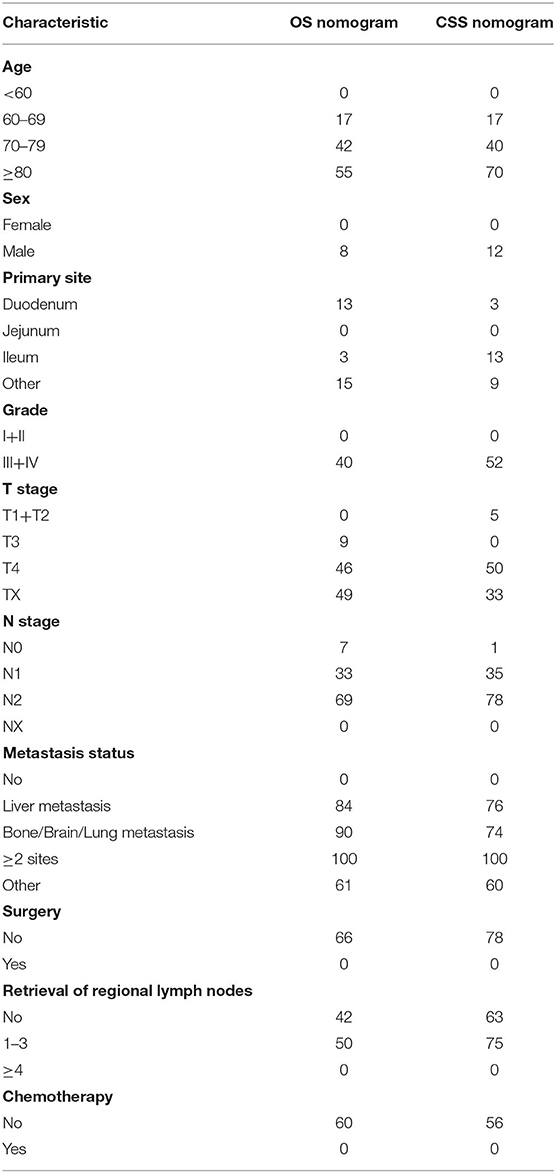
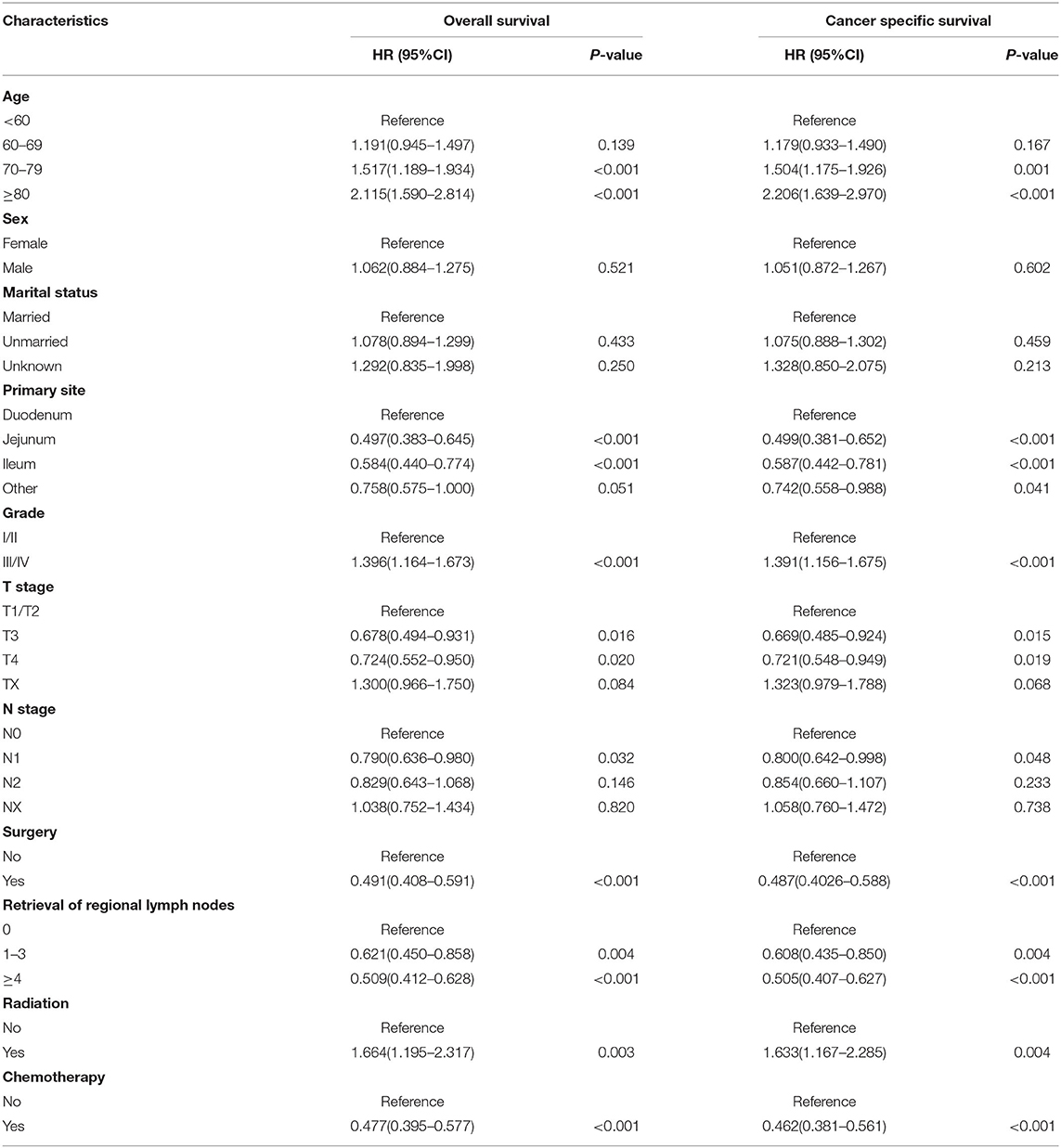
In the training cohort, we determined that age, marital status, primary site, grade, T stage, N stage, metastasis pattern, surgery, retrieval of regional lymph nodes, and chemotherapy are related to OS and CSS by univariate Cox regression analysis (Table 2). In order to further judge whether these factors can be used as independent prognostic factors, we conducted a multivariate Cox regression analysis (Table 3). The results showed that age, grade, T stage, N stage, metastasis pattern, surgery, retrieval of regional lymph nodes, and chemotherapy were identified as independent prognostic factors for OS and CSS, which were further included in the construction of nomogram. From the clinical point of view, we also considered tumor primary site as the parameter of the nomogram (Figure 3). Nomogram showed that the