- 1Department of General Surgery, The Second Hospital of Shanxi Medical University, Taiyuan, China
- 2Department of Targeted Therapy, Shanxi Cancer Hospital, Taiyuan, China
Background: Advanced gastric cancer is the fifth leading cause of cancer-related deaths. Patients with metastatic advanced gastric cancer commonly develop a gastric outlet obstruction that considerably worsens their quality of life. Surgical interventions such as gastrojejunostomy and palliative gastrectomy are commonly administered to alleviate this obstruction. However, whether one intervention is better than another at improving morbidity- and mortality-related outcomes is unclear. Thus, in this meta-analysis, we compare outcomes of palliative gastrectomy and gastrojejunostomy (overall hospital stay length, time to oral intake, survival, and complication rates) in patients with metastatic advanced gastric cancer to identify the best procedure.
Objective: To compare morbidity and mortality outcomes of palliative gastrectomy and gastrojejunostomy in patients with metastatic advanced gastric cancer.
Methods: We followed the PRISMA guidelines to systematically search Web of Science, EMBASE, CENTRAL, Scopus, and MEDLINE for relevant studies. We conducted a random-effects meta-analysis to find differential outcomes between palliative gastrectomy and gastrojejunostomy among variables such as time to oral intake, overall hospital stay length, complication rates, and survival in patients with metastatic advanced gastric cancer.
Results: From 963 studies, we found 7 eligible studies with 642 patients (70.3 ± 4.7 years) who had undergone palliative gastrectomy or gastrojejunostomy. Our meta-analysis revealed an insignificant (p > 0.05) differences in terms of overall survival duration (Hedge's g, 1.22), complication risks (odds ratio, 1.35), and time to oral intake (g, 0.62) and hospital stay length (g, 0.12) between patients undergoing gastrojejunostomy and palliative gastrectomy.
Conclusion: In this present study we observed no statistically significant differences in terms of morbidity and mortality outcomes after palliative gastrectomy and gastrojejunostomy in patients with metastatic advanced gastric cancer. Therefore, no conclusions can be drawn for the variables evaluated. This study provides a preliminary overview of the risks associated with gastrojejunostomy and palliative gastrectomy to help gastroenterologists manage patients with metastatic advanced-stage gastric cancer.
Introduction
Gastric cancer is the fifth most common cancer worldwide (1). According to the American Cancer Society, the severity of gastric cancer depends on the extent of the cancerous tissue's growth across the gastrointestinal layers and/or into the adjacent digestive organs (2). Epidemiological studies have widely reported a high incidence of gastric cancer worldwide (15.5 per 100,000 people) (3), and the Global Burden of Disease study estimates that almost 785,000 patients die annually due to gastric cancer (4, 5).
Most patients with gastric cancer are diagnosed during advanced stages of the disease (6) with extremely poor morbidity- and mortality-related outcomes (7, 8). Patients with metastatic advanced-stage gastric cancer also present gastrointestinal complications, with gastric outlet obstruction being the most common one (9, 10). According to Khullar and DiSario (11), patients with gastric outlet obstruction can have symptomatic manifestations (vomiting, malnutrition, nausea, and dehydration) that depend upon the site (pyloric or proximal duodenum) and the extent of the obstruction. These symptoms impair the patients' ability to receive appropriate oral palliative treatment and considerably worsen their quality of life (12). Surgical interventions such as gastrojejunostomy and palliative gastrectomy have been widely recommended to alleviate symptoms and improve the patients' quality of life (9, 13, 14). Gastrojejunostomy involves the creation of an anastomosis (a bypass) between the stomach and the jejunum to bypass the gastric outlet obstruction (15), and palliative gastrectomy involves the resection of the gastric outlet obstruction to improve stomach emptying (14).
