- 1Department of Anatomy, Faculty of Medicine, Sabaragamuwa University of Sri Lanka, Ratnapura, Sri Lanka
- 2Department of Anatomy, Faculty of Medicine, University of Colombo, Colombo, Sri Lanka
- 3Proteostasis and Neurodegeneration Laboratory, Australian Regenerative Medicine Institute, Monash University, Clayton, VIC, Australia
- 4Department of Pathology, Faculty of Medicine, University of Colombo, Colombo, Sri Lanka
- 5Department of Surgery, Faculty of Medicine, University of Colombo, Colombo, Sri Lanka
- 6Lady Ridgeway Hospital for Children, Colombo, Sri Lanka
Introduction: Cajal like cells (CLCs) in the upper urinary tract have an ability to generate coordinated spontaneous action potentials and are hypothesized to help propel urine from renal pelvis into the ureter. The objective of this review was to describe the variations in the density and distribution of CLCs associated with ureteropelvic junction obstruction (UPJO).
Materials and Methods: Studies comparing the density and distribution of CLCs in the human upper urinary tract in patients with UPJO and healthy controls were included in this systematic review. We searched online electronic databases; Ovid MEDLINE, Scopus, PubMed and Cochrane reviews for the studies published before October 31, 2020. A meta-analysis was conducted to compare the density of CLCs at the ureteropelvic junction (UPJ) in patients with UPJO and matched controls.
Results: We included 20 and seven studies in the qualitative and quantitative synthesis, respectively. In majority (55%) CLCs were located between the muscle layers of the upper urinary tract. The CLC density in the UPJ gradually increased with aging in both healthy subjects and patients with UPJO. The pooled analysis revealed that the density of CLCs at the UPJ was significantly low in patients with UPJO compared to the controls (SMD = −3.00, 95% CI = −3.89 to −2.11, p < 0.01).
Conclusions: The reduction in CLC density at the UPJ in patients with UPJO suggests a contribution from CLCs in the pathogenesis of UPJO. Since age positively correlates with CLC density, it is imperative to carefully match age when conducting case control studies comparing the CLC density and distribution.
Protocol Registration Number: CRD42020219882.
Introduction
Primary ureteropelvic junction obstruction (UPJO) is the most common congenital abnormality causing hydronephrosis in children (1) which affects 1 in 750–1,500 newborns annually (2–4). Structurally, the UPJO is characterized by a narrowed segment of the ureteropelvic junction (UPJ) containing atrophied smooth muscles and a hypertrophied segment proximal to the obstruction with increased collagen deposition (5). The widely accepted theory for the pathogenesis of UPJO is the disruption of coordinated unidirectional smooth muscle contractions, leading to dampening of peristaltic waves that propels urine downward from the renal pelvis to the ureter (6). Nevertheless, the exact mechanism of how these unidirectional contractions are coordinated in healthy ureteropelvic junction remains a mystery. Nearly a century ago Santiago Ramón y Cajal discovered a cell, later named in his honor, which has a regulatory role in smooth muscle contractility. These cells form a plexus that runs between the gut muscle layers, with processes extending from the ganglion cells of Auerbach plexus and nerve terminals residing on the plasmalemma of smooth muscle cells (7). These cells express c-kit (CD177) encoding receptor tyrosine kinase in their cytoplasmic membrane, which allow visualization of them using immunostaining (8). Reduction in the density of intestinal Cajal cells was later found to be associated with motility disorders of the gastrointestinal system such as congenital pyloric stenosis, achalasia cardia, Hirschsprung's disease and chronic intestinal pseudo obstruction (9–12).
Huizinga and Faussone-Pellegrini (13) reported the presence of different subtypes of Cajal cells, termed Cajal like cells (CLCs), outside the gastrointestinal tract with unique ultrastructural characteristics that help distinguish them from other cell types expressing c-kit such as mast cells, glial cells and melanocytes. The CLCs in the urinary tract have a stellate shape or a fusiform cell body with two distinct dendrites (14, 15). Subsequently, CLCs were identified in many organs including urinary tract, vagina, blood vessels and glands (13, 16, 17). The CLCs in the upper urinary tract in guinea pigs generate and amplify action potentials both in the renal pelvis and the ureter (18, 19), suggesting a unique role of CLCs in maintaining a unidirectional flow of urine at the UPJ (20, 21). With the discovery of an intrinsic motility action of the human UPJ (20), the CLCs were considered to be the pacemaker regulating the expulsion of urine at the UPJ. Nonetheless, the postulated role of CLCs in the pathogenesis of UPJO was challenged since the early studies failed to demonstrate a consistent decrease in the density of the CLCs at the UPJ in patients with UPJO (22, 23). These contradicting results led to further studies that primarily focused on the functions of the CLCs which generated clues on the pathogenesis of UPJO.
Despite decades of research, the exact pathogenic mechanism (s) of primary UPJO remains enigmatic. In this review, we provide a comprehensive analysis of the density and distribution of CLCs in the upper urinary tract associated with the UPJO and mechanistic insights to the pathophysiology of this disease. Moreover, we critically evaluate the methodological inaccuracies of certain studies which may have led to false assumptions regarding the association of the density of the CLCs at the UPJ with the UPJO.
