
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 25 October 2021
Sec. Vascular Surgery
Volume 8 - 2021 | https://doi.org/10.3389/fsurg.2021.744721
Background: Acute lower limb ischemia with a motor deficit (Rutherford IIb) needs urgent revascularization to avoid major amputation and mortality. It is unclear whether immediate revascularization without performing CT angiography (CTA) prior to revascularization in Rutherford IIb acute lower limb ischemia (ALI) is associated with better outcomes.
Methods: Retrospective observational study of Rutherford IIb ALI patients treated between 2006 and 2018. A propensity score adjusted analysis was performed to compare outcomes after the performance of CTA examination or not.
Results: Among 681 patients, 260 had Rutherford IIb ALI. CTA prior to revascularization was performed in 131 (50.4%) and increased (p < 0.001) throughout the study period. Open vascular and endovascular surgery was first performed in 147 (56.5%) and 113 (43.5%) patients, respectively. The proportion of endovascular treatment increased while the open vascular surgery decreased during the study period (p = 0.031). In the propensity score adjusted analysis, the performance of CTA was associated with decreased risk of combined major amputation /mortality (odds ratio 0.52, 95% confidence interval 0.27–0.99; p = 0.046) at 1 year.
Conclusion: Performance of CTA was associated with a higher amputation-free survival in revascularized patients with Rutherford IIb ALI. CTA seem to provide guidance in selecting the most appropriate candidates for revascularization and choice of technique.
Although important technical advancements have been made, acute lower limb ischemia (ALI) remains associated with high rates of amputation, mortality (1), and reperfusion injuries (2). Emergent revascularization is especially important in Rutherford IIb (3) ALI patients since motor deficit at presentation is associated with poor prognosis. Historically, Rutherford IIb ALI patients have been sent directly to an operative intervention, circumventing any non-invasive imaging, and is still recommended in the medical literature (4). However, a more modern approach has begun to take form resulting in more clinicians choosing to manage patients with Rutherford IIb ALI, like Rutherford I and IIa ALI, by performing imaging, often computed tomography angiography (CTA), prior to intervention.
Intra-arterial thrombolysis (IAT) was already in the 1990's shown to be equally effective as open surgery for the treatment of ALI (5). Techniques and equipment used in endovascular surgery have evolved rapidly since the 1990's which has resulted in a shift toward a broader use of this less invasive method (6). There are, however, still concerns about using IAT in Rutherford IIb ALI since revascularization is more gradual and takes a longer time compared to open revascularization techniques. Nevertheless, patients with Rutherford IIb ALI do undergo IAT (7), but the efficacy and safety compared to open surgery have not been possible to evaluate. In fact, only 30% of 106 included studies in a recent systematic review on ALI reported the clinical presentation according to the Rutherford classification (8).
The main objective of this study was to evaluate if immediate revascularization without performing CTA prior to revascularization in Rutherford IIb ALI is associated with better outcomes.
This study was approved by the Swedish Ethical Review Authority (Diary nr 2020/00764) and informed consent from patients was waived. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Integrity and privacy of each participant were protected and all data is confidential. Patients undergoing open and endovascular revascularization procedures for ALI in the present tertiary referral hospital between January 1, 2006 and December 31, 2018 were included. The study complies with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement for cohort studies (9). Among 681 patients, 260 (38.2%) with Rutherford IIb (motor deficit in the lower limb) ALI at admission were included in the present study.
In emergencies, multi-detector row CT was performed from 2004 and onward (10). Run-off CTA scanning was done from hemidiaphragms to the forefoot. Iohexol 90 ml 350 mg I/ml (Omnipaque 350 mg I/ml, GE Healthcare Limited Little Chalfont, England) followed by 50 ml saline flush at flow rate 5 ml/s were injected via an 18 G intravenous cannula placed in an antecubital vein. Arterial phase images were obtained 5 s after bolus detection in the suprarenal aorta (threshold 120 HU for Siemens Somatom Definition Flash and threshold 180 HU for Canon Aquilion One). The scanning was done in two series: first from the level of the right atrium to the middle of the femur and secondly from hips to forefoot. The images were reconstructed with 1 and 3 mm thickness in an axial plane. A reconstruction in the coronal and axial plane with 2 mm thickness was also done. Images of the abdomen were reconstructed with 3 mm thickness in the sagittal plane.