metastatic pattern of SBA had the greatest impact on the prognosis, of which liver metastasis accounted for a large proportion, and surgery, N stage, and chemotherapy also made great contribution to prognosis. Age, grade, T stage, and retrieval of reginal lymph nodes showed a moderate effect on OS (Figure 3A). However, in the nomogram for CSS, the metastasis pattern was the greatest contributor, followed by surgery, N stage, and retrieval of reginal lymph nodes (Figure 3B). According to the individual characteristics of patients with SBA, the scores of each variable were added to correspond to the total score to predict the OS and CSS at 3- and 5-years. The detailed score of each variable is shown in Table 4.
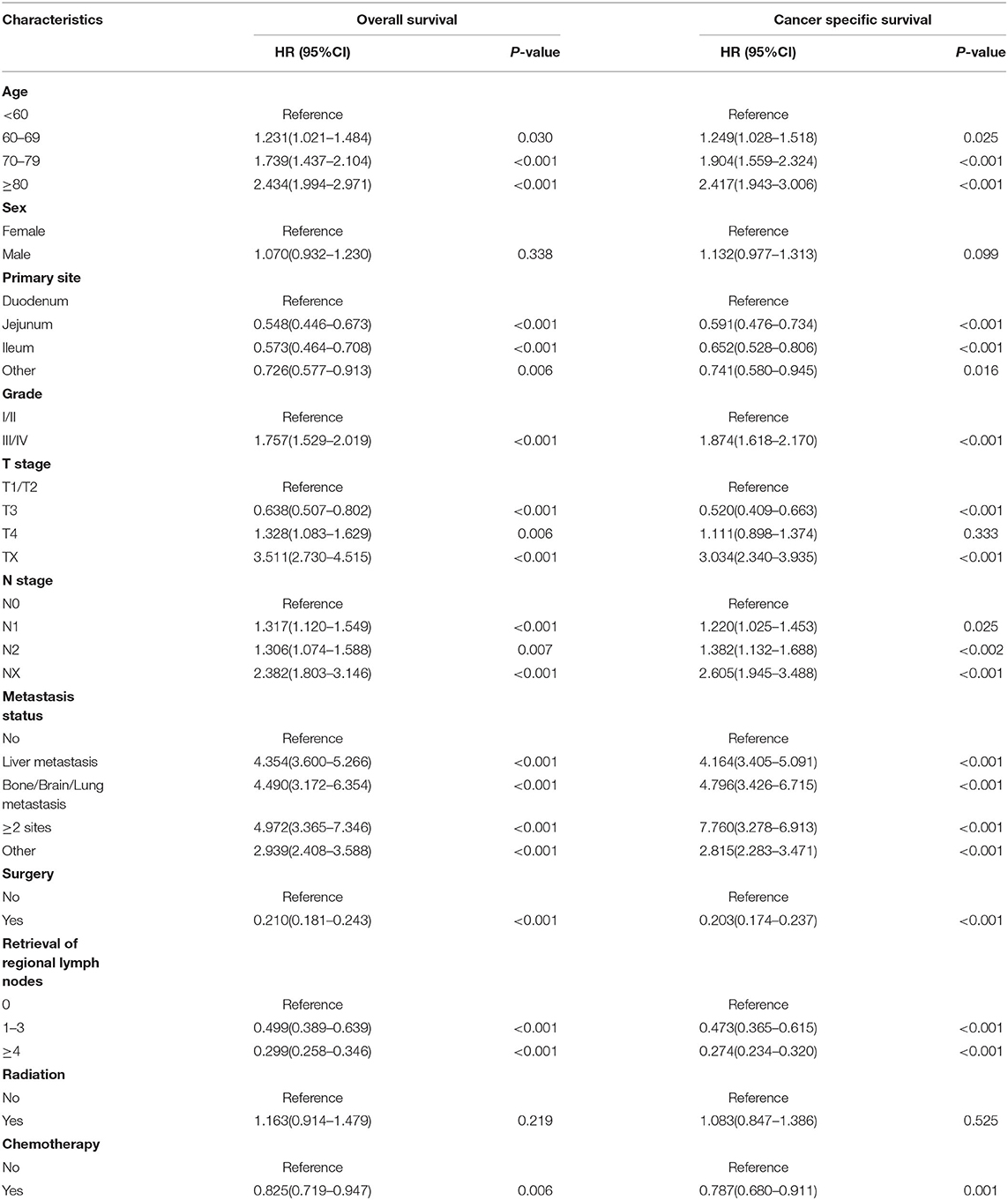
Table 2. Univariate Cox regression analysis for overall survival and cancer-specific survival in small bowel adenocarcinoma (training cohort).
