- 1Department of Anesthesiology, The Ohio State University Medical Center, Columbus, OH, United States
- 2Center for Biostatistics, Department of Biomedical Informatics, The Ohio State University Medical Center, Columbus, OH, United States
Background: Recently formed ileostomies may produce an average of 1,200 ml of watery stool per day, while an established ileostomy output varies between 600–800 ml per day. The reported incidence of renal impartment in patients with ileostomy is 8–20%, which could be caused by dehydration (up to 50%) or high output stoma (up to 40%). There is a lack of evidence if an ileostomy could influence perioperative fluid management and/or surgical outcomes.
Methods: Subjects aged ≥18 years old with an established ileostomy scheduled to undergo an elective non-ileostomy-related major abdominal surgery under general anesthesia lasting more than 2 h and requiring hospitalization were included in the study. The primary outcome was to assess the incidence of perioperative complications within 30 days after surgery.
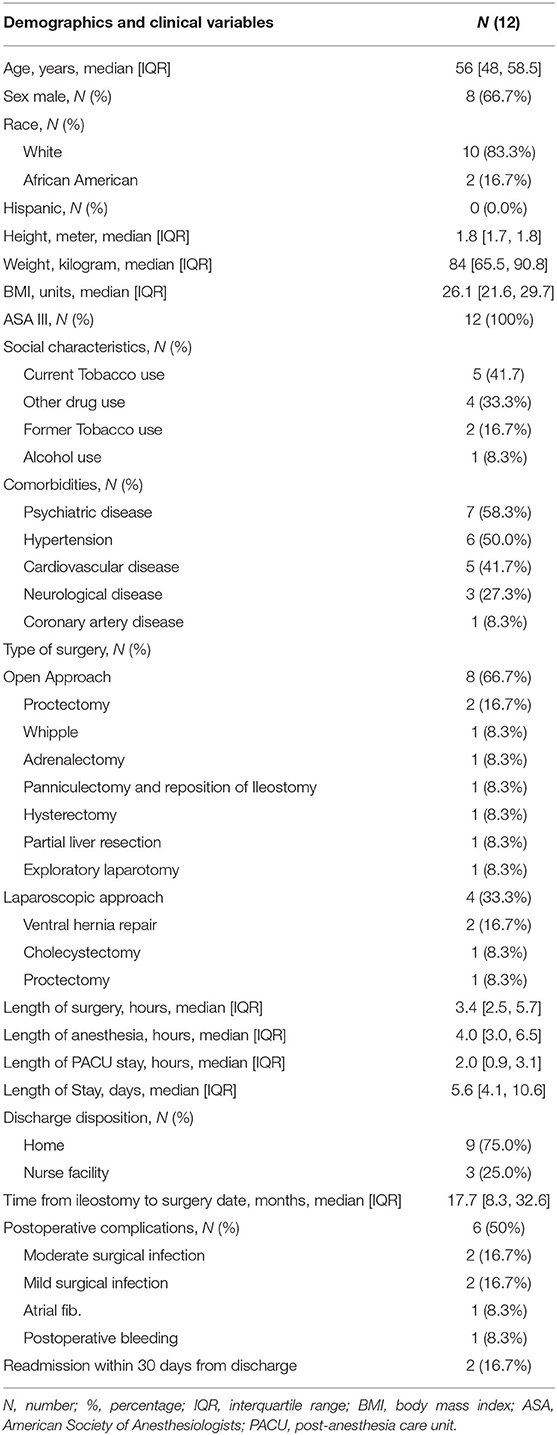
Results: A total of 552 potential subjects who underwent non-ileostomy-related abdominal surgery were screened, but only 12 were included in the statistical analysis. In our study cohort, 66.7% of the subjects were men and the median age was 56 years old (interquartile range [IQR] 48-59). The median time from the creation of ileostomy to the qualifying surgery was 17.7 months (IQR: 8.3, 32.6). The most prevalent comorbidities in the study group were psychiatric disorders (58.3%), hypertension (50%), and cardiovascular disease (41.7%). The most predominant surgical approach was open (8 [67%]). The median surgical and anesthesia length was 3.4 h (IQR: 2.5, 5.7) and 4 h (IQR: 3, 6.5), respectively. The median post-anesthesia care unit (PACU) stay was 2 h (IQR:0.9, 3.1), while the median length of hospital stay (LOS) was 5.6 days (IQR: 4.1, 10.6). The overall incidence of postoperative complications was 50% (n = 6). Two subjects (16.7%) had a moderate surgical wound infection, and two subjects (16.7%) experienced a mild surgical wound infection. In addition, one subject (7.6%) developed a major postoperative complication with atrial fibrillation in conjunction with moderate hemorrhage.