Retrospective cohort studies have compared morbidity- and mortality-related outcomes in patients with advanced gastric cancer undergoing palliative gastrectomy or gastrojejunostomy (9, 13, 14, 16–19). However, the evidence for the impact of these surgical interventions on the survival and complication rates of the patients with advanced gastric cancer is contradictory. Some studies have shown an increased survival in patients undergoing palliative gastrectomy compared with those undergoing gastrojejunostomy (9, 16, 18). However, Okumura et al. (17) reported longer survival times for patients receiving gastrojejunostomy. Similarly, some studies have reported that palliative gastrectomy was associated with higher complication risks (9, 14, 18), while others have reported higher risks for gastrojejunostomy (13, 16, 17). To the best of our knowledge, no systematic review or meta-analysis has compared the morbidity- and mortality-related impact of palliative gastrectomy and gastrojejunostomy in patients with metastatic advanced gastric cancer.
Thus, in this systematic review and meta-analysis, we compare overall survival, hospital stay length, complication rates, and times to oral intake in patients undergoing either palliative gastrectomy or gastrojejunostomy. Our findings should be valuable to gastroenterologists managing patients with metastatic advanced gastric cancer.
Methods
We adhered to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (20) while performing this meta-analysis.
Data Search Strategy
We searched for publications in Web of Science, MEDLINE, CENTRAL, EMBASE, and Scopus (from inception until February 2021) using MeSH keywords including “Gastric cancer,” “gastrectomy,” “palliative gastrectomy,” “gastrojejunostomy,” “morbidity,” and “mortality.” We also searched the bibliography section of the included studies manually to identify further relevant studies. Our inclusion criteria were the following,
a) Studies comparing overall hospital stay length, time to oral intake, and overall survival in patients with advanced gastric cancer undergoing palliative gastrectomy or gastrojejunostomy.
b) Studies comparing complication rates in patients with advanced gastric cancer undergoing palliative gastrectomy or gastrojejunostomy.
c) Studies with human participants.
d) Case-control studies, prospective cohort trials, or retrospective cohort trials.
e) Studies published in peer-reviewed scientific journals.
f) Studies published in English.
The screening of the studies was independently performed by two reviewers. Disagreements were resolved by discussion with a third independent reviewer.
Quality Assessment
We used the Newcastle Ottawa scale (21) to appraise the risk of bias in the included studies. This tool evaluates bias due to selective reporting, confounding factors, measurement of outcomes, and incomplete data availability. Two reviewers independently assessed the methodological quality of the studies, and a third reviewer intervened to arbitrate in case of disagreements.
Data Analysis
A within-group meta-analysis was performed using the Comprehensive Meta-analysis version 2.0 (22). We conducted the meta-analysis based on the random-effects model (23). Odds ratios were calculated to evaluate the complication risks in patients undergoing either palliative gastrectomy or gastrojejunostomy. We also calculated weighted effect sizes (Hedge's g) to evaluate the outcomes (overall hospital stay length, time to oral intake, and survival). We computed I2 statistics to assess the heterogeneity among studies. We considered values between 0 and 25% as having negligible heterogeneity, those between 25 and 75% as having moderate heterogeneity, and those ≥75% as having substantial heterogeneity (24). We used the method listed by Hozo et al. (25) to convert medians and ranges (i.e., mentioned in the descriptive statistics of the included studies) into means and standard deviations for data analysis, respectively. In addition, we used Duval and Tweedy's trim and fill procedure to evaluate publication bias (26). The significance level for this study was determined at 5%.
