- Department of Thoracic and Cardiovascular Surgery, Affiliated Taizhou Hospital of Wenzhou Medical University, Taizhou, China
Objective: The skip N2 metastases were frequent in non-small-cell lung cancer (NSCLC) and the better prognosis of NSCLC with a skip over non-skip N2 lymph node metastases is controversial. The primary aim of this study is to investigate the prognosis effect of skip N2 lymph node metastases on the survival of NSCLC.
Setting: A literature search was conducted in PubMed, EMBASE, and Cochrane Library with the term of “N2” or “mediastinal lymph node” or “mediastinal nodal metastases”, and “lung cancer” and “skip” or “skipping” in the title/abstract field. The primary outcomes of interests are 3- and 5-year survival in NSCLC.
Participants: Patients who underwent complete resection by lobectomy, bilobectomy, or pneumonectomy with systemic ipsilateral lymphadenectomy and were staged as pathologically N2 were included.
Primary and Secondary Outcome Measures: The 3- and 5-year survival of NSCLC was analyzed. The impact of publication year, number of patients, baseline mean age, gender, histology, adjuvant therapy, number of skip N2 stations, and survival analysis methods on the primary outcome were also analyzed.
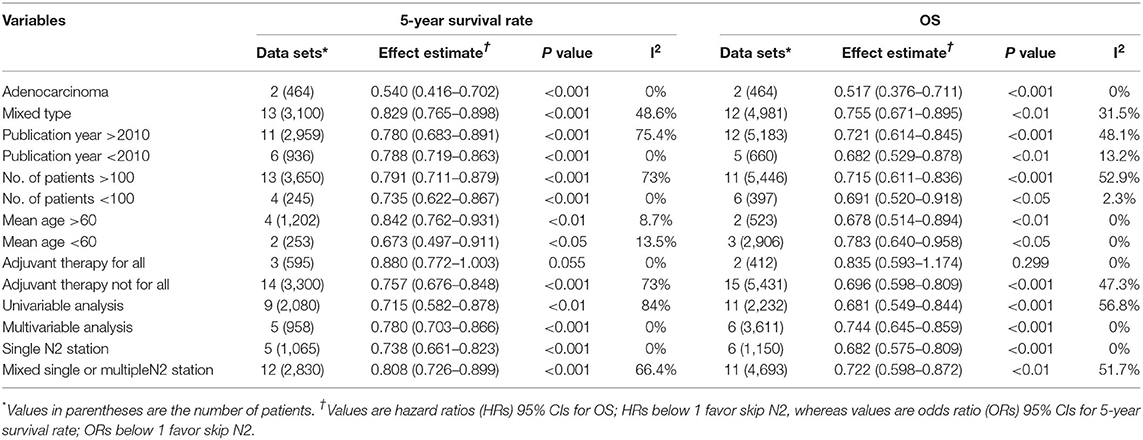
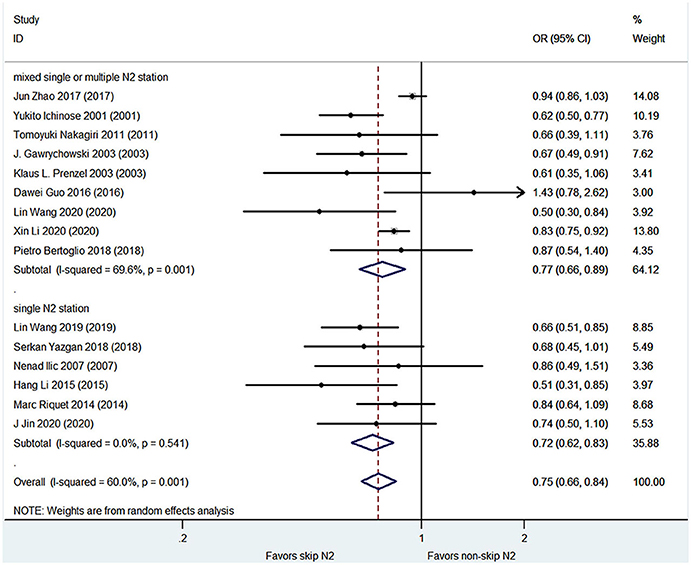
Results: A total of 21 of 409 studies with 6,806 patients met the inclusion criteria and were finally included for the analysis. The skip N2 lymph node metastases NSCLC had a significantly better overall survival (OS) than the non-skip N2 NSCLC [hazard ratio (HR), 0.71; 95% CI, 0.62–0.82; P < 0.001; I2 = 40.4%]. The skip N2 lymph node metastases NSCLC had significantly higher 3- and 5-year survival rates than the non-skip N2 lymph node metastases NSCLC (OR, 0.75; 95% CI, 0.66–0.84; P < 0.001; I2 = 60%; and OR, 0.78; 95% CI, 0.71–0.86; P < 0.001; I2 = 67.1%, respectively).
Conclusion: This meta-analysis suggests that the prognosis of skip N2 lymph node metastases NSCLC is better than that of a non-skip N2 lymph node.
Introduction
Non-small-cell lung cancer (NSCLC) is a leading cause of cancer-related death in the world (1). Approximately 30% of the NSCLC were locally advanced at the time of diagnosis (2). The most important risk factor in completely resected NSCLC is metastasis to the mediastinal lymph nodes (N2). The survival rates of N2-NSCLC after surgical treatment ranged from 19.2 to 40% which means that there was a large heterogeneity among N2-NSCLC (3–5). Skip metastases (pN0N2), pathological N2 NSCLC without hilar lymph nodes involvement (N1), were an important subgroup of N2-NSCLC. It was reported that skip N2 occurred in approximately 17.2–42.7% of surgically resected N2-NSCLC (6, 7). Skip N2 was reported to be an independent prognostic factor for better overall survival (OS) of the NSCLC (8, 9). However, the prognostic impact of skip N2 metastases was still under debate. The aim of this study was to elucidate the prognostic impact of skip N2 lymph node metastases on the OS of NSCLC through a meta-analysis of the published studies.