In the training cohort, the C-index of nomogram for predicting CSS and OS was 0.793 (95% CI 0.785–0.801) and 0.787 (95% CI 0.780–0.794), respectively. However, the C-index of TNM staging for CSS and OS was 0.702 (95% CI 0.692–0.712) and 0.696 (95% CI 0.687–0.705). In the validation cohort, the C-index of nomogram for CSS was 0.796 (95% CI 0.785–0.807) and that for OS was 0.762 (95% CI 0.749–0.775), which were higher than those of TNM staging [CSS: 0.718 (95% CI 0.704–0.732); OS: 0.678 (95% CI 0.663–0.693)]. The higher the C-index, the more suitable of the model for patients with SBA. By comparing the discrimination capability of nomogram with the 7th edition of AJCC TNM staging, ROC analysis showed that the nomograms we constructed also demonstrated a superior survival predictability than the 7th AJCC TNM staging system (Figure 4). Furthermore, the calibration and prediction curves of nomograms for the 3- and 5-year survival rates of patients exhibited a perfect correlation in the training cohort, indicating that nomograms for CSS and OS were well-validated (Figures 5A,C,E,G). In the validation cohort, the results showed the same reliability of the model (Figures 5B,D,F,H). Decision curve analysis indicated that the models had significantly positive net benefits within the risk of death in both cohorts, demonstrating that nomograms had good clinical value in predicting OS and CSS at 3- and 5-years (Figure 6).
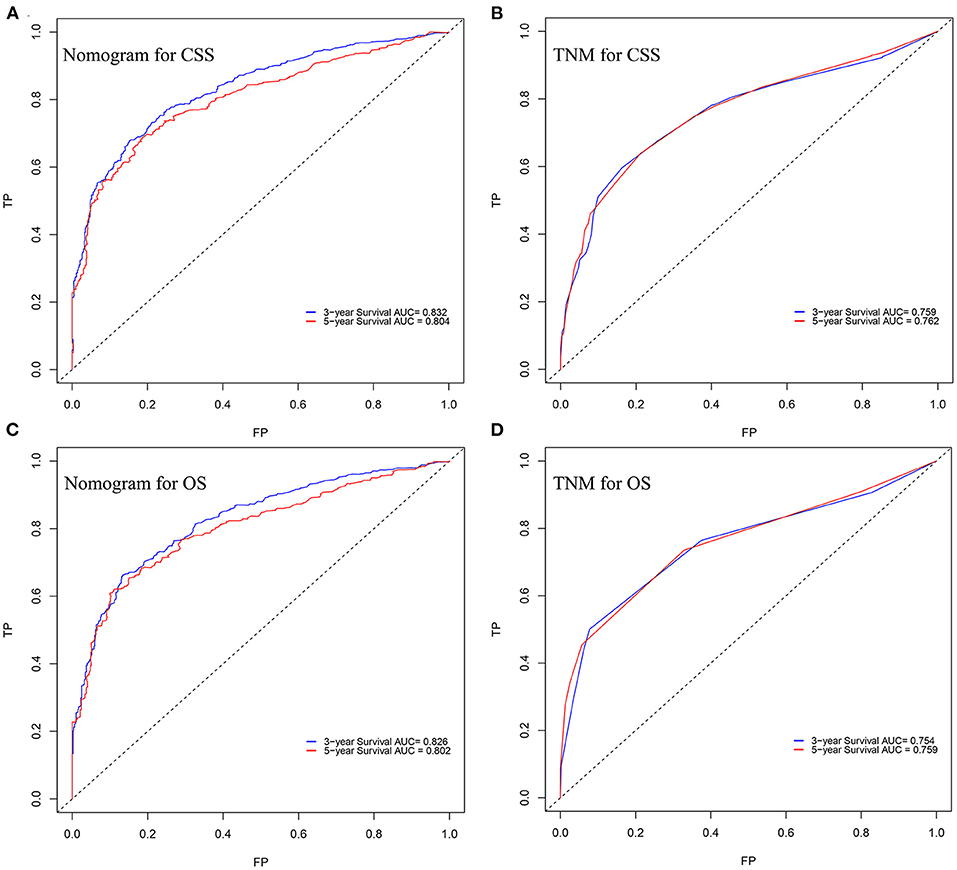
Figure 4. ROC curves of the nomogram for predicting (A) CSS and (B) OS at 3- and 5-year point. ROC curves of the TNM staging for predicting (C) CSS and (D) OS.