Results
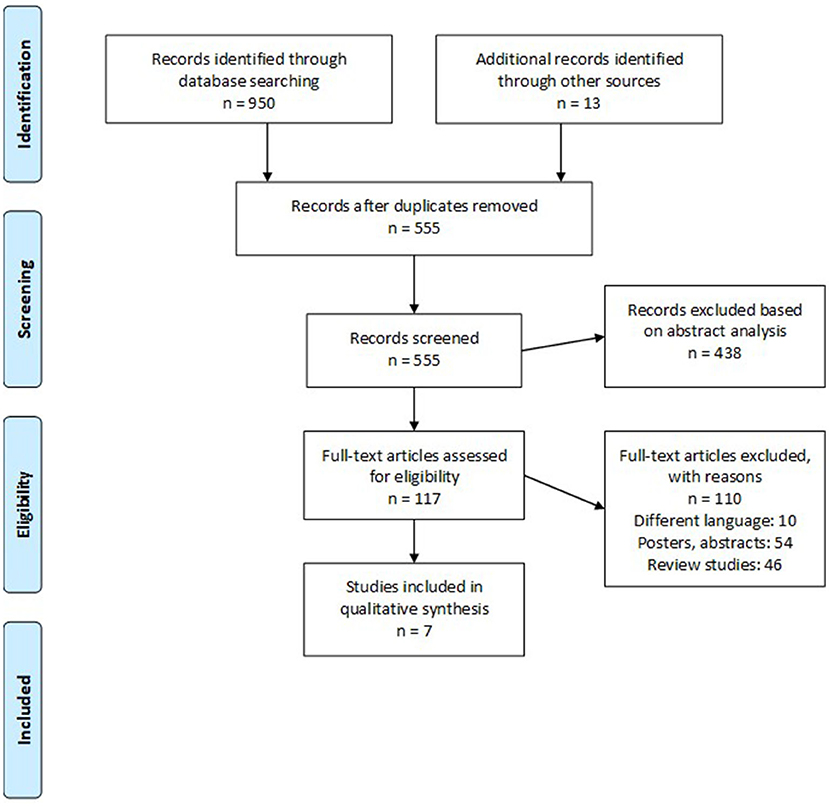
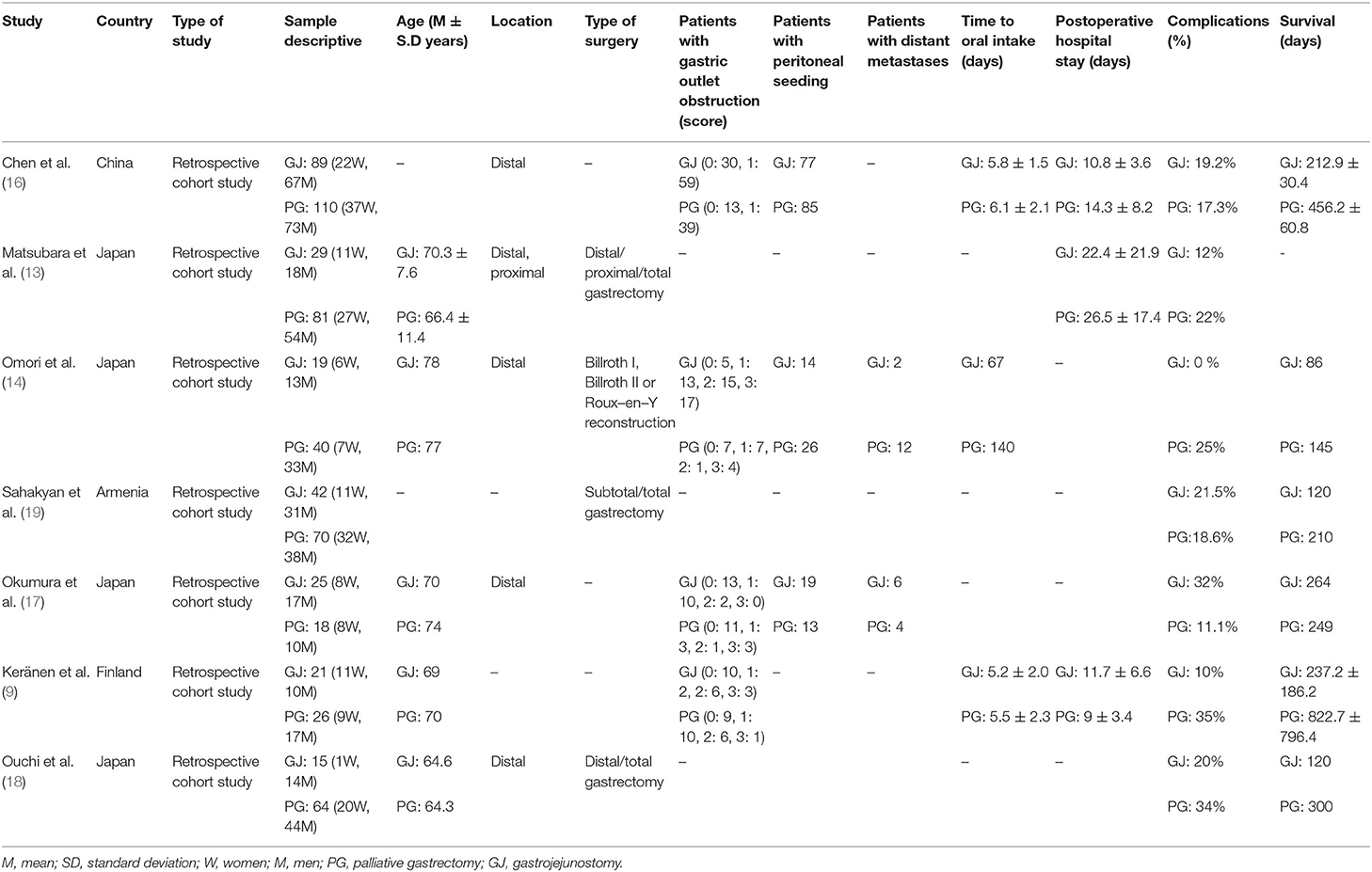
We retrieved 950 studies after our initial database search. We identified an additional 13 studies by screening the reference sections of the included studies. Only seven retrospective cohort studies (9, 13, 14, 16–19) fit our inclusion criteria (Figure 1). Table 1 summarizes the extracted data.
Participant Information
Data from 649 patients (210W, 439M) were included in the 7 studies. We found 240 patients (70W, 170M) who underwent gastrojejunostomy and 409 (140W, 269M) who underwent palliative gastrectomy.
The average age of the participants was 70.3 ± 4.7 years. The average age of patients undergoing gastrojejunostomy was 70.3 ± 4.8 years and the average age of those undergoing palliative gastrectomy was 70.3 ± 5.2 years. Two studies failed to report the age distribution of their sample (16, 19).
Quality Assessment for Cohort Studies
Supplementary Table 1 shows the Newcastle Ottawa scale results for the risks of bias. The overall risk was low and is demonstrated in Supplementary Figure 1.