Symptom duration was defined as the number of hours from symptom onset until the start of the revascularization procedure. Embolic events resulting in ALI was mainly based upon CTA or angiographic appearance of embolic clots, synchronous embolism to other arterial territories, previous arterial embolism, atrial fibrillation, or other source of embolism. Acute on chronic limb ischemia was defined as an acute exacerbation of existing chronic limb ischemia (claudication, rest pain, or foot ulcer). Major amputation was defined as amputation above the foot level. Only major amputation of the limb affected by ALI was taken into consideration. Major bleeding was defined as hemorrhage resulting in the requirement of blood transfusion, surgery, resulting in stroke or cessation of thrombolysis due to bleeding (11). Anemia was defined as hemoglobin <134 g/L in men and <117 g/L in women, and renal insufficiency was present if serum creatinine reached levels >105 μmol/L in men and >90 μmol/L in women.
In five patients, no follow-up data regarding limb status at 1 year was possible to retrieve. Two patients living in a foreign country were not possible to follow up at all. Survival status for 258 (99.2%) patients was searched through the national population registry.
Statistical analysis was performed using IBM SPSS Statistics for Macintosh, version 26.0 (IBM Corp., Armonk, N.Y., USA). Group comparison of nominal data was performed using Pearson's chi-square. Annual time trends for non-invasive imaging prior to revascularization, mode of revascularization (endovascular first therapy or open vascular surgery first), or combined major amputation/mortality at 1 year were assessed using the Kendall's tau-b test. Continuous data were expressed as median and interquartile range (IQR) and comparison between groups was analyzed using the Mann-Whitney U-test. p < 0.05 was considered statistically significant.
A propensity score technique to adjust for multiple risk factors (12, 13) was used since multivariate adjustments by logistic regression are limited by the number of endpoints, and a limited number of covariates should be modeled (14). With this method, several risk factors for the adverse outcome are used to calculate a propensity score, reflecting the differences in risk factors between those examined with CTA or not. In the next step, the propensity score can be used to adjust for differences between those examined with CTA or not in an analysis of outcomes. In the first step, we used a logistic regression model, with age, sex, hypertension, diabetes mellitus, ischemic heart disease, cerebrovascular disease, atrial fibrillation, claudication, renal insufficiency, anemia, existing foot ulcer, treatment with acetyl salicylic acid (ASA), supra or infra-inguinal arterial occlusion, native artery occlusion, bypass graft occlusion, endoprosthesis occlusion, embolus, open or endovascular surgery, former (2006–2012) or latter (2013–2018) study time period as independent variables, and examination with CTA or not as the dependent variable. Propensity score distribution was trimmed and individuals within the 5–95th percentile were included in the analysis to exclude outliers. Missing data were coded as separate dummy variables and included in the propensity score adjusted analysis. The propensity score variable was inserted as another covariate together with examination with CTA or not in the final logistic regression analysis of outcomes.
The proportion of non-invasive imaging prior to revascularization (p < 0.001), CTA prior to revascularization (p < 0.001; Figure 1), endovascular treatment (p = 0.031), and amputation-free survival rate at 1 year (p = 0.002) increased in Rutherford IIb ALI between 2006 and 2018.