Materials and Methods
Search Strategy
A literature search was conducted in PubMed, EMBASE, and Cochrane Library from the date of database inception to Jan 2021. The following search terms were searched in the title/abstract field: “N2” or “mediastinal lymph node” or “mediastinal nodal metastases” and “lung cancer” and “skip” or “skipping”. Only articles in the English language were included. The reference lists of relevant review articles were checked to identify extra relevant articles.
Inclusion and Exclusion Criteria
Inclusion Criteria
Studies must include patients with NSCLC who had undergone complete resection by lobectomy, bilobectomy, or pneumonectomy with systemic ipsilateral lymphadenectomy and were staged as pathological N2.
Studies must provide survival information on the patients with skip and non-skip N2 mediastinal nodal metastases.
Exclusion Criteria
Studies were excluded if the patients underwent the surgical operation of segmentectomy, wedge resection, or lymph node sampling.
Studies were excluded if the patients who were pathological N2 with pleural effusion or distant metastases.
Articles from the same study with duplicated data.
Studies do not provide survival information on patients with a skip or non-skip N2 mediastinal nodal metastases separately.
Data Extraction
Data were extracted independently by two authors (XW, HG). If there was a disagreement, a consensus was achieved by discussion. The following data were extracted from each included article: first author, publication year, study design, inclusion criteria, number of patients, patients and tumor characteristics, operation techniques, survival curve, hazard ratio (HR), 3- and 5-year survival rates.
Quality Assessment
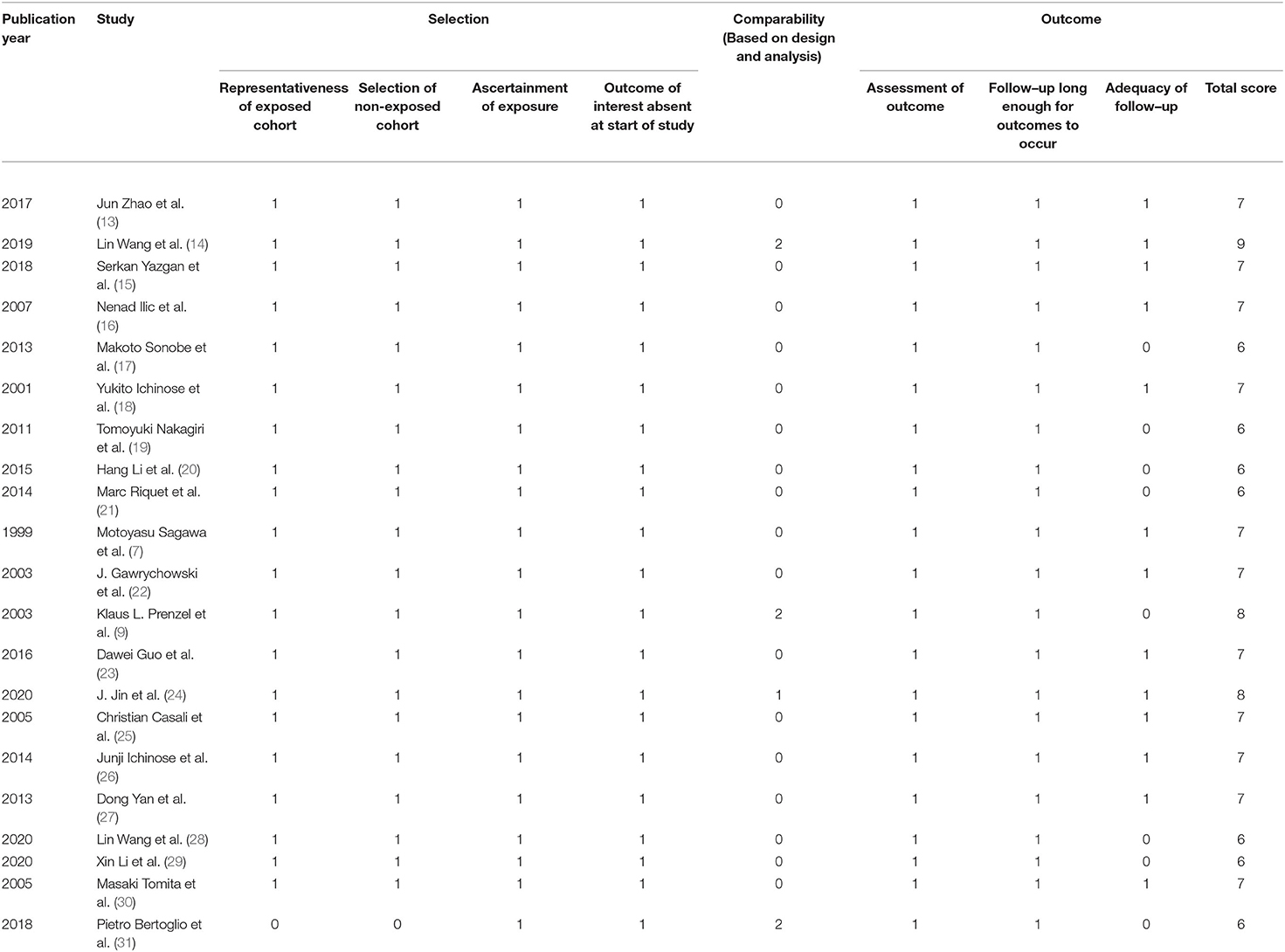
The Newcastle–Ottawa Scale (NOS) was used to access the quality of included studies, with the highest score of 9. A high-quality study was defined as a study with a score of ≥6 (10). The assessment was performed independently by two authors (QH and YY). If necessary, a third author (BC) was consulted to settle disagreements.