Conclusions: Our findings suggest that the presence of a well-established ileostomy might not represent a relevant risk factor for significant perioperative complications related to fluid management or hospital readmission. However, the presence of peristomal skin complications could trigger a higher incidence of surgical wound infections.
Introduction
Temporary or permanent diverting loop ileostomies are commonly created to protect a distal anastomosis with a high risk of anastomotic leakage, resulting in reduced morbidity and mortality (1). Ileostomies are performed as part of either emergency or elective procedures. Having less viscous effluence at a higher volume is associated with early postoperative complications. Recently formed ileostomies may produce an average of 1,200 ml of watery stool per day (2), while an established ileostomy (longer than 1 year) output varies between 600 and 800 ml per day (3, 4). Excessive fluid loss through the stoma and the inability of the small bowel to preserve sodium, chloride, and bicarbonate may result in life-threatening complications such as acute dehydration, electrolyte imbalance, and acid-base disorder (5–8). Furthermore, the loss of excessive amounts of sodium in stools contributes to chronic sodium depletion and dehydration with secondary hyperaldosteronism as compensatory post-ileostomy adaptation (9). Conversely, patients with long-standing ileostomies often experience hypomagnesemia, decreased absorption of folic acid and vitamin B-12 (9).
Acute or chronic intestinal failure is a long-term complication after ileostomy (7, 8, 10). High-output stoma (HOS) is defined as a stoma with an output of 1–2 L over a 24-h period and may develop within the first-month post-ileostomy up to 16% of patients (2, 11). The most common causes of HOS include, but are not limited to, intestinal resection, partial bowel obstruction, chronic bowel dysfunction (dysmotility disorders, radiation enteritis), intra-abdominal sepsis, steroid withdrawal following surgery for inflammatory bowel disease (IBD), prokinetic drugs, and Clostridium difficile infection (2, 12). Dehydration, electrolyte depletion, and acute kidney injury (AKI) are some of the acute complications of HOS, and malnutrition and chronic kidney disease (CKD) are identified as late complications (10, 13–15). The reported incidence of renal impartment in patients with ileostomy is 0.8–20%, and this complication could be caused by dehydration (up to 50%) or HOS (up to 40%) (15). Although, the literature describes dehydration and renal failure as potential complications in this surgical population, there is a lack of evidence if an ileostomy could influence perioperative fluid management and/or surgical outcomes. In addition, there is a lack of information regarding the perioperative consideration and management of patients with an established ileostomy undergoing non-ileostomy related surgeries. Therefore, we designed a retrospective study to assess the incidence of perioperative complications in patients with an established ileostomy undergoing non-ileostomy related surgical procedures.
Materials and Methods
We conducted a retrospective, single-center, observational chart review of subjects with an established ileostomy who underwent non-ileostomy-related major abdominal surgeries under general anesthesia at The Ohio State University Wexner Medical Center between May 1, 2014, and May 31, 2019. After obtaining the approval of our Institutional Review Board (Office of Responsible Research Practices, The Ohio State University), we accessed the electronic medical records to evaluate eligibility and collect perioperative data from eligible subjects.
Participants
Subjects aged ≥18 years old with an established ileostomy who were scheduled to undergo an elective non-ileostomy related major surgery under general anesthesia lasting more than 2 h and requiring hospitalization between May 1, 2014, and May 31, 2019, at The Ohio State University Wexner Medical Center were included in the study. In contrast, prisoners, pregnant or breastfeeding women, subjects with CKD before surgery, newly established ileostomies, and surgical procedures lasting <2 h were excluded from the analysis.
Clinical Outcomes
The primary outcome was to assess the incidence of perioperative complications within 30 days after surgery. The secondary outcomes were to identify the potential risk factors associated with the perioperative complications, including subject demographics and perioperative variables.
Data Collection and Data Management
The following preoperative information was retrieved from the electronic medical records and collected in a data sheet created as part of our electronic data capture system: demographics (age, gender, weight, height, median body mass index [BMI]), preoperative American Society of Anesthesiologists (ASA) physical status, history of smoking, mental disorders, neurologic disease or any other diagnosed cognitive impairment, alcohol or drug abuse, coronary artery disease, active malignancy, and concomitant medications. The perioperative variables collected included time from the ileostomy creation to the surgery date, type of surgery and anesthesia, length of anesthesia, estimated blood loss (EBL), post-anesthesia care unit (PACU) stay, intensive care unit (ICU) stay (if available), vital signs, significant laboratory results, intraoperative administration of vasoactive drugs and fluid administration, perioperative blood transfusion requirements, diuresis, and ostomy output. In addition, other inpatient variables such as discharge disposition, readmissions, and perioperative complications within the first month after surgery were reviewed.