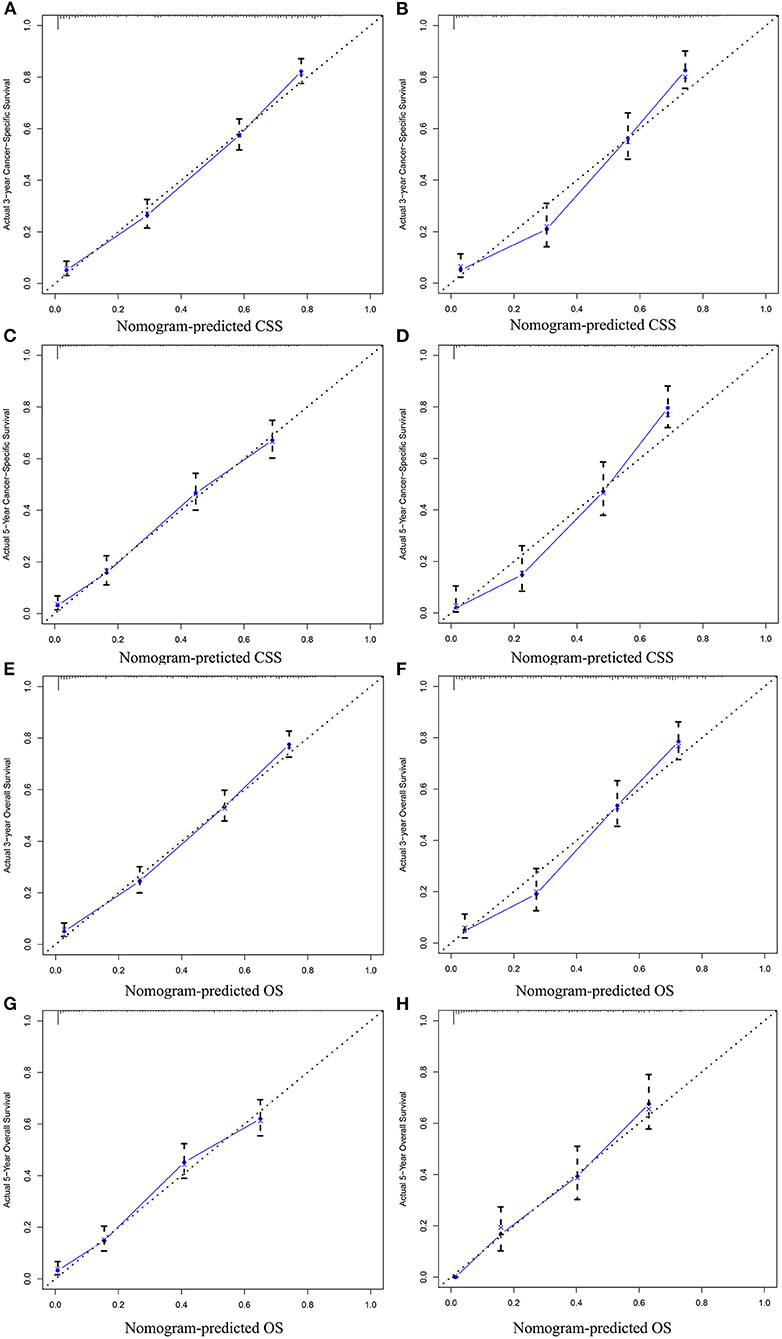
Figure 5. Calibration plots for predicting CSS at 3-year (A) training cohort and (B) validation cohort; calibration plots for predicting CSS at 5-year (C) training cohort and (D) validation cohort; calibration plots for predicting OS at 3-year (E) training cohort and (F) validation cohort; calibration plots for predicting OS at 5-year (G) training cohort, and (H) validation cohort.
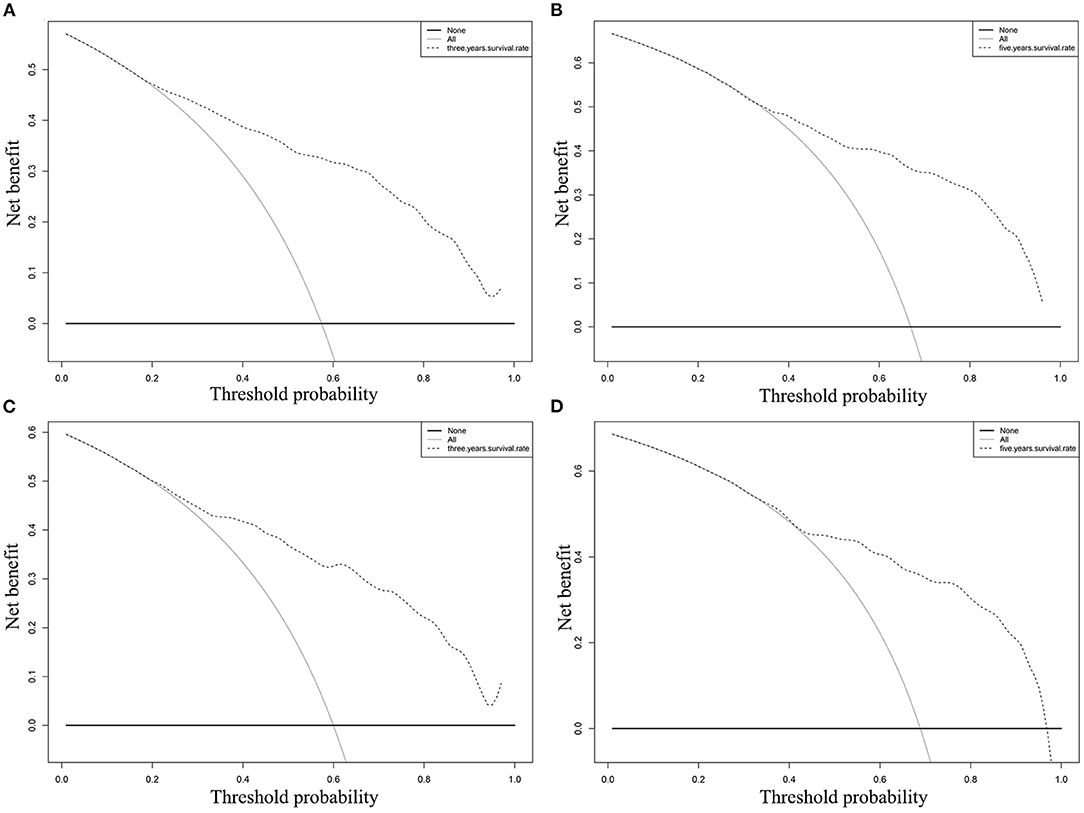
Figure 6. DCA of the nomogram for predicting CSS at (A) 3-year and (B) 5-year point; DCA of the nomogram for predicting OS at (C) 3-year and (D) 5-year point.