Statistical Methods
Stata (version 12.0; StataCorp, College Station, Texas, United States) was used to perform all the statistical analyses. Between-study heterogeneity was calculated using Higgins' I2 statistics (11). A Mantel-Haenszel random-effects meta-analysis was performed for outcomes in consideration of interstudy heterogeneity. Studies with an I2 statistics of >50% were considered of a high degree of heterogeneity. A summary of the odds ratio (OR) and its corresponding 95% CI were computed for the dichotomous outcomes. To reduce the bias caused by different follow-up periods and the timing of censored patients between the two groups, OS in terms of log-transformed HR and 95% CI was analyzed using an inverse variance model. Relevant effect measures were calculated using methods described by Tierney et al. (12). Publication bias was assessed qualitatively using funnel plots and quantitively using Egger's linear regression method. Meta-regression analysis was conducted to evaluate the effects of covariates on the pooled estimates and the heterogeneity across studies with covariates including publication year, baseline mean age, the proportion of men, operation techniques, tumor location, and histological type. The overall effect was considered statistically significant if the two-sided p-value was < 0.05.
Results
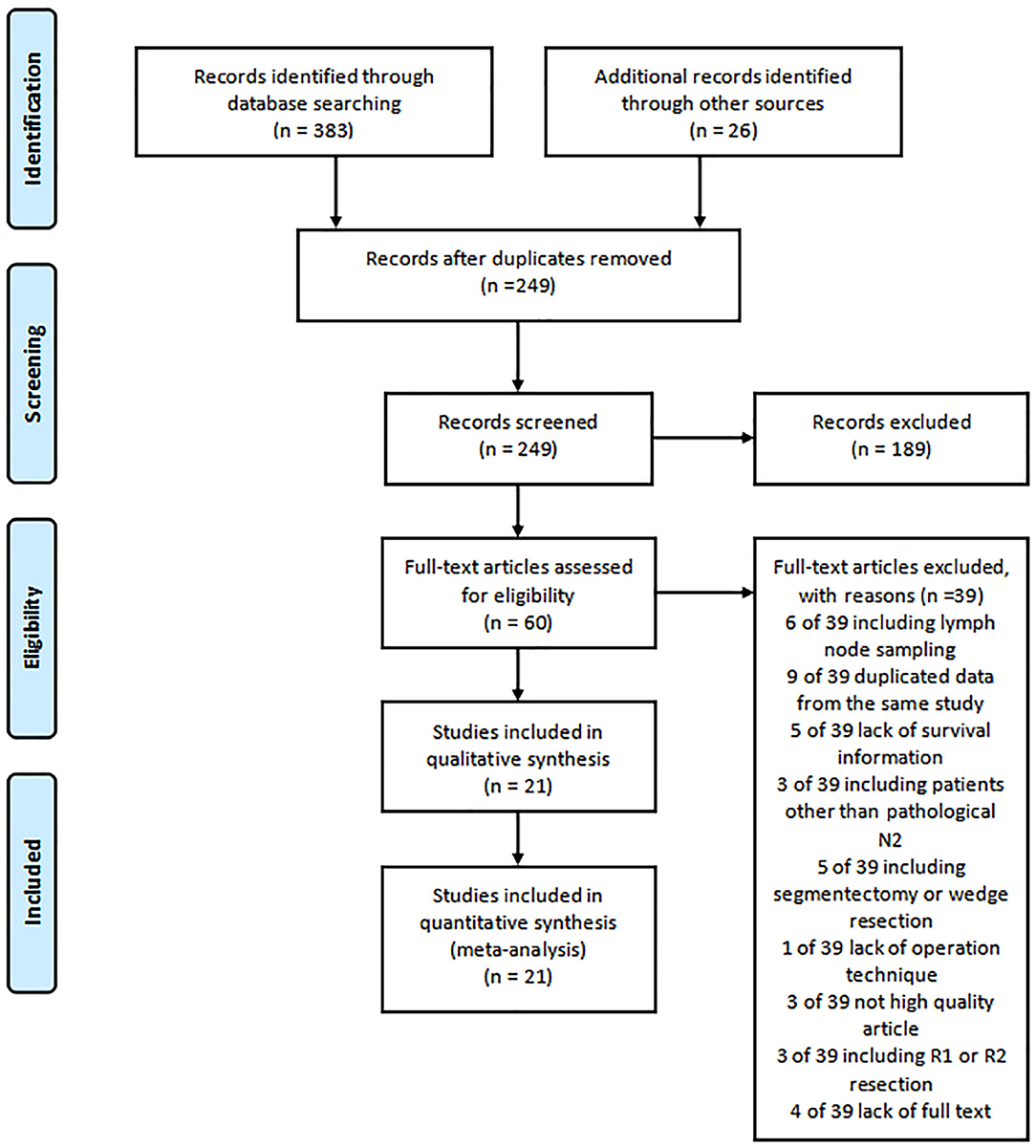
The literature search identified 409 relevant studies for review. Based on the title and abstracts, 60 studies were selected and reviewed for full text. Six studies were excluded for including patients with lymph node sampling. Nine studies were eliminated for the duplicated data. A total of five studies not providing survival information were excluded. Three studies including patients of not pathologically N2 were excluded. Five studies were eliminated because of including operation technique of segmentectomy or wedge resection. Lack of operation information was excluded in one study and three studies were eliminated for low quality scores. Three studies with patients of R1 or R2 resection were excluded. The full text was not available for four studies.