Publication Bias
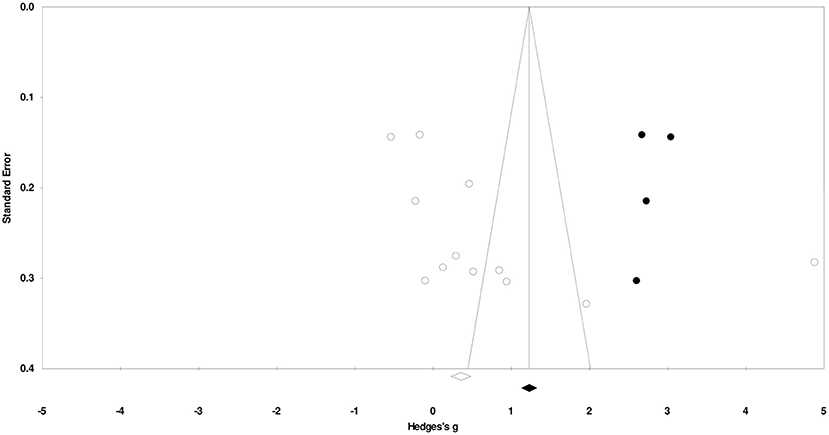
Duval and Tweedy's trim and fill method identified four missing studies on the right side of the mean effect. The overall random effect models determined the point estimates and the 95% confidence intervals for all the combined studies at 0.74 (0.01–1.48). After using the trim and fill method, the imputed point estimates were estimated at 1.25 (0.46–2.05). Figure 2 reports the publication bias results.
Meta-Analysis Report
Time to Oral Intake
The time to oral intake was reported by three studies (9, 14, 16). We observed a moderate insignificant effect on time to oral intake between patients undergoing palliative gastrectomy and gastrojejunostomy (Figure 3) (Hedge's g, 0.62; 95% CI, −0.55 to 1.80; p = 0.30) with negligible heterogeneity (I2, 17.5%).

Figure 3. Forest plot for studies comparing the overall time to oral intake for patients undergoing gastrojejunostomy or palliative gastrectomy.