Risk Factors for SBA Distant Metastasis
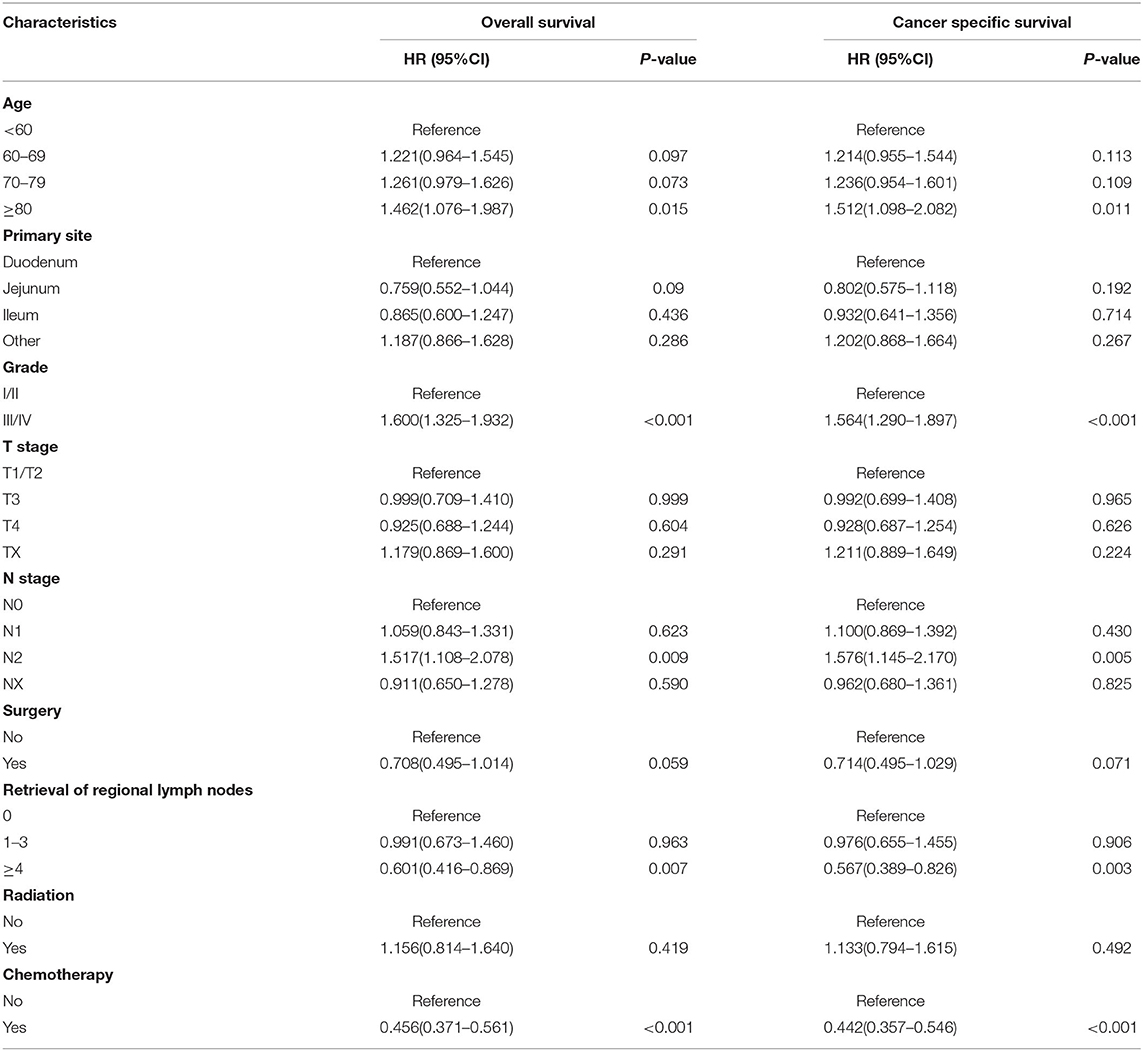
We then identified the risk factors that were significantly associated with distant metastatic SBA. Among the variables studied, advanced age, tumor origin in the duodenum, poor differentiation, lack of surgical treatment at the primary site and regional lymph node dissection, and lack of radiotherapy and chemotherapy were significantly associated with OS and CSS in patients with metastatic SBA (Table 5). Variables that were statistically significant in the univariate analysis were selected for inclusion in the multivariate analysis for OS. As shown in Table 6, advanced age (p = 0.015), poor differentiation (p < 0.001), N2 stage (p = 0.009), absence of regional lymph node dissection (p = 0.007), and not receiving chemotherapy (p < 0.001) were significantly associated with poor prognosis in patients with metastatic SBA. Similar results were observed in the multivariate analysis for CSS.