Statistical Analysis
Continuous variables of demographic data, clinical characteristics, lab values, and hospital readmissions were summarized using descriptive statistics and expressed as median [interquartile range (IQR)] for continuous variables and frequency (percentage) for categorical variables. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
We screened 552 potential subjects who underwent non-ileostomy-related abdominal surgery during hospitalization at the Ohio State University Wexner Medical Center between May 1, 2014, and May 31, 2019. Of this subject list, 540 subjects were excluded due to ileostomy creation during surgery (n = 512), surgery length <2 h (n = 23), and non-abdominal surgeries (n = 5). Therefore, a total of 12 subjects were included in the statistical analysis. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in Figure 1 (16).
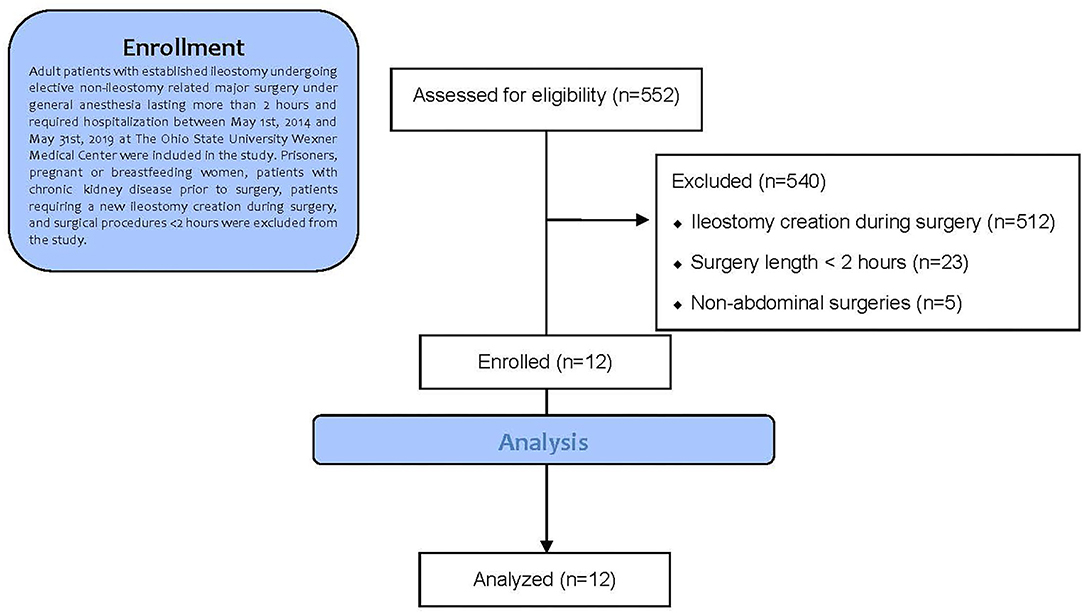
Figure 1. Trial profile according to consolidated standards of reporting trials (CONSORT) guidelines.
In our study cohort, 66.7% of the subjects were men, the median age was 56 years old ([IQR] 48–59), and the median BMI was 26.1 kg/m2 (IQR: 21.6, 29.7). All the subjects had an ASA physical status of 3 and the median time from the creation of ileostomy to the qualifying surgery was 17.7 months (IQR: 8.3, 32.6). The most predominant surgical approach was open (8 [67%] subjects). The most common type of open surgery was proctectomy (2 [16.7%] subjects) and the most common type of laparoscopic surgery was ventral hernia repair (3 [25%] subjects).
The most prevalent comorbidities in the study group were psychiatric disorders (58.3%), hypertension (50%), and cardiovascular disease (41.7%). At the time of surgery, 47.7% were current smokers and 16.7% were former smokers; in addition, around 33.3% used another type of drug and 8.3% consumed alcohol. The median surgical and anesthesia length was 3.4 h (IQR: 2.5, 5.7) and 4 h (IQR: 3, 6.5), respectively. The median PACU stay was 2 h (IQR:0.9, 3.1), while the median length of hospital stay (LOS) was 5.6 days (IQR: 4.1, 10.6) (Table 1).