Duration of Hospital Stay
Three studies reported the overall hospital stay length (9, 13, 16). We observed a small insignificant effect on the duration hospital stay between patients undergoing palliative gastrectomy and gastrojejunostomy (Figure 4) (Hedge's g, 0.12; 95% CI, −0.42 to 0.67; p = 0.66) with negligible heterogeneity (I2, 41.8%).
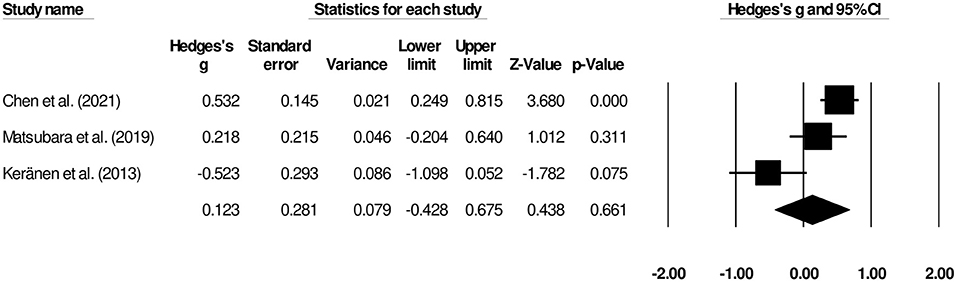
Figure 4. Forest plot for studies evaluating the overall hospital stay length for patients undergoing gastrojejunostomy or palliative gastrectomy.
Complications
The complication rates of patients undergoing gastrojejunostomy or palliative gastrectomy were reported by seven studies (9, 13, 14, 16–19). We observed insignificant differences in terms of risk of complication between patients undergoing palliative gastrectomy and gastrojejunostomy (Figure 5) (odds ratio, 1.35; 95% CI, 0.68–2.68; p = 0.38) with negligible heterogeneity (I2, 16.2%).
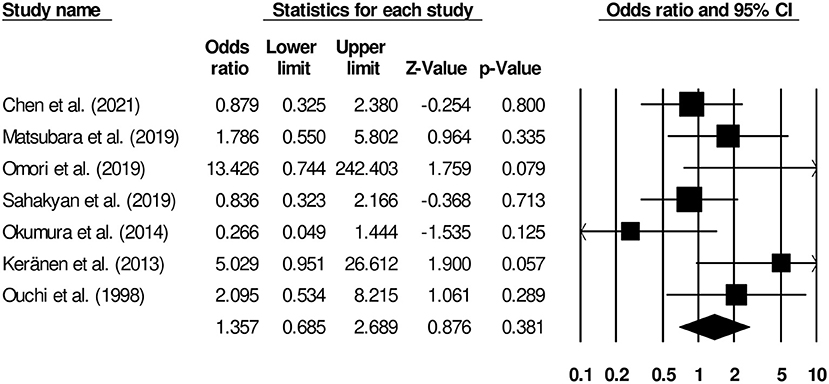
Figure 5. Forest plot for studies comparing complication rates in patients undergoing gastrojejunostomy or palliative gastrectomy.
Survival
The overall survivals were reported in six studies (9, 14, 16–19). We observed a large insignificant effect on overall duration of survival between patients undergoing palliative gastrectomy and gastrojejunostomy (Figure 6) (Hedge's g, 1.22; 95% CI, −0.18 to 2.64; p = 0.08) with negligible heterogeneity (I2, 7.09%).

Figure 6. Forest plot for studies comparing the overall survivals for patients undergoing gastrojejunostomy or palliative gastrectomy.
Discussion
With this systematic review and meta-analysis, we provide comprehensive evidence comparing morbidity- and mortality-related outcomes between patients with metastatic advanced-stage gastric cancer undergoing either palliative gastrectomy or gastrojejunostomy. We observed no statistical significance in terms of the overall survival duration, overall complications, time to oral intake, and overall hospital stay between patients undergoing palliative gastrectomy in those undergoing gastrojejunostomy.