Materials and Methods
Protocol and Registration
We conducted a systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (24). The study protocol was documented in advance in the International Prospective Register of Systematic Reviews (PROSPERO) online database (protocol registration number: CRD42020219882).
Eligibility Criteria
Studies comparing the density and/or distribution of CLCs in the human upper urinary tract in patients with UPJO and controls were included in this systematic review. Only the studies comparing the density of CLCs at the UPJ in patients with UPJO and matched controls were included in the quantitative synthesis. Case reports and animal studies on CLCs were excluded.
Information Sources and Search Strategy
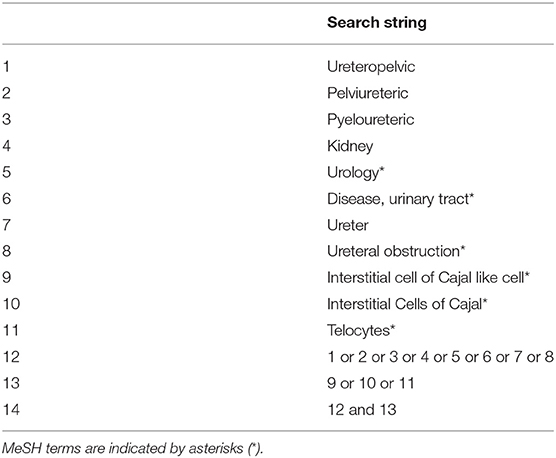
We searched online electronic databases; Ovid MEDLINE (Medical Literature Analysis and Retrieval System), Scopus, PubMed and Cochrane reviews. To obtain additional information, we conducted a manual search of the reference list of the selected articles. The online search strategy was generated by YM. The search comprised of studies listed up to October 31, 2020. We did not set search limits. The PubMed search strategy is provided in Table 1.
Study Selection
Two independent reviewers (US and YM) assessed the eligibility in an unbiased standardized manner. A third reviewer (AM) was involved in case of any disagreements. We screened the total hits obtained by reading “title” and “abstract.” We excluded studies that failed to satisfy the inclusion criteria at this stage. Next, we read the full text of each selected paper to extract data. All relevant articles published in languages other than English were translated into English language before screening. The reviewers determined the final group of articles to be included in the review after an iterative consensus process.
Data Collection Process
We developed a data extraction sheet, pilot-tested it on three randomly selected studies that were consistent with the inclusion criteria and revised it accordingly. One reviewer (US) extracted data from the included studies using this standardized form and another reviewer (YM) checked for the accuracy of data extraction. We extracted the following data from each study: (a) study details (author, country and year published), (b) sample characteristics (age of the study population and sample size), (c) methods (detection and/or quantification of CLC distribution and density) and (d) results (distribution of CLCs in a cross section and along the upper urinary tract and the density of CLCs with its association with disease status (UPJO vs. healthy subjects), age and postoperative outcomes). Ureteropelvic junction was defined as the junction between the renal pelvis and the ureter (20). Despite no clear external feature to locate the UPJ (20, 25), the internal appearance of crowding of mucosal folds forming characteristic “mucosal rosettes” allows its precise localization (20), whereas pathological UPJs in patients with UPJO is visualized intra-operatively as a valve-like appearance (26) preceding a narrowed segment with interrupted development of circular muscle fibers (27). Distribution of CLCs was defined as the location of the CLCs in different layers in the cross section of the ureter or along the upper urinary tract (UPJ, renal pelvis, or ureter). Density was defined as the total number of CLCs per high power field of an optical microscope. We resolved discrepancies in the extracted data by discussion, involving a third reviewer (AM) when necessary. We contacted the corresponding authors of the published manuscripts to obtain additional data such as the age distribution of their study populations and data sets of the measurements.
Risk of Bias in Individual Studies
The methodological quality and the risk of bias of the included studies were assessed independently by two authors (US and YM) using Joanna Briggs Institute (JBI) Critical Appraisal Tool (28). Each criterion was evaluated as “Yes,” “No,” or “Other” (unclear/ not applicable). Overall rating was provided for each study based on the items rated with an affirmative answer and accordingly, the quality score was determined by the range 67–100 (good), 34–66 (average), and 0–33 (bad). The studies meeting the “good” scores were selected for the review.
Quantitative Analysis
We conducted a meta-analysis of studies comparing the density of CLCs at the UPJ in patients with UPJO and matched control. A random effects model was used for the comparisons. Heterogeneity was assessed using the χ2 test on Cochrane's Q statistic and by I2 statistic. The I2 statistic was interpreted as follows: 0–40% might not be important; 30–60% may represent moderate heterogeneity; 50–90% may represent substantial heterogeneity; and 75–100% may represent considerable heterogeneity (29). When appropriate, sensitivity analyses were performed based on the sample size and the age distribution of the study samples to explore the sources of heterogeneity. Data were analyzed using RevMan version 5.4.1 (30). p < 0.05 was considered statistically significant in all analyses.