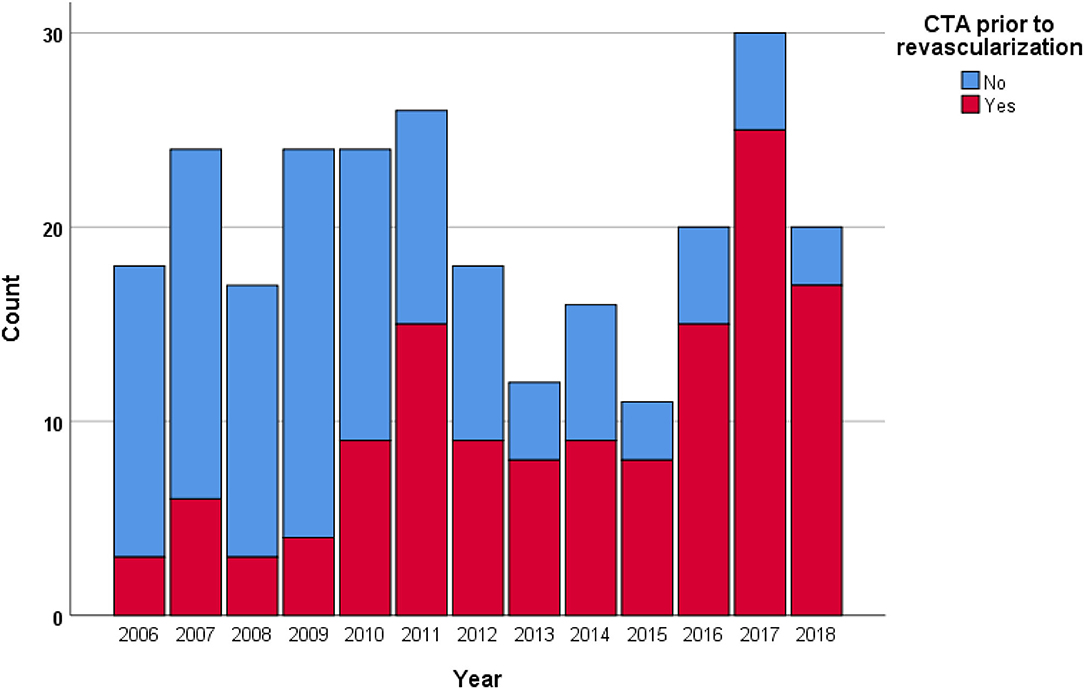
Figure 1. The proportion of computed tomography angiography (CTA) prior to revascularization in Rutherford IIb ALI increased (p < 0.001) throughout the study period.
No imaging and any imaging prior to revascularization were performed in 28% (74/260) and 72% (186/260), respectively. The frequency of duplex, magnetic resonance (MR) angiography, and CTA were 16.2% (n = 42), 8.5% (n = 22), and 50.4% (n = 131), respectively. The use of CTA increased throughout the study period (p < 0.001), while duplex decreased (p = 0.029) and MR angiography was unchanged (p = 0.956). CTA prior to revascularization was done more often (p = 0.023) among those undergoing endovascular (58.4%; 66/113) compared to open surgical treatment (44.2%; 65/147). This significant difference was not maintained in the latter half (2013–2018) of the study period, 76.8% (43/56) vs. 73.6% (39/53), respectively (p = 0.699).
Open vascular and endovascular surgery was first performed in 147 (56.5%) and 113 (43.5%) patients, respectively. The primary open vascular procedures were thromboembolectomy (n = 140) and bypass [Axillo-bifemoral bypass (2), femoro-popliteal bypass below knee (2), femoro-distal bypass (3); n = 7]. The primary endovascular procedures were thrombolysis (n = 102), mechanical thromboembolectomy with the Angiojet® device (MEDRAD, Warrendale, Pennsylvania, USA; n = 5), primarily stent grafting without thrombolysis (n = 4), endovascular aspiration embolectomy (n = 1), and primary hybrid revascularization (thrombendarterectomy of the common femoral artery and stenting of the ipsilateral common iliac artery; n = 1).
Factors associated with no examination with CTA prior to revascularization with Rutherford IIb ALI were higher age (p = 0.011), female gender (p = 0.035), atrial fibrillation (p = 0.011), infra-inguinal as opposed to supra-inguinal occlusion (p < 0.001), native artery occlusion (p = 0.014), embolus (p = 0.033), open vascular as opposed to endovascular surgery (p = 0.023), and former as opposed to latter study period (p < 0.001) (Table 1). Among 37 female elderly (≥80 years) patients with atrial fibrillation and infra-inguinal occlusion, 11 were examined with CTA and 26 were not, and the corresponding combined major amputation/mortality at 1 year was 18.2% (2/11) vs. 50.0% (13/26), respectively (p = 0.072).