The management of gastric outlet obstruction in patients with metastatic advanced-stage gastric cancer is challenging because of cancer's atypical pathophysiological mechanisms, co-existing morbidities, and manifestations (27–29). Malnutrition, dehydration, and the inability to take palliative medications are common manifestations of patients with gastric outlet obstruction (30), and are associated with poor prognoses for short- and long-term morbidity, mortality, and quality of life (31, 32). Surgical interventions such as gastrojejunostomy and palliative gastrectomy are widely recommended to alleviate the symptoms of patients with gastric outlet obstruction (33). Studies have suggested that these surgical interventions can provide symptomatic relief and improve the complication-free survival of even the patients with dismal prognoses (9). However, whether one of these two interventions results in better postoperative prognostic outcomes than the other for patients with advanced-stage gastric cancer remains unclear (16).
We observed that the included studies had reported different complication rates of these interventions. In a cohort representative of the Japanese population, Omori et al. (14) reported that while 10% of patients who underwent palliative gastrectomy developed complications, none of the patients who underwent gastrojejunostomy exhibited any postoperative complications. The postoperative complications in patients undergoing palliative gastrectomy included abdominal abscess, ileus, leakage, and intraabdominal bleeding. Similarly, Keränen et al. (9) and Ouchi et al. (18) also found higher rates of postoperative complications in patients undergoing palliative gastrectomy. The main complication after palliative gastrectomy in those two studies included re-obstruction of the gastrointestinal pathway and bleeding. On the other hand, Okumura et al. (17), Chen et al. (16), and Sahakyan et al. (19) reported a slightly higher number of postoperative complications in patients undergoing gastrojejunostomy than in those undergoing palliative gastrectomy. In our meta-analysis, we found no difference in terms of risks of complications between patients undergoing palliative gastrectomy and gastrojejunostomy (OR, 1.35). Likewise, in subsequent analyses, we observed that the outcomes of both overall hospital stay (Hedge's g, 0.12) and time to oral intake (g, 0.62) were insignificantly different between patients undergoing palliative gastrectomy and gastrojejunostomy. Insignificant increase in morbidity that we observed may be due to complications during palliative gastrectomy that are not as frequent with gastrojejunostomy.
We also synthesized the evidence on the overall postoperative survival of patients with advanced-stage gastric cancer. In a retrospective cohort study of 199 patients, Chen et al. (16) reported that the overall survival of patients undergoing palliative gastrectomy (15.9 months; 95% CI, 10.9–20.9 months) was longer than that for gastrojejunostomy (7.7, 5.7–9.6 months). After applying a propensity score matching system for adequately balancing the selection bias, authors further confirmed longer overall survival for patients undergoing palliative gastrectomy (11.8, 7.3–16.3 months) than for those undergoing gastrojejunostomy (8.5, 4.5–12.4 months). Chen et al. (34) recommended the use of palliative gastrectomy due to its high success rate for peritoneal seeding (the most common metastasis pattern in their cohort of patients) management. Similarly, in a cohort of 47 Finnish patients, Keränen et al. (9) found that the palliative gastrectomy improved the survival and the complication-free survival (palliative gastrectomy, 223 days and gastrojejunostomy, 121 days) of patients with advanced gastric cancer to a greater extent than gastrojejunostomy or endoscopic stenting. The authors mentioned that among those three surgical interventions, palliative gastrectomy was the only independent prognostic factor in their multivariate survival analysis. In our meta-analysis, we report no differences in the overall survival outcome of patients with advanced-stage gastric cancer undergoing palliative gastrectomy and gastrojejunostomy (g, 1.22).
We are aware of the limitations of our systematic review and meta-analysis. First, we did not pre-register it in a systematic review repository such as PROSPERO York or Joanna Briggs Institute due to the extended registration times during the COVID-19 pandemic. We understand this may raise concerns about the validity of our findings (35), but we decided not to wait more than a year to complete the registration process. Second, the relative paucity of data in the studies included may have created biases in our analysis of the overall times to oral intake and hospital stay lengths. We included data from three different studies in the comparative analysis with small sample sizes (356 for hospital stay length and 305 for time to oral intake). Therefore, we cannot rule out the possibility of a type II error (36). Third, the retrospective nature of each included study is another limitation of the paper. Fourth, we did not conduct extensive subgroup analysis and meta-regression to further elucidate the effects of the various confounders on the final outcomes. Thus, future studies need to address these limitations because risk stratification guidelines for reducing the morbidity- and mortality-related outcomes in patients with metastatic advanced-stage gastric cancer are urgently needed.