Results
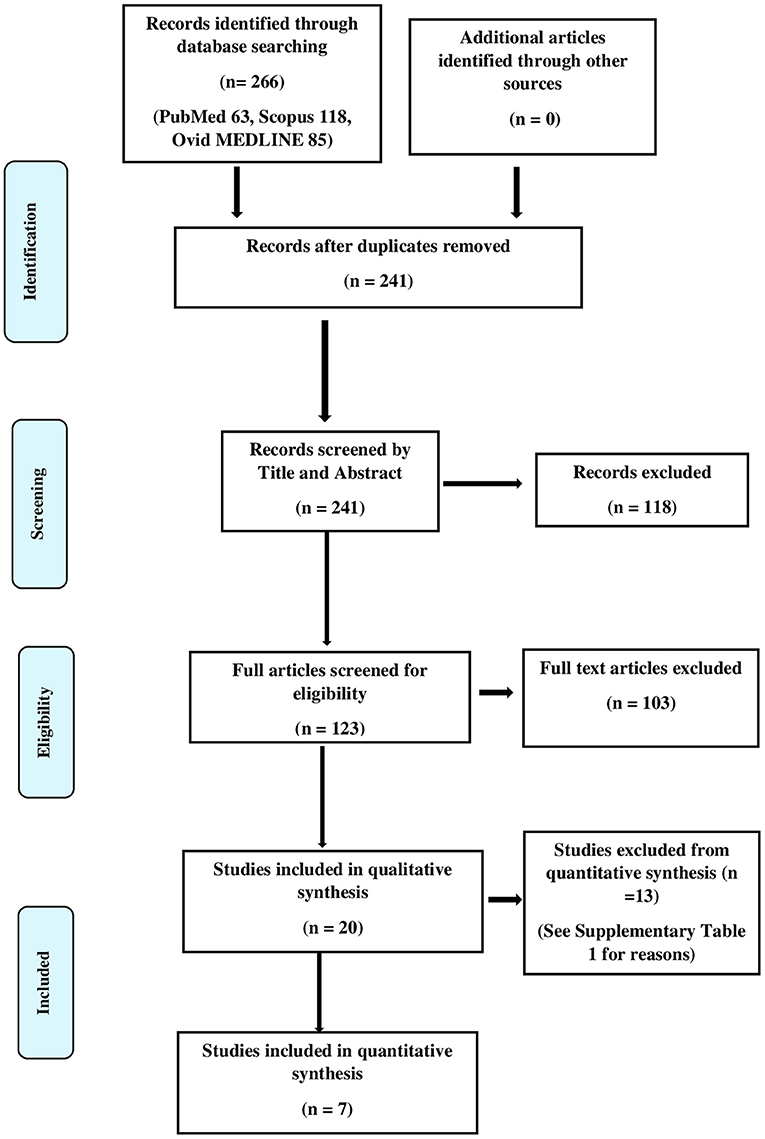
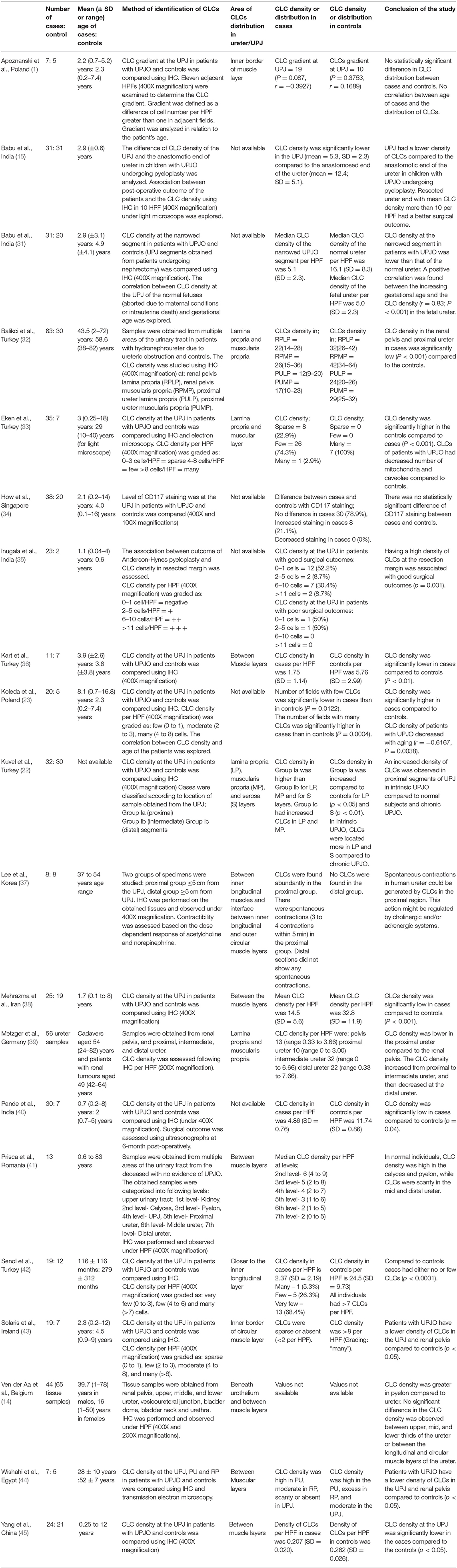
We found a total of 266 hits in the initial literature search, and after 50 duplicates were removed, 241 articles remained. We did not find additional articles after manual screening. We obtained full texts that had potential for the final review and included twenty of these studies in the final qualitative synthesis. Figure 1 illustrates the PRISMA flow diagram of the search. The results of the qualitative synthesis are summarized in Table 2. Of them, five studies presented the density of CLC as an ordinal variable (e.g., low, medium, and high density) as opposed to a continuous variable viz, the absolute number of CLCs per high power field, hence were subsequently excluded from the quantitative synthesis. The reasons of excluding articles from the quantitative synthesis are provided in the Supplementary Table 1. The risk of bias assessment is provided in the Supplementary Tables 2, 3. Of the studies included in the qualitative synthesis, eleven were conducted exclusively among children, while eight pooled results of adults and children. One study did not provide the age distribution of the subjects. The studies were conducted in Turkey, Poland, India, Egypt, Belgium, Germany, Korea, Singapore, Iran, Romania, Ireland, and China.