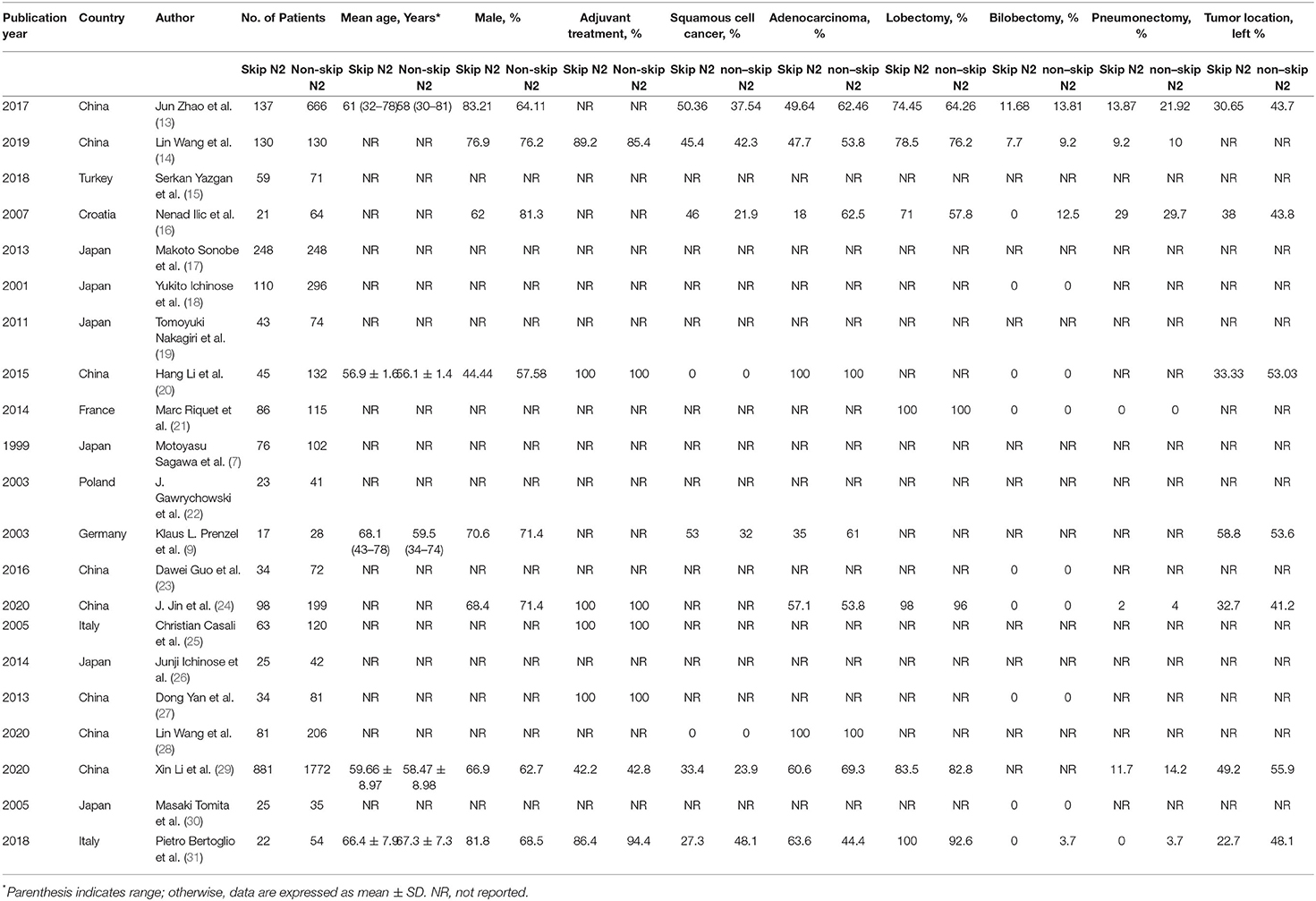
A total of 21 studies with 6,806 patients were included for the meta-analysis (Figure 1) (7, 9, 13–31). All the studies were retrospective studies. The clinical information of 2,258 patients of skip N2 and 4,548 patients of non-skip N2 were retrieved for further analysis. A total of 21 studies were performed in China (8), Japan (6), Italy (2), Germany (1), France (1), Turkey (1), Poland (1), and Croatia (1). All the studies were published between 1999 and 2020. Seven studies were published before 2010. Baseline characteristics in one study were adequately matched for age, gender, surgical procedure, tumor size, histology, T stage, and use of adjuvant therapy. The detailed baseline information was summarized in Table 1. The results of the quality assessment of the studies were shown in Table 2.
Overall Survival
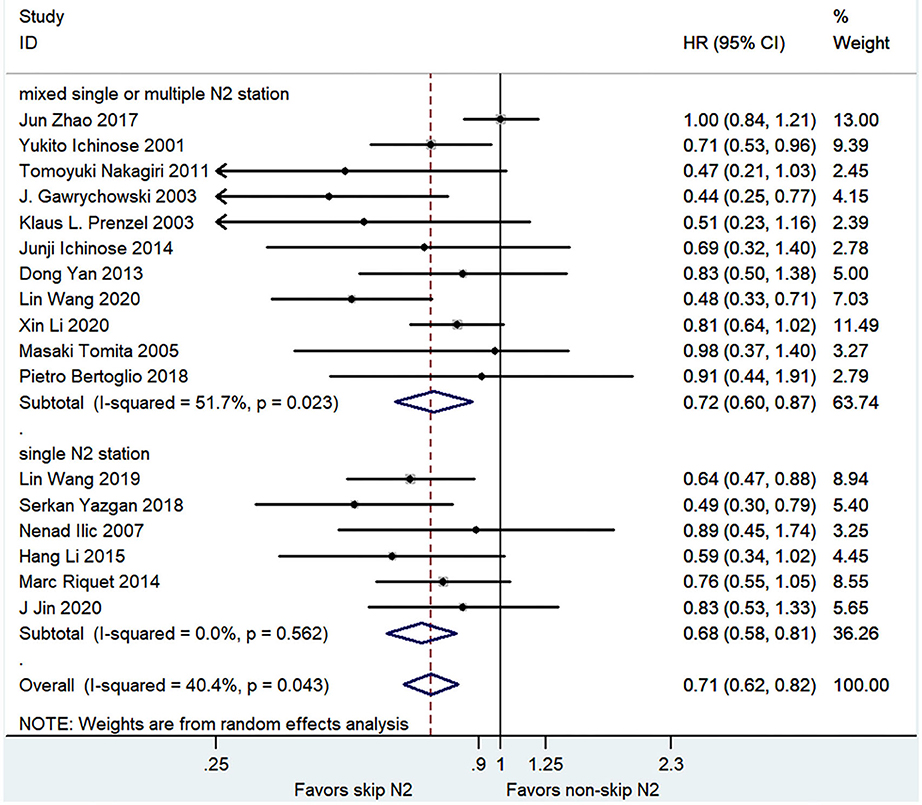
The pooled analysis demonstrated that the skip N2 group had a significantly better OS than the non-skip N2 group with a moderate heterogeneity (HR, 0.71; 95% CI, 0.62–0.82; P < 0.001; I2 = 40.4%; Figure 2). The skip N2 group still had a significantly better OS in the pooled analysis of multivariable analysis study (HR, 0.74; 95% CI, 0.65–0.86; P < 0.001; I2 = 0%; Table 3) and univariable analysis study (HR, 0.68; 95% CI, 0.55–0.84; P < 0.001; I2 = 56.8%; Table 3). The univariable studies had a high degree of heterogeneity. The skip N2 group had a significantly better OS in the pooled analysis of single skip N2 station and mixed single or multiple N2 station (HR, 0.68; 95% CI, 0.58–0.81; P < 0.001; I2 = 0% and HR, 0.72; 95% CI, 0.60–0.87; P < 0.01; I2 = 51.7%, respectively; Figure 2).