In conclusion, our preliminary evidence suggests that there are no significant differences in terms of morbidity and mortality outcomes between patients with metastatic advanced gastric cancer undergoing palliative gastrectomy and outlet obstruction than for those undergoing gastrojejunostomy. Thus, no conclusions can be drawn for the effectiveness of either of the surgical interventions.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
CL and HF conceived and designed the study and wrote the paper. CL, HF, and WC were involved in literature search and data collection. WC and LC analyzed the data. CL and LC reviewed and edited the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.723065/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
3. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. (2020) 5:17–30. doi: 10.1016/S2468-1253(19)30333-4
4. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. (2020) 8:e180–90. doi: 10.1016/S2214-109X(19)30488-7
5. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Cancer Today. Available online at: http://gco.iarc.fr/today/home (Accessed on Jun 7, 2021)
6. Wang F-H, Shen L, Li J, Zhou Z-W, Liang H, Zhang X-T, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun Lond Engl. (2019) 39:10. doi: 10.1186/s40880-019-0349-9
7. Takeno S, Noguchi T, Kikuchi R, Sato T, Uchida Y, Yokoyama S. Analysis of the survival period in resectable stage IV gastric cancer. Ann Surg Oncol. (2001) 8:215–21. doi: 10.1007/s10434-001-0215-1
8. Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. (2020) 18:534–42. doi: 10.1016/j.cgh.2019.07.045
9. Keränen I, Kylänpää L, Udd M, Louhimo J, Lepistö A, Halttunen J, et al. Gastric outlet obstruction in gastric cancer: a comparison of three palliative methods. J Surg Oncol. (2013) 108:537–41. doi: 10.1002/jso.23442
10. Shone DN, Nikoomanesh P, Smith-Meek MM, Bender JS. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol. (1995) 90:1769–70.
11. Khullar SK, DiSario JA. Gastric outlet obstruction. Gastrointest Endosc Clin N Am. (1996) 6:585–603. doi: 10.1016/S1052-5157(18)30356-8
12. Schmidt C, Gerdes H, Hawkins W, Zucker E, Zhou Q, Riedel E, et al. A prospective observational study examining quality of life in patients with malignant gastric outlet obstruction. Am J Surg. (2009) 198:92–9. doi: 10.1016/j.amjsurg.2008.09.030
13. Matsubara D, Konishi H, Kubota T, Kosuga T, Shoda K, Shiozaki A, et al. Comparison of clinical outcomes of gastrojejunal bypass and gastrectomy in patients with metastatic gastric cancer. Anticancer Res. (2019) 39:2545–51. doi: 10.21873/anticanres.13376
14. Omori H, Tanizawa Y, Makuuchi R, Irino T, Bando E, Kawamura T, et al. Role of palliative resection in patients with incurable advanced gastric cancer who are unfit for chemotherapy. World J Surg. (2019) 43:571–9. doi: 10.1007/s00268-018-4816-2
15. Sigmon DF, Lopez PP. Gastrojejunostomy. In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing (2021). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK560493/ (accessed Jun 5, 2021)
16. Chen X-J, Chen G-M, Wei Y-C, Yu H, Wang X-C, Zhao Z-K, et al. Palliative Gastrectomy versus Gastrojejunostomy for advanced Gastric cancer with outlet obstruction: a propensity score matching analysis. BMC Cancer. (2021) 21:188. doi: 10.1186/s12885-021-07904-7
17. Okumura Y, Yamashita H, Aikou S, Yagi K, Yamagata Y, Nishida M, et al. Palliative distal gastrectomy offers no survival benefit over gastrojejunostomy for gastric cancer with outlet obstruction: retrospective analysis of an 11-year experience. World J Surg Oncol. (2014) 12:364. doi: 10.1186/1477-7819-12-364