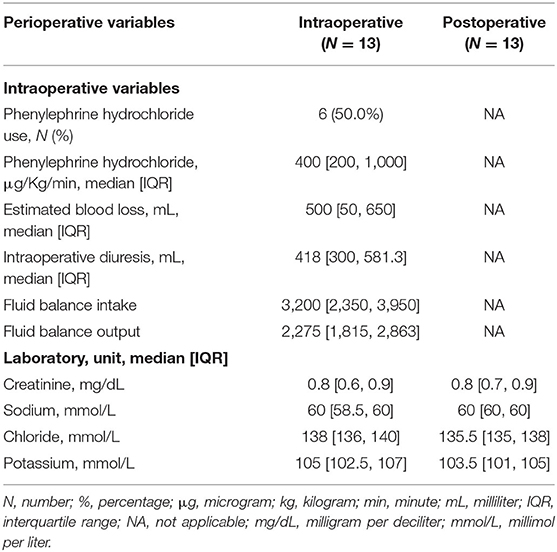
The median and IQRs for intraoperative variables were as follows: fluid administration 3,200 ml (IQR: 2,350, 3,950 ml), fluid balance output 2,275 ml (IQR: 1,815, 2,863 ml), EBL 500 ml (IQR: 50, 650), and diuresis 418 ml (IQR: 300, 581.3). Six subjects (50%) required the administration of vasopressors (phenylephrine hydrochloride) to maintain adequate values of perfusion pressures during surgery, mainly after the induction of anesthesia; the median dose of phenylephrine hydrochloride used was 400 μg/kg/min (IQR: 200, 1,000) (Table 2). The intra- and postoperative median values of serum creatinine, sodium, chloride, and potassium were within normal ranges (reported in Table 2).
The overall incidence of major and minor postoperative complications was 33.3% (n = 4) and 16.7% (n = 2), respectively. Two subjects (16.7%) had a moderate surgical wound infection, two subjects (16.7%) experienced a mild surgical wound infection (did not require surgical drainage and subsided with antibiotic therapy). In addition, one subject (7.6%) developed a major postoperative complication with atrial fibrillation in conjunction with moderate hemorrhaging that required two units of blood transfusion. All the patients were discharged in stable conditions, nine subjects (75%) were discharged home, while three subjects (25%) were discharged to nursing facilities. The readmission rate at 30 days after discharge was 16.7 % and no deaths were reported (Table 1).
Discussion
This article provides the first-ever retrospective observational cohort study that assessed the incidence of perioperative complications and analyzed clinical outcomes in subjects with established ileostomy scheduled to undergo elective non-ileostomy-related major abdominal surgery under general anesthesia lasting more than 2 h. The incidence of postoperative complications in our cohort was 46.1%. The postoperative complications that we found in our study (surgical wound infection, acute bleeding, and atrial fibrillation) could not be directly attributed to the existence of an established ileostomy at the time of the surgeries of the subjects. Two subjects (16.7%) were readmitted within 30 days of discharge for the management of untreatable pain due to an extension of underlying malignancy. Furthermore, the reported incidence of postoperative complications after extensive abdominal surgeries could reach up to 45%, especially in patients undergoing surgery due to gastrointestinal, hepatobiliary, and/or pancreatic malignancies (17–20).
In a recent retrospective cohort study, Li et al. reported a significant association between ileostomy creation, the onset of AKI, and progression to CKD in patients undergoing colorectal cancer surgery (14). In addition, the authors identified ileostomy as an important predictor for AKI-related readmissions (Odds ratio [OR]: 10.3; 95% CI: 3.9–27.2) and severe CKD after a year (OR: 4.1; 95% CI: 1.4–11.9) (14). Likewise, this significant association between ileostomy, AKI, and progression to CKD has been reported in other studies (21–23). In our patient cohort, the incidence of postoperative complications was 50% having mild or moderate surgical wound infection, acute bleeding, and/or atrial fibrillation were reported.
Messaris et al. reviewed 603 patients undergoing colorectal surgery and diverting loop ileostomy to identify predictive factors for readmission (7). The main preoperative diagnoses were irritable bowel syndrome (50.9%) and rectal cancer (16.1%) (7). The authors reported a total incidence of readmission at 60 days after surgery of 16.9%, with dehydration being the most common cause (43.1%), especially in those patients receiving diuretics as concomitant medication (7). We found a similar readmission rate at 30 days (16.7%) after surgery in our patient setting. However, the main identified cause of readmission in our study was cancer-related severe pain associated with the progression of the malignant disease.