3- and 5-year Survival Rates
The pooled analysis demonstrated that the skip N2 group had significantly higher 3- and 5-year survival rates than the non-skip N2 group (OR, 0.75; 95% CI, 0.66–0.84; P < 0.001; I2 = 60%; and OR, 0.78; 95% CI, 0.71–0.86; P < 0.001; I2 = 67.1%, respectively; Figures 3, 4) with a high heterogeneity. The mean 3- and 5-year survival rates in the skip N2 group were 60.4 and 43.4% and those of the non-skip N2 group were 46.6 and 25.3%, respectively.
Sensitivity Analysis and Subgroup Analysis
The sensitivity analyses for OS and 5-year survival are summarized in Table 3. The sensitive analysis including publication year, number of patients, baseline mean age, the proportion of male, histology of adenocarcinoma or other, with or without adjuvant therapy, single or mixed single and multiple skip N2 station and analysis method of multivariable or univariable showed a survival benefit for skip N2 over non-skip N2, consistent with evidence from the primary outcome analysis except in the subgroup of adjuvant therapy.
Meta-Regression Analysis
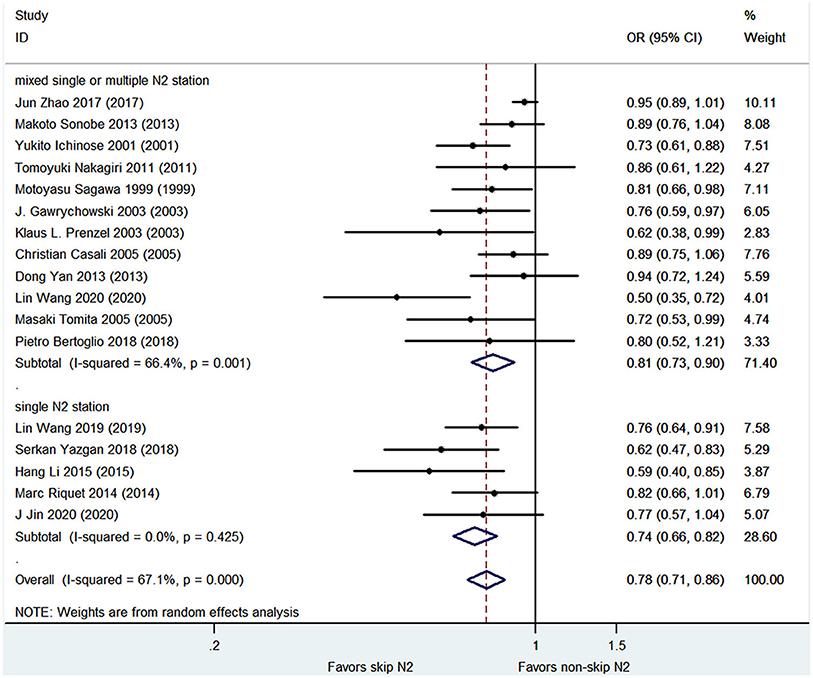
Meta-regression analysis showed a trend for publication year, baseline proportion of male, histology of adenocarcinoma or squamous cell, operation technique of lobectomy, tumor location of the left side. The trend was not statistically significant except for the histology of the squamous cells (P < 0.05) (Figure 5). The contribution of different study characteristics to the heterogeneity was calculated. The proportion of heterogeneity ranged from −22.67 to 100% for all the covariates. The remaining heterogeneity was small (τ2 range from 0 to 0.0316).
Figure 5. Meta regression analysis for OS. Bubble plot with fitted meta regression line of the log HR for (A) publication year, (B) baseline proportion of male, (C) baseline proportion of adenocarcinoma, (D) baseline proportion of squamous cell cancer, and (E) baseline proportion of lobectomy, (F) baseline proportion of the left side tumor location.
Publication Bias
The funnel plots of OS and 3- and 5-year survival outcomes for skip and non-skip N2 groups are shown in Figure 6. There was publication bias for OS and 5-year survival outcomes (Egger's p-value, < 0.05) but no bias for 3-year survival outcome (Egger's P > 0.05).