Discussion
The malignant small bowel tumor is a rare gastrointestinal tumor, accounting for about 5% of all gastrointestinal tumors (20). Small bowel adenocarcinoma is one of the most common histological subtypes, which has been found to have a very bad prognosis (21). The AJCC TNM staging system neglects taking some significant factors into consider, though it is applied to predict the survival of patients with SBA. Although recent study has constructed a prognostic model for SBA, this study ignores the impact of adjuvant chemotherapy, radiotherapy, lymph node dissection, and tumor metastasis patterns on the survival of patients with SBA (22). Compared with previous prognostic models, our study established the most comprehensive nomogram for patients with SBA. Concordance index, calibration curves, ROC, and DCA all proved good predictive ability and clinical application of nomograms. The new model showed more predictability than the 7th edition of AJCC TNM staging system both in the training test and in the validation set. We further identify the risk factors of patients with metastatic SBA.
Age, tumor grade, T stage, and N stage were shown to be independent prognostic factors for OS and CSS in patients with SBA, consistent with previous studies (6, 20, 23, 24). Old age was one of the factors for the poor prognosis of many malignant tumors, which may be attributed to the decline of the patient's body resistance. Poorly differentiated or undifferentiated of tumors often led to the malignant progression; thus, it was the factor affecting the survival. In addition, our model indicated that T4 and N2 were the important risk factors for patients with SBA. In a prospective study based on 347 patients with SBA, T4 (p = 0.001) was correlated with a higher risk of death, which was an independent prognostic factor for OS in patients with SBA (25). Another retrospective study also emphasized T4 as an independent prognostic factor (26). Previous retrospective studies have shown that lymph node involvement has adverse effects on patients with SBA (7). It should be noted that surgery, number of lymph node dissection, and chemotherapy have strong prognostic values for patients with CSS and OS, demonstrating the positive effect of surgical treatment and systemic chemotherapy on patients with SBA. At present, surgery remains the main treatment option for patients with SBA, while radical surgical resection and adequate lymph node dissection are important means to improve the prognosis of patients. In many past studies, adjuvant chemotherapy could significantly improve OS and disease-free survival (DFS) (27, 28). Another retrospective study pointed out that adjuvant chemotherapy did not improve the OS and DFS of patients with SBA after surgery (29). In our study, patients receiving chemotherapy had a lower risk of survival (HR = 0.502, P < 0.001), suggesting that adjuvant chemotherapy has a positive effect on improving the prognosis of patients with SBA. However, radiotherapy had no significant effect on the prognosis of SBA based on the Cox analysis. For solid tumors, there were still uncertainties in dose selection, time, and toxicity of radiotherapy (30). Due to the rare incidence of small intestinal adenocarcinoma, the analysis of the effect of therapies was limited by the small sample size, so the effect of radiation on patients with SBA was still controversial (21, 23, 26, 28). In addition, studies have shown that the primary tumor site was an independent prognostic factor in patients with SBA (31, 32). This may be attributed to the fact that the early diagnosis rate of SBA that originated in the duodenum was higher than that of jejunum and ileum tumors, and they were treated relatively early. In univariate Cox analysis, the primary tumor site was significant, but multivariate regression analysis suggested negative results.
Among the included variables, the metastatic pattern was a significant independent prognostic factor and was positively correlated with the risk of death of the patient. The results of survival analysis were consistent. Small bowel adenocarcinoma patients with more than two metastasis sites had a significantly negative prognosis in this model. The study showed poor OS in patients with metastatic SBA, consistent with our study (33). Based on the results of univariate Cox analysis, multivariate Cox regression again emphasized that poorly differentiated, N2, and absence of chemotherapy were negatively associated with OS and CSS in metastatic SBA patients. A systematic review of seven prospective studies revealed that adjuvant chemotherapy was effective for unresectable or metastatic SBA (34). In a retrospective analysis of patients with metastatic SBA, patients who received palliative chemotherapy had a median OS of 9.3 months, significantly better than those who did not receive chemotherapy (35). These studies suggested that adjuvant chemotherapy had a certain benefit in improving the prognosis of metastatic SBA. However, the current SBA chemotherapy regimens mostly refer to other gastrointestinal malignancies, and the efficacy of different chemotherapy regimens varies greatly. In a multicenter retrospective analysis, oxaliplatin-based chemotherapy regimens were found to significantly improve the prognosis of patients with metastatic SBA compared with cisplatin (36). Limitations of our study were the lack of detailed protocols for chemotherapy and targeted therapy regimens and the absence of a history of Crohn's disease.