Distribution of Cajal-Like Cells
Majority (11/20, 55%) of the studies found CLCs between the inner longitudinal and outer circular muscle layers or in close proximity to the muscle layers (7/20, 35%), while others found these cells to be present both in the lamina propria and serosal layers (1/20, 5%) in addition to the muscle layers (Supplementary Table 4).
The reported distribution of the CLCs in different parts of the upper urinary system were controversial. Wishahi et al. (44) reported that the CLC density gradually increased from renal pelvis to proximal ureter in healthy subjects, while two studies found a decrease in CLC density from UPJ to distal ureter (37, 41). Conversely, Metzger et al. (39) reported that the CLC density gradually increased from the pelvis to the intermediate ureter, and then reduced at the distal ureter, while Ven Der Aa et al. (14) could not find a statistically significant difference in CLC density between upper, mid and distal thirds of the ureter.
Most studies (31–33, 42, 45) reported a lower density of CLCs in the UPJ of the patients with UPJO compared to the controls (Table 2). On the contrary, Koleda et al. (23), and Kuvel et al. (22) found a comparatively higher density of CLCs at the UPJ in patients. How et al. (34) found no statistically significant difference between the CLC density in the UPJ between the cases and controls. Apoznanski et al. (1) explored the density of CLCs in affected patients with UPJO by quantifying the density of CLCs in adjacent high-power fields of the UPJ and calculated the gradient of CLCs. They found no significant differences of the CLC gradient between cases and controls (1).
Age Related Changes in Cajal-Like Cells
The CLC density increases in the UPJ with the advancement of the gestational age of the fetal ureter (31). Nevertheless according to Koleda et al. (23) the density gradually decrease as the age advanced into childhood. Based on the studies included in the quantitative synthesis, a line diagram was drawn to illustrate the CLC density at the UPJ and we found an increase in CLC density with age in both healthy and those affected with UPJO (31, 32, 36, 42) (Figure 2). Further, the affected subjects consistently had a low CLC density compared to the healthy controls.
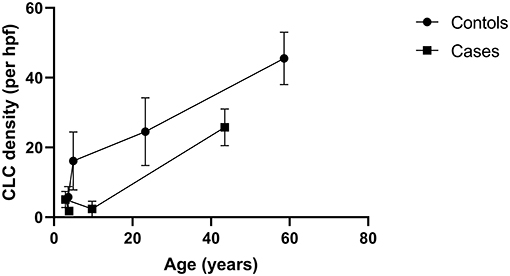
Figure 2. A line diagram demonstrating the associations of density of Cajal like cells (CLCs) at the ureteropelvic junction and age. The diagram illustrates that the CLC density increases with age in both healthy and those affected with ureteropelvic junction obstruction. Data from four studies were used to create the chart (31, 32, 36, 42).
Cajal-Like Cell Contribution to Post-operative Outcome
Two studies exploring the association between the post-operative outcome of Anderson-Hynes pyeloplasty and the CLC density at the resected margin of ureter, showed that patients with a higher density of CLCs had a better surgical functional outcome (15, 35). Nevertheless, Pande et al. (40) found no correlation between the CLC density and the post-operative functional outcome.
Meta-Analysis
Seven studies reporting the mean difference of the density of CLC in the UPJ per high power field in patient with UPJO and controls were included in the meta-analysis. In the pooled analysis, the density of CLCs was significantly low in patients with UPJO (standardized mean difference = −3.00, 95% confidence interval = −3.89 to −2.11, p < 0.01) (Figure 3). The funnel plot of the selected studies is provided in the Supplementary Figure 1. We detected a considerable heterogeneity in this comparison (χ2 = 41.03, I2 = 85%, df = 6, p < 0.01). We performed a sensitivity analysis by including studies conducted on children only (aged <14 years) (n = 5) (31, 36, 38, 40, 45). The studies including both children (<2 years) and elders (>70 years) were excluded (32, 42). Nonetheless, the sensitivity analysis found a standardized mean difference of −2.93 (95% CI = −4.14 to −1.73) with a considerable heterogeneity (χ2 = 32.71, I2 = 88%, df = 4, p < 0.01) (Supplementary Figure 2). We were unable to perform a subgroup analysis comparing pediatric and adult populations since none of the included studies had a homogeneous adult population. To explore the effect of sample sizes, we performed another sensitivity analysis after including studies with at least 10 samples per group (n = 5) (31, 32, 38, 42, 45). The results showed a standardized mean difference of −2.56 (95% CI = −3.14 to −1.97) with a substantial heterogeneity (χ2 = 11.40, I2 = 65%, df = 4, p < 0.01) (Supplementary Figure 3). Subsequently, we combined the two sensitivity analyses by including studies of children with a large sample size (as defined above) (n = 3) (31, 38). In this analysis we found a standardized mean difference of −2.11 (95% CI= −2.53 to −1.68) with no heterogeneity (χ2 = 0.56, I2 = 0%, df = 2, p < 0.01) (Supplementary Figure 4).