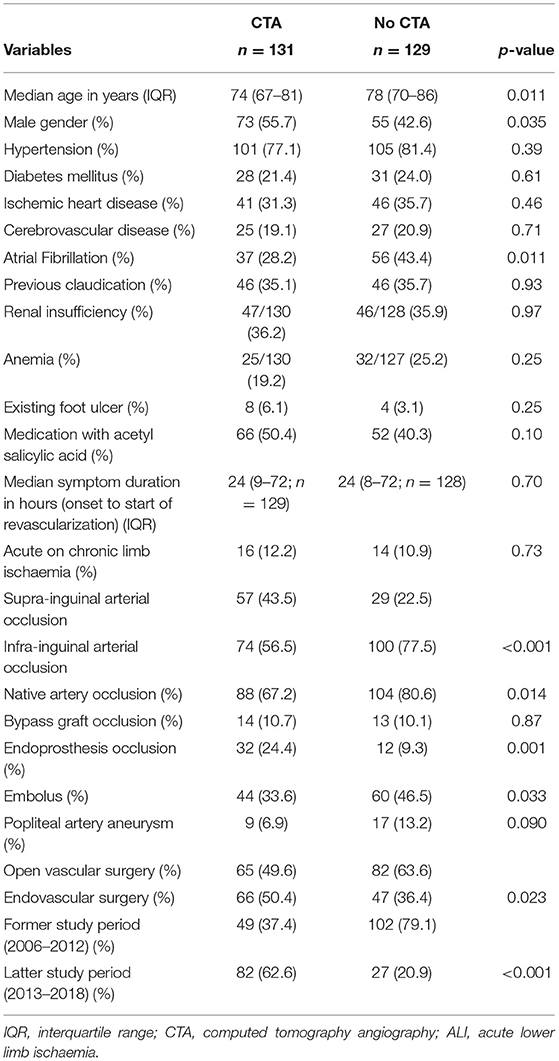
Table 1. Characteristics in patients undergoing computed tomography angiography (CTA) or not prior to revascularization in patients with Rutherford IIb acute lower limb ischemia (ALI).
The overall major amputation rate, mortality, and combined major amputation/mortality at 1 year was 14.9% (38/255), 28.7% (74/258), and 38.7% (99/256), respectively. The major amputation rate, mortality, and combined major amputation at 1 year for those examined with CTA prior to revascularization were 10.2% (13/128), 18.5% (24/130), and 25.8% (33/128), respectively, compared with 19.7% (25/127; p = 0.033), 39.1% (50/128; p < 0.001), and 51.6% (66/128; p < 0.001), respectively, for those not examined with CTA prior to revascularization.
There was a trend that performance of CTA was associated with a decreased risk of major amputation (OR 0.44, 95% CI 0.18–1.08; p = 0.74) at 1 year. Performance of CTA was associated with decreased risk of combined major amputation/mortality (OR 0.52, 95% CI 0.27–0.99; p = 0.046) at 1 year (Table 2).
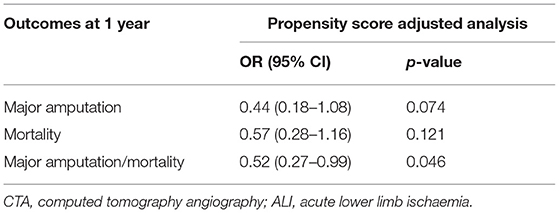
Table 2. Propensity score adjusted analysis of outcomes in patients with Rutherford IIb ALI examined with CTA prior to revascularization or not.
Performance of CTA was associated with a higher amputation-free survival at 1 year in patients with Rutherford IIb ALI. CTA seem to provide guidance in selecting the most appropriate candidates for revascularization and choice of technique. The favorable results associated with increased use of CTA prior to revascularization in Rutherford IIb ALI justify imaging of the arterial tree in Rutherford IIa (sensory deficit) or I ALI prior to invasive therapy.