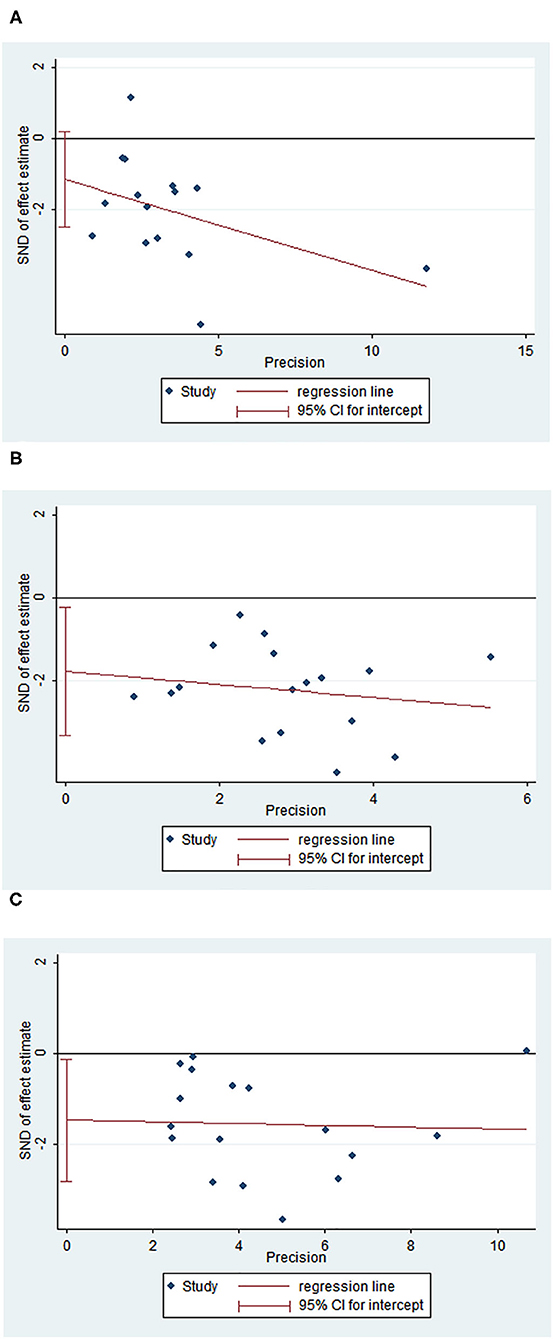
Figure 6. Funnel plot of OS, 3- and 5-year survival outcomes. (A) Egger plot for 3-year survival rates; (B) Egger plot for 5-year survival rates; (C) Egger plot for OS.
Discussion
Pathological N2 involvement is an important prognostic factor for NSCLC. Skip N2 disease is an important subclassification of N2 disease and accounts for 17.2–42.7% of resected N2 NSCLC (6, 7). The impact of skip N2 metastases on survival remains controversial. Several studies found a better OS for skip N2 disease than the non-skip N2 disease (17, 32). However, other studies did not find a significant difference in 5-year OS between the two groups (33, 34). Recently, the International Association for the Study of Lung Cancer suggested that the skip N2 should be treated as a new pN subclassification because of its better survival (35, 36). However, the evidence was based on the analysis of the patients from the cancer registry database and the clinical characteristics and operation details were not provided which still casts doubt on the conclusion. The present meta-analysis demonstrated that the skip N2 disease provided a better survival for NSCLC with a pooled analysis of the 24 studies.
The relationship is close in anatomy between N1 and N2 disease and the prognosis is also similar in some subgroups of N1 and N2 disease. It was reported that patients of N1 with hilar nodal involvement and patients of single N2 station metastasis had a comparable prognosis (37). Another recent study found that the 5-year OS was similar between patients with N1 metastasis and single skip N2 station metastasis, significantly better than the patients with single non-skip N2 station metastasis (15). Our study showed that the better prognostic impact is consistent in the single N2 station metastasis subgroup.
Multiple N2 stations involvement was reported to be a poor prognostic factor for survival (14, 31, 38). The new 8th tumor, node, metastasis (TNM) staging system for lung cancer did not further classify the N2b group into multiple skip N2 stations and multiple non-skip N2 station diseases. Our result showed that the influence of skip N2 in multistation metastases was not significant for OS although there were only two studies providing the survival information on the subgroup of N2 multistation metastases. Large studies may be needed to further elaborate the prognostic factor of multiskip N2 stations on survival.
Adenocarcinoma is an important histology subtype of NSCLC and the incidence has increased fast and accounted for almost one-half of all lung cancer in recent years (35, 39, 40). There was a higher risk of lymph node metastasis associated with adenocarcinoma compared with squamous cell cancer (41). Histological and molecular heterogeneity also existed in adenocarcinoma (42). The subgroup analysis of our study showed that skip N2 was also a benefit prognostic factor for adenocarcinoma.
The result of our study supported the 8th edition of the American Joint Committee on Cancer TNM staging system which classified the N2 into N2a1 (skip single station involved), N2a2 (non-skip single station involved), and N2b (multiple stations involved). Our result suggested that these patients with skip N2 metastases should be carefully recognized and surgery is an optimal option for these patients.
Limitations
At first, this study is the first meta-analysis focusing on the prognosis of skip N2 lymph node metastasis on NSCLC and provides a convincing result that the prognosis of skip N2 lymph node metastasis NSCLC is better compared with that of non-skip N2 lymph node metastasis. Second, all the studies were retrospective studies. Third, the baseline clinical characteristics and adjuvant therapy in skip N2 and non-skip N2 were not matched among studies. Fourth, a high degree of heterogeneity and publication bias was found. Despite the use of meta-regression and numerous sensitivity analyses, significant heterogeneity remained. The results should be interpreted with caution. At last, most of the patients were Asians and the result may not be applicable for other ethnicities.