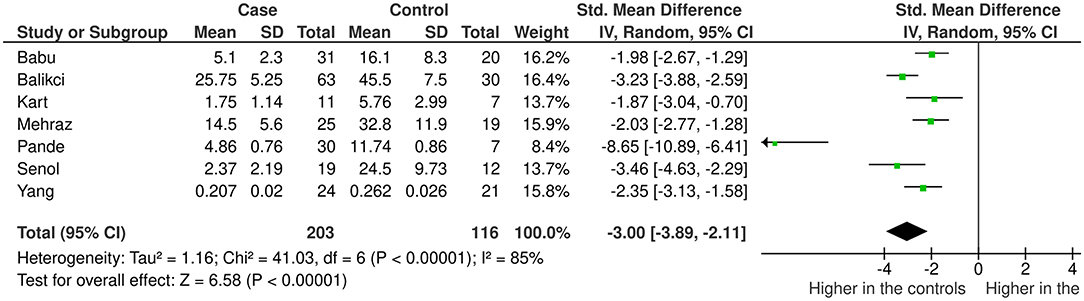
Figure 3. The study characteristics and standardized mean differences of the density of the interstitial cells of Cajal like cells at the ureteropelvic junction in patients with ureteropelvic junction obstruction and matched controls.
Discussion
UPJO is the partial or intermittent blockage of urinary flow from the renal pelvis into the ureter, governed by either an anatomical derangement or in most instances a functional disturbance (5, 26, 46–48). About a decade ago, dilemma on the pathophysiology of UPJO brought myogenic theory to light, which suggests that uncoordinated muscular contractions at the UPJ leads to a functional obstruction of antegrade urine flow (49). The discovery of CLCs in the upper urinary tract which could propagate action potentials in the UPJ, intrigued researchers to investigate into their role in UPJO (49).
Cajal Like Cells in the Upper Urinary Tract
Ureteric wall consists of a transitional epithelium, lamina propria, inner longitudinal, and outer circular muscle layers and a serosa. In most studies, CLCs were located between the inner longitudinal and outer circular muscle layers (Supplementary Table 4). Few studies found CLCs in the lamina propria (14, 22, 39), while a single study detected CLCs in the serosa (22). Cajal cells in the intestines, are located near the myenteric plexus and submucosal plexus, between longitudinal and circular muscle layers, between inner and outer circular muscle layers and within interlamellar connective tissues of circular muscles (50). They are, however not often observed in serosa. Similarly, in ureter, CLCs are not readily located in the serosa in most instances but are present considerably more in the lamina propria. These cells are believed to play a coordinator role of impulse transmission between the sensory nerve endings and smooth muscle cells (18, 19), hence are located in areas richly innervated by sensory nerves. Ureteric innervation is to the muscular and subepithelial layers (51) where the nerve endings reside, therefore the deficiency in CLCs in serosal layer could be due to the lack of sensory nerve endings in the serosa.
Majority of the studies suggest that the overall CLC density at UPJ is reduced in individuals with UPJO compared to controls (33, 38, 40, 42, 45), which is consistent with the results of our quantitative synthesis. A constellation of gastrointestinal motility diseases including achalasia cardia (52) and Hirschsprung's disease (53) are associated with depletion of Cajal cells, while reduction of Cajal cell density in small intestinal segments of inflammation or obstruction significantly improves when treating the pathology causing inflammation or removal of obstruction (54, 55). Abstracting from this knowledge, a theory was postulated on the lack of CLCs in the UPJ as a contributor of failed peristaltic wave propagation across the UPJ in UPJO. However, the observational nature of these studies lacked the ability to derive a direct causation, but only an association. This putative role of CLCs was projected to doubt by Koleda et al. (23) and Kuvel et al. (22) with their description of an increase in the CLC density in the UPJ in affected individuals. Interestingly, in Koleda et al. (23) study, the age of the cases was markedly higher compared to the controls which could have contributed to the rising CLCs in cases, since there is a gradual increase of the CLC density with age in normal individuals as well as in patients with UPJO (Figure 2). Similarly, data was lacking on the age of the subjects in Kuvel et al. (22) study. Due to this reason, it may not be prudent to derive meaningful comparisons of the CLC density in cases and controls from the latter two studies. Although CLC density increases in the urinary tract with aging (Figure 2), the Cajal cell number and volume reduces steadily in colon and stomach (56). Furthermore, Cajal cell loss and aging increases slow waves conduction velocity in the stomach (57) resulting in delayed gastric emptying. Nevertheless it is possible that other syncytial factors have an interdependent role with Cajal cells giving rise to slow wave velocity changes (57).