Dehydration, electrolyte alterations, infections, obstruction, prolapse, hernias, AKI, and stoma-related complications are commonly found in patients with established ileostomies (12, 14, 24–27). Colon resections and ileostomy creation may lead to volume depletion, subsequent acid-base and electrolyte instability. Most of these complications usually occur within the first 2 postoperative months (26, 28). Additionally, the time of the ileostomy has been directly associated with an increased risk of enteral ostomy-related complications (25, 29, 30). Even though the median time elapsed from the ileostomy creation and the non-ileostomy surgery in our study was 17.7 months (IQR: 8.3, 32.6), we found no evidence of postoperative ostomy-related complications (e.g., HOS), electrolyte imbalance, and impaired renal function in our patient setting. However, we should consider that due to the median time elapsed from the ileostomy creation and the non-ileostomy targeted surgery on this study of 17.7 months, our sample population had a more robust compensated physiological state regarding intravascular volume depletion and electrolyte imbalance in comparison with patients assessed in other studies during the acute period of ileostomy adaptation (2 months after ileostomy creation). Additionally, 50% of our patients required an intraoperative administration of vasopressors to maintain their hemodynamic stability, mainly during the induction of anesthesia. These episodes of hypotension could be attributed to the combined effect of anesthesia-related effects on cardiovascular function (e.g., heart contractility, arterial/venous vasodilation) in addition to occult hypovolemia, a frequent finding in patients with long-term ileostomies (31, 32). Anesthesia-induced venodilation is widely recognized as one of the main causes of relative hypovolemia, increasing venous compliance ensuing a subsequent decrease in venous return, preload, and response to vasopressors (33). This temporary state of relative hypovolemia could be clinically undetected by anesthesia care providers, resulting in potential oxygen delivery and/or tissue perfusion impairment (33). Therefore, it is important to recognize the presence of occult intravascular volume depletion and correct it, as a measure to attenuate the cardiovascular impact of the anesthetic drugs and reduce the incidence of intraoperative hypotension. In a recent publication, Ejaz et al. (34) identified intra- and post-operative major blood loss requiring blood transfusion, and tachypnea as perioperative risk factors linked to postoperative surgical site infection (SSI) after major abdominal surgery, while other common clinical situations like intraoperative hypothermia, hyperthermia, bradycardia, tachycardia, hypotension, and hypertension were not associated with SSIs. Babazade et al. (35) retrospectively studied 2,531 patients who underwent colorectal surgery and did not find any correlation between intraoperative hypotension and SSI after colorectal surgery. Yilmaz et al. (36) conducted a retrospective, cohort study in 5,896 patients who sustained colorectal surgery and concluded that postoperative hypotension was not associated with SSI. Conversely, the two subjects (16.7%) that developed moderate surgical wound infection did not present with sustained intraoperative episodes of hypotension or recorded hypotension during PACU or ward hospitalization, while the patient that experienced atrial fibrillation after postoperative bleeding that required blood transfusion did not develop a surgical wound infection.
A recent meta-analysis conducted by Gavriilidis et al. compared the incidence of SSI among patients that underwent loop transverse colostomy (N = 628) vs. loop ileostomy (N = 906) (37). The study found a lower incidence of SSI among the subjects that underwent loop ileostomy (1%; 8/575; p < 0.001) when compared with the loop transverse colostomy group (5%; 14/299) (37). On the other hand, the shaving or constant pulling of the hair adhesives around the stoma during the replacement of the appliances leads to the presence of folliculitis (caused by Staphylococcus aureus) that could trigger the postoperative SSI complication in this vulnerable surgical population (38, 39). Our study showed a high incidence (33.4%) of SSI (mild or moderate) that might be related to the aforementioned peristomal skin complication.
The most common comorbidity found in our study was psychiatric disorders (58.3%). This finding is supported by robust evidence that showed an increased incidence of psychosocial conditions in individuals with a stoma, which has a negative impact on their quality of life (40–42).
A retrospective study conducted by Vergara-Fernandez et al. assessed the potential predictors of high output ileostomy-related complications (1). The study included 102 adult patients undergoing colorectal surgery with primary low pelvic anastomosis and diverting loop ileostomy at elective or emergency settings. The authors concluded that patients with a history of ulcerative colitis and those with a current ileostomy output >1 L/day at discharge were more likely to develop high output-related complications (1).
We acknowledge the limitations in our study that should be considered. First, the single-center and retrospective design of the study limited our analysis, as well our ability to identify reliable statistical significance in the primary and secondary objectives. Second, the small sample size (12 patients) may increase the probability of a Type II error biasing the accuracy of our findings. Third, and perhaps the most significant limitation, we found a high variability of the median time from the creation of ileostomy to surgery date (17.7 months, IQR: 8.3, 32.6). This could have had an important impact on our findings considering that most of the ileostomy-related complications occur during the first 12 months after ileostomy creation. Lastly, certain limitations linked to the retrospective nature of our study (e.g., data not collected and/or measured) should also be considered.