Conclusion
The present meta-analysis suggests that the skip N2 lymph node metastases are a prognostic factor for better survival in N2-NSCLC.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
XW drafted the manuscript. BC revised the manuscript. HG and XW extracted the data and XW did the analysis. QH and YY assessed the quality of the included studies. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by the Taizhou Municipal Science and Technology Bureau (No. 1801ky09) and the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No. 2019KY773).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: Cancer J Clinic. (2021) 71:7–33. doi: 10.3322/caac.21654
2. Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thoracic Oncol. (2010) 5:29–33. doi: 10.1097/JTO.0b013e3181c5920c
3. Hsieh CP, Fu JY, Liu YH, Yang CT, Hsieh MJ, Tsai YH, et al. Prognostic factors in resectable pathological N2 disease of non-small cell lung cancer. Biomed J. (2015) 38:329–35. doi: 10.4103/2319-4170.145765
4. Vansteenkiste JF, De Leyn PR, Deneffe GJ, Stalpaert G, Nackaerts KL, Lerut TE, et al. Survival and prognostic factors in resected N2 non-small cell lung cancer: a study of 140 cases. Leuven lung cancer group. Ann Thoracic Surg. (1997) 63:1441–50. doi: 10.1016/S0003-4975(97)00314-7
5. Detterbeck F. What to do with “Surprise” N2?: intraoperative management of patients with non-small cell lung cancer. J Thoracic Oncol. (2008) 3:289–302. doi: 10.1097/JTO.0b013e3181630ebd
6. Li GL, Zhu Y, Zheng W, Guo CH, Chen C. Analysis of factors influencing skip lymphatic metastasis in pN(2) non-small cell lung cancer. Chin J Cancer Res. (2012) 24:340–5. doi: 10.1007/s11670-012-0273-x
7. Sagawa M, Sakurada A, Fujimura S, Sato M, Takahashi S, Usuda K, et al. Five-year survivors with resected pN2 nonsmall cell lung carcinoma. Cancer. (1999) 85:864–8. doi: 10.1002/(sici)1097-0142(19990215)85:4<864::aid-cncr13>3.0.co;2-q
8. Guerrera F, Renaud S, Tabbó F, Voegeli AC, Filosso PL, Legrain M, et al. Epidermal growth factor receptor mutations are linked to skip N2 lymph node metastasis in resected non-small-cell lung cancer adenocarcinomas. Eur J Cardio-Thoracic Surg. (2017) 51:680–8. doi: 10.1093/ejcts/ezw362
9. Prenzel KL, Mönig SP, Sinning JM, Baldus SE, Gutschow CA, Grass G, et al. Role of skip metastasis to mediastinal lymph nodes in non-small cell lung cancer. J Surg Oncol. (2003) 82:256–60. doi: 10.1002/jso.10219
10. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online at: http://wwwohrica/programs/clinical_epidemiology/oxfordasp (2011).
11. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
12. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
13. Zhao J, Li J, Li N, Gao S. Clinical significance of skipping mediastinal lymph node metastasis in N2 non-small cell lung cancer. J Thorac Dis. (2018) 10:1683–8. doi: 10.21037/jtd.2018.01.176
14. Wang L, Zhan C, Gu J, Xi J, Lin Z, Xue L, et al. Role of skip mediastinal lymph node metastasis for patients with resectable non-small-cell lung cancer: a propensity score matching analysis. Clin Lung Cancer. (2019) 20:e346–e55. doi: 10.1016/j.cllc.2018.12.007
15. Yazgan S, Ucvet A, Gursoy S, Samancilar O, Yagci T. Single-station skip-N2 disease: good prognosis in resected non-small-cell lung cancer (long-term results in skip-N2 disease). Interact Cardiovasc Thorac Surg. (2019) 28:247–52. doi: 10.1093/icvts/ivy244
16. Ilic N, Petricevic A, Arar D, Kotarac S, Banovic J, Ilic NF, et al. Skip mediastinal nodal metastases in the IIIa/N2 non-small cell lung cancer. J Thorac Oncol. (2007) 2:1018–21. doi: 10.1097/JTO.0b013e318158d471
17. Sonobe M, Date H, Wada H, Okubo K, Hamakawa H, Teramukai S, et al. Prognostic factors after complete resection of pN2 non-small cell lung cancer. J Thorac Cardiovasc Surg. (2013) 146:788–95. doi: 10.1016/j.jtcvs.2013.04.043
18. Ichinose Y, Kato H, Koike T, Tsuchiya R, Fujisawa T, Shimizu N, et al. Overall survival and local recurrence of 406 completely resected stage IIIa-N2 non-small cell lung cancer patients: questionnaire survey of the Japan Clinical Oncology Group to plan for clinical trials. Lung cancer. (2001) 34:29–36. doi: 10.1016/S0169-5002(01)00207-0
19. Nakagiri T, Sawabata N, Funaki S, Inoue M, Kadota Y, Shintani Y, et al. Validation of pN2 sub-classifications in patients with pathological stage IIIA N2 non-small cell lung cancer. Interact Cardiovasc Thorac Surg. (2011) 12:733–8. doi: 10.1510/icvts.2010.249896
20. Li H, Hu H, Wang R, Li Y, Shen L, Sun Y, et al. Lung adenocarcinoma: Are skip N2 metastases different from non-skip? J Thorac Cardiovasc Surg. (2015) 150:790–5. doi: 10.1016/j.jtcvs.2015.03.067
21. Riquet M, Rivera C, Pricopi C, Arame A. Pimpec-Barthes FLJEjoc-tsojotEAfC-tS. Is the lymphatic drainage of lung cancer lobe-specific? Surg Appraisal. (2014) 47:543–9. doi: 10.1093/ejcts/ezu226
22. Gawrychowski J, Gabriel A, Lackowska B. Heterogeneity of stage IIIA non-small cell lung cancers (NSCLC) and evaluation of late results of surgical treatment. Eur J Surg Oncol. (2003) 29:178–84. doi: 10.1053/ejso.2002.1321