Though immunohistochemical studies have failed to establish differences of the expression levels of neuronal markers in UPJO (34), it is suggested that a defective innervation at the UPJ in intrinsic obstruction could contribute to increase in CLC density causing increased peristaltic activity as a result of an attempt to overcome peristaltic failure (22). In chronic UPJO from tumors or ureteric stones up-regulation of c-kit expression is not observed to overcome the obstruction (22). The excitatory impulses are generated from a single site of origin propelling urine into the ureter (58). However, when more than one impulse generator sites are present, they block the conduction of waves of excitation (58). This suggests that if there is a change in distribution of impulse generating CLCs in UPJ, it may contribute to alteration of impulse generation leading to intrinsic UPJO. This hypothesis is supported by a study conducted on rabbits where increased frequency of spontaneous mechanical activity of the UPJ was observed during obstruction (59). Researchers pondered on the distributional changes in CLCs in the pathogenesis of UPJO, to which Apoznanski et al. (1) answered by demonstrating no distributional gradient changes in the CLCs in UPJO compared to the age of the affected. However, this study included only seven cases. In addition, we noted that there is a marked deficiency in studies that embark on the distributive changes in the CLCs in affected and healthy UPJ.
CLCs do not possess a primary action potential generation ability in animals, but form a conduit for transmission of signals (60) (Animal study findings related to CLCs are summarized in Supplementary Table 5). Guinea pig renal system, which resembles similar anatomy to humans, shows pacemaker oscillations at the pelvicalyceal junction and UPJ, while oscillations are absent in the ureter (19). When the proximal pacemaker drive is blocked either by pharmacological means or by transection, the distal regions take over the waves of excitation (61), suggesting the presence of pacemaker potential generation mechanism in the mid and distal ureter. These findings corroborate the results of the human studies where CLCs, the potential pacemakers of the renal tract, are not restricted to the renal pelvis and UPJ, but also found in the mid and distal ureter to coordinate unidirectional peristaltic activity.
Limitations
Few studies (23, 33, 34, 43) could not be incorporated in the quantitative synthesis when the CLC density was not presented as a continuous variable with means and standard deviations as summary statistics. The marked variability of the study designs, especially the wide range of age and limited sample sizes contributed to the high heterogeneity of the quantitative synthesis.
Quality of Evidence
This systematic review followed the standard recommended methodology set out by PRISMA guidelines. Two reviewers independently assessed the studies for potential sources of bias and a standard approach of data extraction was employed, thus reducing the risk of performance bias in the review and data extraction errors. PRISMA checklist of the review is presented in Supplementary Table 6.
Conclusions
Cajal like cells are predominantly distributed between the muscle layers of the upper urinary tract. However, the distribution of CLCs along the urinary tract from the renal pelvis toward the lower ureter is subjected to controversy. The CLC density at the UPJ is significantly low in patients with UPJO compared to the controls, suggesting a pivotal contribution by CLCs in the pathogenesis of UPJO. The CLC density gradually increases with aging in both healthy subjects and patients with UPJO, which could potentially bias the results of the anatomical studies when age is not strictly matched in cases and controls. Careful matching of age in cases and controls, avoiding large age ranges and using an adequate sample size are necessary when performing future studies.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
US conceptualized the study. YM developed the search strategy. US, YM, and AM extracted data. YM conducted the meta-analysis. US, YM, UL, and AM wrote the first draft of the manuscript. All authors were involved in drafting and commenting on the paper and have approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Authors thank the Faculty of Medicine, University of Colombo.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.721143/full#supplementary-material
References
1. Apoznanski W, Koleda P, Wozniak Z, Rusiecki L, Szydelko T, Kalka D, et al. The distribution of interstitial cells of Cajal in congenital ureteropelvic junction obstruction. Int Urol Nephrol. (2013) 45:607–12. doi: 10.1007/s11255-013-0454-7
2. Degheili JA, Termos S, Moussa M, Aoun B. Ureteropelvic junction obstruction: diagnosis, treatment and prognosis. In: Advances in Medicine and Biology. New York, NY: Nova Medicine and Health (2020). p. 75–7.
3. Nguyen HT, Benson CB, Bromley B, Campbell JB, Chow J, Coleman B, et al. Multidisciplinary consensus on the classification of prenatal and postnatal urinary tract dilation (UTD classification system). J Pediatric Urol. (2014) 10:982–98. doi: 10.1016/j.jpurol.2014.10.002
4. Gopal M, Peycelon M, Caldamone A, Chrzan R, El-Ghoneimi A, Olsen H, et al. Management of ureteropelvic junction obstruction in children—a roundtable discussion. J Pediatric Urol. (2019) 15:322–29. doi: 10.1016/j.jpurol.2019.05.010
5. Pinter A, Horvath A, Hrabovszky Z. The relationship of smooth muscle damage to age, severity of pre-operative hydronephrosis and post-operative outcome in obstructive uropathies. Br J Urol. (1997) 80:227–33. doi: 10.1046/j.1464-410X.1997.00311.x
6. Murnaghan G. The dynamics of the renal pelvis and ureter with reference to congenital hydronephrosis. Br J Urol. (1958) 30:321. doi: 10.1111/j.1464-410X.1958.tb03525.x