18. Ouchi K, Sugawara T, Ono H, Fujiya T, Kamiyama Y, Kakugawa Y, et al. Therapeutic significance of palliative operations for gastric cancer for survival and quality of life. J Surg Oncol. (1998) 69:41–3. doi: 10.1002/(SICI)1096-9098(199809)69:1<41::AID-JSO8>3.0.CO;2-K
19. Sahakyan MA, Gabrielyan A, Aghayan DL, Yesayan S, Petrosyan H, Chobanyan A, et al. Gastrectomy for metastatic gastric cancer: a 15-year experience from a developing country. Indian J Surg Oncol. (2019) 10:527–34. doi: 10.1007/s13193-019-00943-4
20. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
21. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
22. Bax L, Yu L-M, Ikeda N, Moons KGM A. systematic comparison of software dedicated to meta-analysis of causal studies. BMC Med Res Methodol. (2007) 7:40. doi: 10.1186/1471-2288-7-40
23. Higgins JPT, Thompson SG, Spiegelhalter DJ A. re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. (2009) 172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x
24. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 21:1539–58. doi: 10.1002/sim.1186
25. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
26. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
27. Leiting JL, Grotz TE. Advancements and challenges in treating advanced gastric cancer in the West. World J Gastrointest Oncol. (2019) 11:652–64. doi: 10.4251/wjgo.v11.i9.652
28. Lordick F, Allum W, Carneiro F, Mitry E, Tabernero J, Tan P, et al. Unmet needs and challenges in gastric cancer: the way forward. Cancer Treat Rev. (2014) 40:692–700. doi: 10.1016/j.ctrv.2014.03.002
29. Rawicz-Pruszyński K, van Sandick JW, Mielko J, Ciseł B, Polkowski WP. Current challenges in gastric cancer surgery: European perspective. Surg Oncol. (2018) 27:650–6. doi: 10.1016/j.suronc.2018.08.004
30. Jaffin BW, Kaye MD. The prognosis of gastric outlet obstruction. Ann Surg. (1985) 201:176–9. doi: 10.1097/00000658-198502000-00007
31. Chau I, Fuchs CS, Ohtsu A, Barzi A, Liepa AM, Cui ZL, et al. Association of quality of life with disease characteristics and treatment outcomes in patients with advanced gastric cancer: exploratory analysis of RAINBOW and REGARD phase III trials. Eur J Cancer Oxf Engl. (2019) 107:115–23. doi: 10.1016/j.ejca.2018.11.013
32. Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, et al. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer Oxf Engl. (2014) 50:1330–44. doi: 10.1016/j.ejca.2014.01.029
33. Ly J, O'Grady G, Mittal A, Plank L, Windsor JA A. systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc. (2010) 24:290–7. doi: 10.1007/s00464-009-0577-1
34. Nie R-C, Chen S, Yuan S-Q, Chen X-J, Chen Y-M, Zhu B-Y, et al. Significant role of palliative gastrectomy in selective gastric cancer patients with peritoneal dissemination: a propensity score matching analysis. Ann Surg Oncol. (2016) 23:3956–63. doi: 10.1245/s10434-016-5223-2
35. PLoS Medicine Editors. Best practice in systematic reviews: the importance of protocols and registration. PLoS Med. (2011) 8:e1001009. doi: 10.1371/journal.pmed.1001009
Keywords: gastric cancer, gastrojejunal bypass, palliative resection, complications, morbidity, mortality
Citation: Lin C, Fan H, Chen W and Cui L (2021) Palliative Gastrectomy vs. Gastrojejunostomy for Advanced Gastric Cancer: A Systematic Review and Meta-Analysis. Front. Surg. 8:723065. doi: 10.3389/fsurg.2021.723065
Received: 10 June 2021; Accepted: 26 October 2021;
Published: 26 November 2021.
Edited by:
Cuneyt Kayaalp, Inönü University, TurkeyReviewed by:
M. Noushif, Aster MIMS Hospital, IndiaKonstantinos Perivoliotis, University Hospital of Larissa, Greece
Copyright © 2021 Lin, Fan, Chen and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingzhi Cui, Y3VpbHoxMjMmI3gwMDA0MDsxMjYuY29t
 Chunfang Lin1
Chunfang Lin1 Lingzhi Cui
Lingzhi Cui