23. Guo D, Ni Y, Lv X, Zhang Z, Ye P. Distribution and prognosis of mediastinal lymph node metastases of nonsmall cell lung cancer. J Cancer Res Therapeut. (2016) 12:120–5. doi: 10.4103/0973-1482.191613
24. Jin J, Xu Y, Hu X, Chen M, Fang M, Hang Q, et al. Postoperative radiotherapy option based on mediastinal lymph node reclassification for patients with pN2 non-small-cell lung cancer. Current Oncol. (2020) 27:e283–e93. doi: 10.3747/co.27.5899
25. Casali C, Stefani A, Natali P, Rossi G, Morandi U. Prognostic factors in surgically resected N2 non-small cell lung cancer: the importance of patterns of mediastinal lymph nodes metastases. Eur J Cardio-Thorac Surg. (2005) 28:33–8. doi: 10.1016/j.ejcts.2005.03.016
26. Ichinose J, Murakawa T, Hino H, Konoeda C, Inoue Y, Kitano K, et al. Prognostic impact of the current Japanese nodal classification on outcomes in resected non-small cell lung cancer. Chest. (2014) 146:644–9. doi: 10.1378/chest.14-0159
27. Yan D, Wei P, An G, Chen W. Prognostic potential of ERCC1 protein expression and clinicopathologic factors in stage III/N2 non-small cell lung cancer. J Cardiothorac Surg. (2013) 8:149. doi: 10.1186/1749-8090-8-149
28. Wang L, Ye G, Xue L, Zhan C, Gu J, Xi J, et al. Skip N2 metastasis in pulmonary adenocarcinoma: good prognosis similar to N1 disease. Clin Lung Cancer. (2020) 21:e423–e34. doi: 10.1016/j.cllc.2020.02.027
29. Li X, Li X, Fu X, Liu L, Liu Y, Zhao H, et al. Survival benefit of skip metastases in surgically resected N2 non-small cell lung cancer: A multicenter observational study of a large cohort of the Chinese patients. Eur J Surg Oncol. (2020) 46:1874–81. doi: 10.1016/j.ejso.2019.12.015
30. Tomita M, Matsuzaki Y, Shimizu T, Hara M, Ayabe T, Onitsuka T. Vascular endothelial growth factor expression in pN2 non-small cell lung cancer: lack of prognostic value. Respirology. (2005) 10:31–5. doi: 10.1111/j.1440-1843.2005.00655.x
31. Bertoglio P, Ricciardi S, Alì G, Aprile V, Korasidis S, Palmiero G, et al. N2 lung cancer is not all the same: an analysis of different prognostic groups. Interact Cardiovasc Thorac Surg. (2018) 27:720–6. doi: 10.1093/icvts/ivy171
32. Yoshino I, Yokoyama H, Yano T, Ueda T, Takai E, Mizutani K, et al. Skip metastasis to the mediastinal lymph nodes in non-small cell lung cancer. Ann Thorac Surg. (1996) 62:1021–5. doi: 10.1016/0003-4975(96)00470-5
33. Tateishi M, Fukuyama Y, Hamatake M, Kohdono S, Ishida T, Sugimachi K. Skip mediastinal lymph node metastasis in non-small cell lung cancer. J Surg Oncol. (1994) 57:139–42. doi: 10.1002/jso.2930570302
34. De Rosa V, Iommelli F, Monti M, Fonti R, Votta G, Stoppelli MP, et al. Reversal of warburg effect and reactivation of oxidative phosphorylation by differential inhibition of EGFR signaling pathways in non-small cell lung cancer. Clin Cancer Res. (2015) 21:5110–20. doi: 10.1158/1078-0432.CCR-15-0375
35. Nicholson AG, Chansky K, Crowley J, Beyruti R, Kubota K, Turrisi A, et al. The international association for the study of lung cancer lung cancer staging project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. (2016) 11:300–11. doi: 10.1016/j.jtho.2015.10.008
36. Kim H, Ahn YC, Pyo H, Oh D, Noh JM, Sun JM, et al. Do new pN subclassifications proposed by IASLC's lung cancer staging project agree with ypN categories after trimodality therapy for initial N2 disease? J Thorac Oncol. (2016) 11:2202–7. doi: 10.1016/j.jtho.2016.07.005
37. Asamura H, Suzuki K, Kondo H, Tsuchiya R. Where is the boundary between N1 and N2 stations in lung cancer? Ann. Thorac Surg. (2000) 70:1839–45. doi: 10.1016/S0003-4975(00)01817-8
38. Asamura H, Chansky K, Crowley J, Goldstraw P, Rusch VW, Vansteenkiste JF, et al. The international association for the study of lung cancer lung cancer staging project: proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J Thorac Oncol. (2015) 10:1675–84. doi: 10.1097/JTO.0000000000000678
39. Parkin DM, Ferlay J, Curado MP, Bray F, Edwards B, Shin HR, et al. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer. (2010) 127:2918–27. doi: 10.1002/ijc.25517
40. Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. (2014) 84:13–22. doi: 10.1016/j.lungcan.2014.01.009
41. Deng HY, Zeng M, Li G, Alai G, Luo J, Liu LX, et al. Lung adenocarcinoma has a higher risk of lymph node metastasis than squamous cell carcinoma: a propensity score-matched analysis. World J Surg. (2019) 43:955–62. doi: 10.1007/s00268-018-4848-7
Keywords: skip N2, NSCLC, non-skip N2, lymph node metastases, survival
Citation: Wang X, Guo H, Hu Q, Ying Y and Chen B (2021) The Impact of Skip vs. Non-Skip N2 Lymph Node Metastasis on the Prognosis of Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Surg. 8:749156. doi: 10.3389/fsurg.2021.749156
Received: 29 July 2021; Accepted: 07 September 2021;
Published: 12 October 2021.
Edited by:
Hasan Fevzi Batirel, Marmara University, TurkeyReviewed by:
Kazunori Okabe, Bell Land General Hospital, JapanAlper Toker, West Virginia University, United States
Copyright © 2021 Wang, Guo, Hu, Ying and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baofu Chen, Y2hlbmJmQGVuemVtZWQuY29t
 Xinxin Wang
Xinxin Wang Haixie Guo
Haixie Guo