7. Cajal R. Histologie du système nerveux de l'Homme et des Vertébrés. Grand sympathique. Paris, Maloine (1911). p. 2.
8. Miliaras D, Karasavvidou F, Papanikolaou A, Sioutopoulou D. KIT expression in fetal, normal adult, and neoplastic renal tissues. J Clin Pathol. (2004) 57:463–6. doi: 10.1136/jcp.2003.013532
9. Huizinga JD, Thuneberg L, Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal as targets for pharmacological intervention in gastrointestinal motor disorders. Trends Pharmacol Sci. (1997) 18:393–403. doi: 10.1016/S0165-6147(97)90668-4
10. Sanders KM, Ördög T, Koh S, Torihashi S, Ward S. Development and plasticity of interstitial cells of Cajal. Neurogastroenterol Motil. (1999) 11:311–38. doi: 10.1046/j.1365-2982.1999.00164.x
11. Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal in human gut and gastrointestinal disease. Microsc Res Tech. (1999) 47:344–60. doi: 10.1002/(SICI)1097-0029(19991201)47:5 <344::AID-JEMT6>3.0.CO;2-1
12. Wedel T, Spiegler J, Soellner S, Roblick UJ, Schiedeck TH, Bruch HP, et al. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology. (2002) 123:1459–67. doi: 10.1053/gast.2002.36600
13. Huizinga JD, Faussone-Pellegrini MS. About the presence of interstitial cells of Cajal outside the musculature of the gastrointestinal tract. J Cell Mol Med. (2005) 9:468–73. doi: 10.1111/j.1582-4934.2005.tb00372.x
14. Van Der Aa F, Roskams T, Blyweert W, Ost D, Bogaert G, De Ridder D. Identification of kit positive cells in the human urinary tract. J Urol. (2004) 171:2492–6. doi: 10.1097/01.ju.0000125097.25475.17
15. Babu R, Vittalraj P, Sundaram S, Manjusha MP, Ramanan V, Sai V. Comparison of different pathological markers in predicting pyeloplasty outcomes in children. J Pediatric Surg. (2020) 55:1616–20. doi: 10.1016/j.jpedsurg.2019.08.015
16. Shafik A, El-Sibai O, Shafik I, Shafik AA. Immunohistochemical identification of the pacemaker cajal cells in the normal human vagina. Arch Gynecol Obstet. (2005) 272:13–6. doi: 10.1007/s00404-005-0725-3
17. Gherghiceanu M, Hinescu M, Andrei F, Mandache E, Macarie C, Faussone-Pellegrini MS, et al. Interstitial Cajal-like cells (ICLC) in myocardial sleeves of human pulmonary veins. J Cell Mol Med. (2008) 12:1777–81. doi: 10.1111/j.1582-4934.2008.00444.x
18. Constantinou C, Silvert M, Gosling J. Pacemaker system in the control of ureteral peristaltic rate in the multicalyceal kidney of the pig. Investig Urol. (1977) 14:440.
19. Klemm MF, Exintaris B, Lang RJ. Identification of the cells underlying pacemaker activity in the guinea-pig upper urinary tract. J Physiol. (1999) 519:867–84. doi: 10.1111/j.1469-7793.1999.0867n.x
20. Shafik A, Al-Sherif AM. Ureteropelvic junction: a study of its anatomical structure and function. Eur Urol. (1999) 36:150–7. doi: 10.1159/000067987
21. Lang RJ, Tonta MA, Zoltkowski BZ, Meeker WF, Wendt I, Parkington HC. Pyeloureteric peristalsis: role of atypical smooth muscle cells and interstitial cells of Cajal-like cells as pacemakers. J Physiol. (2006) 576:695–705. doi: 10.1113/jphysiol.2006.116855
22. Kuvel M, Canguven O, Murtazaoglu M, Albayrak S. Distribution of Cajal like cells and innervation in intrinsic ureteropelvic junction obstruction. Arch Ital Urol Androl. (2011) 83:128–32.
23. Koleda P, Apoznanski W, Wozniak Z, Rusiecki L, Szydelko T, Pilecki W, et al. Changes in interstitial cell of Cajal-like cells density in congenital ureteropelvic junction obstruction. Int Urol Nephrol. (2012) 44:7–12. doi: 10.1007/s11255-011-9970-5
24. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
25. Standring S. Gray's Anatomy: The Anatomical Basis of Clinical Practice. Philadelphia: Elsevier (2020).
26. Foote J, Blennerhassett J, Wiglesworth F, Mackinnon K. Observations on the ureteropelvic junction. J Urol. (1970) 104:252–7. doi: 10.1016/S0022-5347(17)61710-5