Conclusions
The perioperative management of patients with established ileostomies undergoing major abdominal surgeries might be challenging to anesthesia care providers and surgeons. Despite AKI and dehydration is the most common complications after ileostomy is formed, occult hypovolemia and electrolyte imbalance assessment must be treated and/or corrected prior to anesthesia induction to avoid other short- and long-term complications, regardless of the active length of the functioning ileostomy. Hence, hemodynamic and laboratory parameters should be closely monitored throughout the perioperative period. Our findings suggest that the presence of a well-established (longer than a year) ileostomy might not represent a relevant risk factor for significant perioperative complications related to fluid management or hospital readmission in patients undergoing abdominal surgery unrelated to a functioning ileostomy. However, the presence of peristomal skin complications could trigger a higher incidence of surgical wound infection. Nevertheless, we are not able to form robust conclusions due to the small sample size; thus, future prospective trials focusing on short-term outcomes and complications in this patient setting may better identify the pros and cons of different perioperative approaches.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Office of Responsible Research Practices—The Ohio State University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
AU, TW, ME-V, JF-D, and LL: conceptualization. AU, ME-V, LP, HS, JF-D, and AG-Z: data curation. AU, ME-V, and MA-R: formal analysis. AU, TW, ME-V, LP, HS, JF-D, AG-Z, and LL: investigation. AU, LP, HS, and LL: methodology. AU, TW, and LL: project administration, resources, supervision, and visualization. AU, TW, MA-R, and LL: validation. AU, TW, ME-V, and JF-D: writing—original draft. AU, TW, ME-V, LP, HS, JF-D, AG-Z, MA-R, and LL: writing review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors gratefully acknowledge Alicia Nahhas, BS, and Joshua-Paolo Reyes, BS for their editing collaboration (they provided authorization to be named on this publication). The project described was supported by a grant (no. UL1TR002733) from the National Center for Advancing Translational Sciences, awarded to the Ohio State University Center for Clinical and Translational Science via an internal grant to Dr. Li for biostatistics support.
Abbreviations
HOS, high-output stoma; IBD, inflammatory bowel disease; AKI, acute kidney injury; CKD, chronic kidney disease; ASA, American Society of Anesthesiologists; BMI, body mass index; EBL, estimated blood loss; PACU, post-anesthesia care unit; ICU, intensive care unit; IQR, Interquartile Range; CONSORT, Consolidated Standards of Reporting Trials; LOS, length of hospital stay; OR, Odds ratio; SSI, surgical site infection.
References
1. Vergara-Fernández O, Trejo-Avila M, Santes O, Solórzano-Vicuña D, Salgado-Nesme N. Predictors of dehydration and acute renal failure in patients with diverting loop ileostomy creation after colorectal surgery. World J Clinic cases. (2019) 7:1805. doi: 10.12998/wjcc.v7.i14.1805
2. Baker ML, Williams RN, Nightingale JM. Causes and management of a high-output stoma. Colorectal Dis. (2011) 13:191–7. doi: 10.1111/j.1463-1318.2009.02107.x
3. Goodey A, Colman S. Safe management of ileostomates with high-output stomas. Br J Nurs. (2016) 25:S4–s9. doi: 10.12968/bjon.2016.25.22.S4
4. Rowan AM, Moughan PJ, Wilson MN. Endogenous amino acid flow at the terminal ileum of adult humans determined following the ingestion of a single protein-free meal. J Sci Food Agric. (1993) 61:439–42. doi: 10.1002/jsfa.2740610410
5. Hallböök O, Matthiessen P, Leinsköld T, Nyström PO, Sjödahl R. Safety of the temporary loop ileostomy. Colorectal Dis. (2002) 4:361–4. doi: 10.1046/j.1463-1318.2002.00398.x
6. Kellum JA, Bellomo R, Ronco CB. Renal disease and failure. Kidney. (2009) 18:80–85. doi: 10.1213/ANE.0000000000004641
7. Messaris E, Sehgal R, Deiling S, Koltun WA, Stewart D, McKenna K, et al. Dehydration is the most common indication for readmission after diverting ileostomy creation. Dis Colon Rect. (2012) 55:175–80.