In summary, we have established and verified two clinically effective nomograms predicting OS and CSS for patients with primary SBA at 3- and 5-years, based on a large number of people. The prognosis of metastatic SBA is poor. Advanced age, poor differentiation, and N2 are the risk factors for metastatic SBA. Adjuvant chemotherapy can improve the prognosis of metastatic SBA to a certain extent.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
YG, HD, and YL designed the research and wrote the manuscript. YG and HD processed and analyzed the data. DW assisted in the data processing. YL conceived of the study and coordinated the study. All authors gave final approval for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.759162/full#supplementary-material
References
1. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Small bowel adenocarcinoma, version 1.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2019) 17:1109–33. doi: 10.6004/jnccn.2019.0043
2. Giuffrida P, Vanoli A, Di Sabatino A. Survival in Crohn's disease-associated small bowel adenocarcinoma. Gut. (2021) 70:997–8. doi: 10.1136/gutjnl-2020-322364
3. Aparicio T, Svrcek M, Henriques J, Afchain P, Lièvre A, Tougeron D, et al. Panel gene profiling of small bowel adenocarcinoma: results from the NADEGE prospective cohort. Int J Cancer. (2021) 148:1731–42. doi: 10.1002/ijc.33392
4. Latham A, Shia J, Patel Z, Reidy-Lagunes DL, Segal NH, Yaeger R, et al. Characterization and clinical outcomes of DNA mismatch repair-deficient small bowel adenocarcinoma. Clin Cancer Res. (2021) 27:1429–37. doi: 10.1158/1078-0432.CCR-20-2892
5. Pedersen KS, Foster NR, Overman MJ, Boland PM, Kim SS, Arrambide KA, et al. ZEBRA: a multicenter phase II study of pembrolizumab in patients with advanced small-bowel adenocarcinoma. Clin Cancer Res. (2021) 27:3641–8. doi: 10.1158/1078-0432.CCR-21-0159
6. Sakae H, Kanzaki H, Nasu J, Akimoto Y, Matsueda K, Yoshioka M, et al. The characteristics and outcomes of small bowel adenocarcinoma: a multicentre retrospective observational study. Br J Cancer. (2017) 117:1607–13. doi: 10.1038/bjc.2017.338
7. Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. (2004) 101:518–26. doi: 10.1002/cncr.20404
8. Halfdanarson TR, McWilliams RR, Donohue JH, Quevedo JF. A single-institution experience with 491 cases of small bowel adenocarcinoma. Am J Surg. (2010) 199:797–803. doi: 10.1016/j.amjsurg.2009.05.037
9. Wang S, Yang L, Ci B, Maclean M, Gerber DE, Xiao G, et al. Development and validation of a nomogram prognostic model for SCLC patients. J Thorac Oncol. (2018) 13:1338–48. doi: 10.1016/j.jtho.2018.05.037
10. Liao Y, Yin G, Fan X. The positive lymph node ratio predicts survival in T(1-4)N(1-3)M(0) non-small cell lung cancer: a nomogram using the SEER database. Front Oncol. (2020) 10:1356. doi: 10.3389/fonc.2020.01356
11. He Y, Zhu Z, Chen Y, Chen F, Wang Y, Ouyang C, et al. Development and validation of a novel diagnostic nomogram to differentiate between intestinal tuberculosis and Crohn's disease: a 6-year prospective multicenter study. Am J Gastroenterol. (2019) 114:490–9. doi: 10.14309/ajg.0000000000000064
12. Tanadini-Lang S, Rieber J, Filippi AR, Fode MM, Streblow J, Adebahr S, et al. Nomogram based overall survival prediction in stereotactic body radiotherapy for oligo-metastatic lung disease. Radiother Oncol. (2017) 123:182–8. doi: 10.1016/j.radonc.2017.01.003
13. Liang W, He J, Shen Y, Shen J, He Q, Zhang J, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung cancer: a population study of the US SEER database and a Chinese multi-institutional registry. J Clin Oncol. (2017) 35:1162–70. doi: 10.1200/JCO.2016.67.5140
14. Doll KM, Rademaker A, Sosa JA. Practical guide to surgical data sets: surveillance, epidemiology, and end results (SEER) database. JAMA Surg. (2018) 153:588–9. doi: 10.1001/jamasurg.2018.0501
15. Abdel-Rahman O. Challenging a dogma: five-year survival does not equal cure in all colorectal cancer patients. Expert Rev Anticancer Ther. (2018) 18:187–92. doi: 10.1080/14737140.2018.1409625
16. Moskowitz CS. Using free-response receiver operating characteristic curves to assess the accuracy of machine diagnosis of cancer. Jama. (2017) 318:2250–1. doi: 10.1001/jama.2017.18686
17. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. (1996) 15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
18. Zhang Z, Rousson V, Lee WC, Ferdynus C, Chen M, Qian X, et al. Decision curve analysis: a technical note. Ann Transl Med. (2018) 6:308. doi: 10.21037/atm.2018.07.02
19. Van Calster B, Wynants L, Verbeek JFM, Verbakel JY, Christodoulou E, Vickers AJ, et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol. (2018) 74:796–804. doi: 10.1016/j.eururo.2018.08.038