The increased proportion of endovascular therapy throughout the study period in Rutherford IIb ALI is not surprising due to the endovascular profile of the present study center. The increasing use of stent and stent graft implantations demands more CTA for detection of endoprosthesis restenosis or occlusions (15) and for subsequent treatment planning. Vascular surgeons have adapted to the fact that CTA has become an integral part of their diagnostic arsenal. Parallel to this, the availability of fast high-quality CT scanners around the clock has increased greatly during the latter part of the study period, making CTA a very useful tool for precise mapping of the extent of the occlusions and stenoses in the lower limb arteries in ALI (16). Hence, these factors have very likely contributed to that more patients with Rutherford IIb ALI were managed by CTA prior to revascularization during the latter half of the study period.
The selection for any treatment might have progressed toward a more restrictive manner regarding revascularization attempts during the latter study period. The significant increase in preoperative CTA during the latter period probably contributed to a higher number of patients with ALI being increasingly refrained from revascularization. It is not uncommon that CTA identifies patients with complex arterial occlusive lesions, multiple arterial emboli to the viscera and limbs, and/or poor run-off in CTA as well as important extravascular findings which make them unsuitable as surgical candidates for revascularization. In a recent report, 38 out of 141 patients with ALI had extravascular findings on CTA of immediate clinical relevance (17). For instance, four patients had previously unknown advanced cancer disease, which is a contraindication for thrombolysis. It is highly warranted to estimate the proportion of patients refrained from revascularization attempt based on CTA findings and/or due to poor performance status prior to the onset of ALI.
The study results showed, typically, that elderly female patients with atrial fibrillation and infra-inguinal occlusion more likely were managed by open vascular surgery in the former study period without having an examination with CTA prior to revascularization. It was not possible to show that this subgroup of patients had a statistically higher combined major amputation/mortality at 1 year, probably due to a statistical type 2 error. The extensiveness and profoundness of motor deficit in the patients with Rutherford IIb ALI were not studied, but it is likely that those with extensive motor deficit were more urgently managed and perhaps more often underwent open vascular surgery without being examined with a CTA prior to revascularization. Interestingly, there was no difference in utilization of emergency CTA at the workup stage between those undergoing open vascular surgery and thrombolysis in the latter half of the study period.
There are limitations to consider when interpreting the results of this retrospective observational study. Even though this study only included patients with Rutherford IIb ALI, it should be acknowledged that the severity and extent of paralysis may vary, which induces treatment selection bias. Similarly, the unknown status of medical therapy and smoking during follow up has introduced residual confounding (18, 19). The proportion of patients with Rutherford IIb ALI turned down for revascularization after CTA during the study period was unknown. The present study was conducted in an endovascular-oriented center with high availability to CT scanners around the clock, which may affect the generalizability of study results.
Performance of CTA was associated with a higher amputation-free survival in revascularized patients with Rutherford IIb ALI. CTA seem to provide guidance in selecting the most appropriate candidates for revascularization and choice of technique. The results of the present study could be considered when forming or updating future guidelines on the diagnosis of patients with ALI, recommending immediate CTA in patients with Rutherford IIb ALI.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Swedish Ethical Review Authority. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
ES was involved in the design of the study, data gathering, data analysis, and writing of the manuscript. RS-B and SA was involved in the design of the study, data gathering, data analysis, and critical review of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Henke PK. Contemporary management of acute limb ischemia: factors associated with amputation and in-hospital mortality. Semin Vasc Surg. (2009) 22:34–40. doi: 10.1053/j.semvascsurg.2009.01.002
2. Björck M, Earnshaw JJ, Acosta S, Bastos Goncalvez F, Cochennec F, Debus S, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Acute Limb Ischaemia. Eur J Vasc Endovasc Surg. (2020) 59:173–218. doi: 10.1016/j.ejvs.2019.09.006
3. Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards dealing with lower limb ischemia: revised version. J Vasc Surg. (1997) 26:517–38. doi: 10.1016/S0741-5214(97)70045-4
4. Wallace A, Pershad Y, Saini A, Alzubaidi S, Naidu S, Knuttinen G, et al. Computed tomography angiography evaluation of acute limb ischemia. Vasa. (2019) 48:57–64. doi: 10.1024/0301-1526/a000759
5. Darwood R, Berridge DC, Kessel DO, Robertson I, Forster R. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database Syst Rev. (2018) 8:CD002784. doi: 10.1002/14651858.CD002784.pub3
6. Acosta S, Kuoppala, M. Update on intra-arterial thrombolysis in patients with lower limb ischemia. J Cardiovasc Surg. (2015) 56:317–24.
7. Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or peripheral arterial surgery (TOPAS) investigators. N Engl J Med. (1998) 338:1105–11. doi: 10.1056/NEJM199804163381603
8. Ebben H, Jongkind V, Wisselink W, Hoksbergen AWJ, Yeung KK. Catheter directed thrombolysis protocols for peripheral arterial occlusions: a systematic review. Eur J Vasc Endovasc Surg. (2019) 57:667–75. doi: 10.1016/j.ejvs.2018.11.018
9. von Elm E, Altman DG, Egger M, Pocock SJ, Götzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. (2007) 4:e296. doi: 10.1371/journal.pmed.0040296
10. Acosta S, Wadman M, Syk I, Elmståhl S, Ekberg O. Epidemiology and prognostic factors in acute superior mesenteric artery occlusion. J Gastrointest Surg. (2010) 14:628–35. doi: 10.1007/s11605-009-1130-1
11. Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J. Thromb Haemost. (2010) 8:202–4. doi: 10.1111/j.1538-7836.2009.03678.x
12. Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol. (2003) 158:280–7. doi: 10.1093/aje/kwg115
13. Martens EP, de Boer A, Pestman WR, Belitser SV, Stricker BH, Klungel OH. Comparing treatment effects after adjustment with multivariable Cox proportional hazards regression and propensity score methods. Pharmacoepidemiol Drug Saf. (2008) 17:1–8. doi: 10.1002/pds.1520
14. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. (2007) 165:710–8. doi: 10.1093/aje/kwk052
15. Kuoppala M, Åkeson J, Acosta S. Outcome after thrombolysis for occluded endoprosthesis, bypasses and native arteries in patients with lower limb ischemia. Thromb Res. (2014) 134:23–8. doi: 10.1016/j.thromres.2014.02.030
16. Itoga N, Kim T, Sailer A, Fleischmann D, Mell MW. Lower extremity CT angiography can help predict technical success of endvascular revascularization in the superficial femoral and popliteal artery. J Vasc Surg. (2017) 66:835–43. doi: 10.1016/j.jvs.2017.02.031
17. Preuss A, Elgeti T, Hamm B, Werncke T. Extravascular incidental findings in run-off CT angiography in patients with acute limb ischemia: incidence and clinical relevance. Clin Radiol. (2015) 70:622–9. doi: 10.1016/j.crad.2015.02.014
18. Venermo M, Sprynger M, Desormais I, Bjorck M, Brodmann M, Cohnert T, et al. Editor's Choice - Follow-up of Patients After Revascularisation for Peripheral Arterial Diseases: A Consensus Document From the European Society of Cardiology Working Group on Aorta and Peripheral Vascular Diseases and the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. (2019) 58:641–53. doi: 10.1016/j.jvs.2019.10.017
19. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. (2006) 113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526
Keywords: acute lower limb ischaemia, motor deficit, Rutherford classification, revascularization, computed tomography angiography
Citation: Saphir E, Svensson-Björk R and Acosta S (2021) Performance of Computed Tomography Angiography Before Revascularization Is Associated With Higher Amputation-Free Survival in Rutherford IIb Acute Lower Limb Ischaemia. Front. Surg. 8:744721. doi: 10.3389/fsurg.2021.744721
Received: 20 July 2021; Accepted: 24 September 2021;
Published: 25 October 2021.
Edited by:
Håkan Pärsson, Linköping University, SwedenReviewed by:
Rosa María Moreno Carriles, Hospital Universitario Princess, SpainCopyright © 2021 Saphir, Svensson-Björk and Acosta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Acosta, U3RlZmFuLmFjb3N0YUBtZWQubHUuc2U=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.