8. Paquette IM, Solan P, Rafferty JF, Ferguson MA, Davis BR. Readmission for dehydration or renal failure after ileostomy creation. Diseases of the colon & rectum. (2013) 56:974. doi: 10.1097/DCR.0b013e31823d0ec5
9. Shabbir J, Britton D. Stoma complications: a literature overview. Colorectal Dis. (2010) 12:958–64. doi: 10.1111/j.1463-1318.2009.02006.x
10. Nightingale J, Woodward JM. Guidelines for management of patients with a short bowel. Gut. (2006) 55:iv1–12. doi: 10.1136/gut.2006.091108
11. Mountford CG, Manas DM, Thompson NP. A practical approach to the management of high-output stoma. Frontline Gastroenterol. (2014) 5:203–7. doi: 10.1136/flgastro-2013-100375
12. Murken DR, Bleier JI. Complications and Dilemmas in Colorectal Surgery: Ostomy-Related Complications. Clin Colon Rectal Surg. (2019) 32:176. doi: 10.1055/s-0038-1676995
13. Villafranca JJ, López-Rodríguez C, Abilés J, Rivera R, Adán NG, Navarro PU. Protocol for the detection and nutritional management of high-output stomas. Nutr J. (2015) 14:1–7. doi: 10.1186/s12937-015-0034-z
14. Li L, Lau KS, Ramanathan V, Orcutt ST, Sansgiry S, Albo D, et al. Ileostomy creation in colorectal cancer surgery: risk of acute kidney injury and chronic kidney disease. J Surg Res. (2017) 210:204–12. doi: 10.1016/j.jss.2016.11.039
15. Beck-Kaltenbach N, Voigt K, Rumstadt B. Renal impairment caused by temporary loop ileostomy. Int J Colorectal Dis. (2011) 26:623–6. doi: 10.1007/s00384-010-1086-3
16. Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. (1996) 276:637–9. doi: 10.1001/jama.1996.03540080059030
17. Thompson JS, Baxter BT, Allison JG, Johnson FE, Lee KK, Park WY. Temporal patterns of postoperative complications. Arch Surg. (2003) 138:596–603. doi: 10.1001/archsurg.138.6.596
18. Jakobson T, Karjagin J, Vipp L, Padar M, Parik AH, Starkopf L, et al. Postoperative complications and mortality after major gastrointestinal surgery. Medicina. (2014) 50:111–7. doi: 10.1016/j.medici.2014.06.002
19. Simões CM, Carmona MJ, Hajjar LA, Vincent JL, Landoni G, Belletti A, et al. Predictors of major complications after elective abdominal surgery in cancer patients. BMC Anesthesiol. (2018) 18:1–8. doi: 10.1186/s12871-018-0516-6
20. Woodfield J, Deo P, Davidson A, Chen TY, van Rij A. Patient reporting of complications after surgery: what impact does documenting postoperative problems from the perspective of the patient using telephone interview and postal questionnaires have on the identification of complications after surgery? BMJ open (2019) 9:e028561. doi: 10.1136/bmjopen-2018-028561
21. Fielding A, Woods R, Moosvi SR, Wharton RQ, Speakman CT, Kapur S, et al. Renal impairment after ileostomy formation: a frequent event with long-term consequences. Colorectal Disease. (2020) 22:269–78. doi: 10.1111/codi.14866
22. Collaborative S. Prognostic model to predict postoperative acute kidney injury in patients undergoing major gastrointestinal surgery based on a national prospective observational cohort study. BJS open. (2019) 3:549. doi: 10.1002/bjs5.50199
23. Kee YK, Kim H, Jhee JH, Han SH, Yoo TH, Kang SW, et al. Incidence of and risk factors for delayed acute kidney injury in patients undergoing colorectal surgery. Am J Surg. (2019) 218:907–12. doi: 10.1016/j.amjsurg.2019.03.027
24. Weise WJ, Serrano FA, Fought J, Gennari FJ. Acute electrolyte and acid-base disorders in patients with ileostomies: a case series. Am J Kidney Dis. (2008) 52:494–500. doi: 10.1053/j.ajkd.2008.04.015
25. Rubio-Perez I, Leon M, Pastor D, Dominguez JD, Cantero R. Increased postoperative complications after protective ileostomy closure delay: AN institutional study. World J Gastrointest Surg. (2014) 6:169. doi: 10.4240/wjgs.v6.i9.169
26. Jayarajah U, Samarasekara AM, Samarasekera DN. A study of long-term complications associated with enteral ostomy and their contributory factors. BMC Res Notes. (2016) 9:1–6. doi: 10.1186/s13104-016-2304-z
27. Poskus E, Kildusis E, Smolskas E, Ambrazevicius M, Strupas K. Complications after loop ileostomy closure: a retrospective analysis of 132 patients. Visceral Medicine. (2014) 30:276–80. doi: 10.1159/000366218
28. Duchesne JC, Wang YZ, Weintraub SL, Boyle M. Stoma complications: A multivariate analysis/discussion. Am Surg. (2002) 68:961.