20. Aparicio T, Zaanan A, Svrcek M, Laurent-Puig P, Carrere N, Manfredi S, et al. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis. (2014) 46:97–104. doi: 10.1016/j.dld.2013.04.013
21. Delaunoit T, Neczyporenko F, Limburg PJ, Erlichman C. Small bowel adenocarcinoma: a rare but aggressive disease. Clin Colorectal Cancer. (2004) 4:241–8; discussion 249–51. doi: 10.3816/CCC.2004.n.023
22. Zheng Z, Zhou X, Zhang J, Zhao B, Chen C, Liu X, et al. Nomograms predict survival of patients with small bowel adenocarcinoma: a SEER-based study. Int J Clin Oncol. (2021) 26:387–98. doi: 10.1007/s10147-020-01813-8
23. Koo DH, Yun SC, Hong YS Ryu MH, Lee JL, Chang HM, et al. Systemic chemotherapy for treatment of advanced small bowel adenocarcinoma with prognostic factor analysis: retrospective study. BMC Cancer. (2011) 11:205. doi: 10.1186/1471-2407-11-205
24. Hong SH, Koh YH, Rho SY, Byun JH, Oh ST, Im KW, et al. Primary adenocarcinoma of the small intestine: presentation, prognostic factors and clinical outcome. Jpn J Clin Oncol. (2009) 39:54–61. doi: 10.1093/jjco/hyn122
25. Aparicio T, Henriques J, Manfredi S, Tougeron D, Bouché O, Pezet D, et al. Small bowel adenocarcinoma: results from a nationwide prospective ARCAD-NADEGE cohort study of 347 patients. Int J Cancer. (2020) 147:967–77. doi: 10.1002/ijc.32860
26. Huffman BM, Jin Z, Yadav S, Patel S, Nagorney DM, Truty MJ, et al. Novel prognostic factors in resected small bowel adenocarcinoma. Clin Colorectal Cancer. (2019) 18:218–25. doi: 10.1016/j.clcc.2019.05.002
27. Li N, Shen W, Deng W, Yang H, Ma Y, Bie L, et al. Clinical features and the efficacy of adjuvant chemotherapy in resectable small bowel adenocarcinoma: a single-center, long-term analysis. Ann Transl Med. (2020) 8:949. doi: 10.21037/atm-20-1503
28. Ecker BL, McMillan MT, Datta J, Mamtani R, Giantonio BJ, Dempsey DT, et al. Efficacy of adjuvant chemotherapy for small bowel adenocarcinoma: a propensity score-matched analysis. Cancer. (2016) 122:693–701. doi: 10.1002/cncr.29840
29. Aydin D, Sendur MA, Kefeli U, Unal OU, Tastekin D, Akyol M, et al. Evaluation of prognostic factors and adjuvant chemotherapy in patients with small bowel adenocarcinoma who underwent curative resection. Clin Colorectal Cancer. (2017) 16:220–7. doi: 10.1016/j.clcc.2016.08.002
30. Tzeng CW, Fiveash JB, Heslin MJ. Radiation therapy for retroperitoneal sarcoma. Expert Rev Anticancer Ther. (2006) 6:1251–60. doi: 10.1586/14737140.6.8.1251
31. Wilhelm A, Galata C, Beutner U, Schmied BM, Warschkow R, Steffen T, et al. Duodenal localization is a negative predictor of survival after small bowel adenocarcinoma resection: a population-based, propensity score-matched analysis. J Surg Oncol. (2018) 117:397–408. doi: 10.1002/jso.24877
32. Falcone R, Romiti A, Filetti M, Roberto M, Righini R, Botticelli A, et al. Impact of tumor site on the prognosis of small bowel adenocarcinoma. Tumori. (2019) 105:524–8. doi: 10.1177/0300891619839297
33. Rompteaux P, Gagnière J, Gornet JM, Coriat R, Baumgaertner I, Lecomte T, et al. Resection of small bowel adenocarcinoma metastases: results of the ARCAD-NADEGE cohort study. Eur J Surg Oncol. (2019) 45:331–5. doi: 10.1016/j.ejso.2018.11.012
34. Nishikawa Y, Hoshino N, Horimatsu T, Funakoshi T, Hida K, Sakai Y, et al. Chemotherapy for patients with unresectable or metastatic small bowel adenocarcinoma: a systematic review. Int J Clin Oncol. (2020) 25:1441–9. doi: 10.1007/s10147-020-01703-z
35. Legué LM, Bernards N, Lemmens VE, de Hingh IH, Creemers GJ, van Erning FN. Palliative chemotherapy for patients with synchronous metastases of small-bowel adenocarcinoma: a reflection of daily practice. Unit Eur Gastroenterol J. (2019) 7:1380–8. doi: 10.1177/2050640619858211
Keywords: small bowel adenocarcinoma, cancer-specific survival, overall survival, nomogram, metastasis
Citation: Gu Y, Deng H, Wang D and Li Y (2021) Metastasis Pattern and Survival Analysis in Primary Small Bowel Adenocarcinoma: A SEER-Based Study. Front. Surg. 8:759162. doi: 10.3389/fsurg.2021.759162
Received: 16 August 2021; Accepted: 25 October 2021;
Published: 07 December 2021.
Edited by:
Sanjit Mukherjee, National Institutes of Health (NIH), United StatesReviewed by:
Yi Liao, Affiliated Hospital of Southwest Medical University, ChinaYuqian Feng, Zhejiang Chinese Medical University, China
Copyright © 2021 Gu, Deng, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yumin Li, bGl5bUBsenUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yanmei Gu
Yanmei Gu Haixiao Deng1†
Haixiao Deng1† Yumin Li
Yumin Li