29. Alves A, Panis Y, Lelong B, Dousset B, Benoist S, Vicaut E. Randomized clinical trial of early versus delayed temporary stoma closure after proctectomy. J Br Surg. (2008) 95:693–8. doi: 10.1002/bjs.6212
30. Keane C, Park J, Öberg S, Wedin A, Bock D, O'Grady G, et al. Functional outcomes from a randomized trial of early closure of temporary ileostomy after rectal excision for cancer. J Br Surg. (2019) 106:645–52. doi: 10.1002/bjs.11092
31. Justiniano CF, Temple LK, Swanger AA, Xu Z, Speranza JR, Cellini C, et al. Readmissions with dehydration after ileostomy creation: rethinking risk factors. Diseases of the colon and rectum. (2018) 61:1297. doi: 10.1097/DCR.0000000000001137
32. Chen SY, Stem M, Cerullo M, Canner JK, Gearhart SL, Safar B, et al. Predicting the risk of readmission from dehydration after ileostomy formation: The DRIP score. Dis Colon Rectum. (2018) 61:1410. doi: 10.1097/DCR.0000000000001217
33. Noel-Morgan J, Muir W. Anesthesia-Associated Relative Hypovolemia: Mechanisms, Monitoring, and Treatment Considerations. Front Vet Sci. (2018) 5: doi: 10.3389/fvets.2018.00053
34. Ejaz A, Schmidt C, Johnston FM, Frank SM, Pawlik TM. Risk factors and prediction model for inpatient surgical site infection after major abdominal surgery. Journal of Surgical Research. (2017) 217:153–9. doi: 10.1016/j.jss.2017.05.018
35. Babazade R, Yilmaz HO, Zimmerman NM, Stocchi L, Gorgun E, Kessler H, et al. Association between intraoperative low blood pressure and development of surgical site infection after colorectal surgery. Ann Surg. (2016) 264:1058. doi: 10.1097/SLA.0000000000001607
36. Yilmaz HO, Babazade R, Leung S, Zimmerman NM, Makarova N, Saasouh W, et al. Postoperative hypotension and surgical site infections after colorectal surgery: a retrospective cohort study. Anesthesia & Analgesia. (2018) 127:1129–36. doi: 10.1213/ANE.0000000000003666
37. Gavriilidis P, Azoulay D, Taflampas P. Loop transverse colostomy versus loop ileostomy for defunctioning of colorectal anastomosis: a systematic review, updated conventional meta-analysis, and cumulative meta-analysis. Surg Today. (2019) 49:108–17. doi: 10.1007/s00595-018-1708-x
38. Almutairi D, LeBlanc K, Alavi A. Peristomal skin complications: what dermatologists need to know. Int J Dermatol. (2018) 57:257–64. doi: 10.1111/ijd.13710
39. Woo KY, Sibbald RG, Ayello EA, Coutts PM, Garde DE. Peristomal skin complications and management. Adv Skin Wound Care. (2009) 22:522–32. doi: 10.1097/01.ASW.0000305497.15768.cb
40. White CA, Hunt J. Psychological factors in postoperative adjustment to stoma surgery. Ann R Coll Surg Engl. (1997) 79:3.
41. Zangenberg MS, El-Hussuna A. Psychiatric morbidity after surgery for inflammatory bowel disease: A Systematic review. World J Gastroenterol. (2017) 23:8651. doi: 10.3748/wjg.v23.i48.8651
Keywords: ileostomy, complications, abdominal surgery, established, perioperative
Citation: Uribe AA, Weaver TE, Echeverria-Villalobos M, Periel L, Shi H, Fiorda-Diaz J, Gonzalez-Zacarias A, Abdel-Rasoul M and Li L (2021) Perioperative Morbidity and Complications in Patients With an Established Ileostomy Undergoing Major Abdominal Surgery: A Retrospective Study. Front. Surg. 8:757269. doi: 10.3389/fsurg.2021.757269
Received: 11 August 2021; Accepted: 25 October 2021;
Published: 08 December 2021.
Edited by:
Mahesh C. Misra, All India Institute of Medical Sciences, IndiaReviewed by:
Narendra Pandit, Birat Medical College, NepalAkshay Sood, University of Texas MD Anderson Cancer Center, United States
Copyright © 2021 Uribe, Weaver, Echeverria-Villalobos, Periel, Shi, Fiorda-Diaz, Gonzalez-Zacarias, Abdel-Rasoul and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto A. Uribe, QWxiZXJ0by5VcmliZUBvc3VtYy5lZHU=
 Alberto A. Uribe
Alberto A. Uribe Tristan E. Weaver
Tristan E. Weaver Marco Echeverria-Villalobos
Marco Echeverria-Villalobos Luis Periel1
Luis Periel1 Mahmoud Abdel-Rasoul
Mahmoud Abdel-Rasoul Lin Li
Lin Li