27. Wein AJ, Kavoussi LR, Dmochowski R, Partin AW, Peters CA. Campbell-Walsh Urology. North York, ON: Elsevier (2020).
28. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: systematic reviews of etiology and risk. Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute. (2017). p. 2019–05. doi: 10.46658/JBIRM-17-06
29. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley and Sons (2019). doi: 10.1002/9781119536604
31. Babu R, Vittalraj P, Sundaram S, Shalini S. Pathological changes in ureterovesical and ureteropelvic junction obstruction explained by fetal ureter histology. J Pediatric Urol. (2019) 15:e247. doi: 10.1016/j.jpurol.2019.02.001
32. Balikci Ö, Turunç T, Bal N. Çelik H, Özkardeş H. Comparison of Cajal-like cells in pelvis and proximal ureter of kidney with and without hydronephrosis. Int Braz J Urol. (2015) 41:1178–84. doi: 10.1590/S1677-5538.IBJU.2014.0427
33. Eken A, Erdogan S, Kuyucu Y, Seydaoglu G, Polat S, Satar N. Immunohistochemical and electron microscopic examination of Cajal cells in ureteropelvic junction obstruction. Can Urol Assoc J. (2013) 7:E311–6. doi: 10.5489/cuaj.1247
34. How GY, Chang KTE, Jacobsen AS, Yap TL, Ong CCP, Low Y, et al. Neuronal defects an etiological factor in congenital pelviureteric junction obstruction? J Pediatric Urol. (2018) 14:e57. doi: 10.1016/j.jpurol.2017.07.014
35. Inugala A, Reddy RK, Rao BN, Reddy SP, Othuluru R, Kanniyan L, et al. Immunohistochemistry in ureteropelvic junction obstruction and its correlation to postoperative outcome. J Indian Assoc Pediatric Surg. (2017) 22:129–33. doi: 10.4103/jiaps.JIAPS_254_16
36. Kart Y, Karakuş OZ, Ateş O, Hakgüder G, Olguner M, Akgür FM. Altered expression of interstitial cells of Cajal in primary obstructive megaureter. J Pediatric Urol. (2013) 9:1028–31. doi: 10.1016/j.jpurol.2013.02.003
37. Lee HW, Baak CH, Lee MY, Kim YC. Spontaneous contractions augmented by cholinergic and adrenergic systems in the human ureter. Korean J Physiol Pharmacol. (2011) 15:37–41. doi: 10.4196/kjpp.2011.15.1.37
38. Mehrazma M, Tanzifi P, Rakhshani N. Changes in structure, interstitial Cajal-like cells and apoptosis of smooth muscle cells in congenital ureteropelvic junction obstruction. Iran J Pediatr. (2014) 24:105–10. doi: 10.1097/01.PAT.0000454556.77877.27
39. Metzger R, Schuster T, Till H, Stehr M, Franke FE, Dietz HG, et al. Cajal-like cells in the human upper urinary tract. J Urol. (2004) 172:769–72. doi: 10.1097/01.ju.0000130571.15243.59
40. Pande T, Dey SK, Chand K, Kinra P. Influence of interstitial cells of cajal in congenital ureteropelvic junction obstruction. J Indian Assoc Pediatric Surg. (2020) 25:231–5. doi: 10.4103/jiaps.JIAPS_115_19
41. Prişcǎ RA, Loghin A, Gozar HG, Moldovan C, Moso T, Derzsi Z, et al. Morphological aspects and distribution of interstitial cells of Cajal in the human upper urinary tract. Turk Patoloji Derg. (2014) 30:100–4. doi: 10.5146/tjpath.2014.01242
42. Senol C, Onaran M, Gurocak S, Gonul II, Tan MO. Changes in Cajal cell density in ureteropelvic junction obstruction in children. J Pediatric Urol. (2016) 12:e85. doi: 10.1016/j.jpurol.2015.08.010
43. Solari V, Piotrowska AP, Puri P. Altered expression of interstitial cells of Cajal in congenital ureteropelvic junction obstruction. J Urol. (2003) 170:2420–2. doi: 10.1097/01.ju.0000097401.03293.f0
44. Wishahi M, Mehena A, Elganzoury H, Badawy M, Hafiz E, El-Leithy T. Telocytes and Cajal cells distribution in renal pelvis, ureteropelvic junction (UPJ), and proximal ureter in normal upper urinary tract and UPJ obstruction: reappraisal of the etiology of UPJ obstruction. Folia Morphol. (2020). doi: 10.5603/FM.a2020.0119
45. Yang X, Zhang Y, Hu J. The expression of Cajal cells at the obstruction site of congenital pelviureteric junction obstruction and quantitative image analysis. J Pediatric Surg. (2009) 44:2339–42. doi: 10.1016/j.jpedsurg.2009.07.061
46. Murakumo M, Nonomura K, Yamashita T, Ushiki T, Abe K, Koyanagi T. Structural changes of collagen components and diminution of nerves in congenital ureteropelvic junction obstruction. J Urol. (1997) 157:1963–8. doi: 10.1016/S0022-5347(01)64910-3
47. Sui G, Rothery S, Dupont E, Fry C, Severs N. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int. (2002) 90:118–29. doi: 10.1046/j.1464-410X.2002.02834.x
48. Mut T, Acar Ö, Oktar T, Kiliçaslan I, Esen T, Ander H, et al. Intraoperative inspection of the ureteropelvic junction during pyeloplasty is not sufficient to distinguish between extrinsic and intrinsic causes of obstruction: correlation with histological analysis. J Pediatric Urol. (2016) 12:223.e221–223. e226. doi: 10.1016/j.jpurol.2016.02.016
49. Lang RJ, Hashitani H. Pacemaker mechanisms driving pyeloureteric peristalsis: modulatory role of interstitial cells. Adv Exp Med Biol. (2019) 1124:77–101. doi: 10.1007/978-981-13-5895-1_3
50. Veress B, Ohlsson B. Spatial relationship between telocytes, interstitial cells of Cajal and the enteric nervous system in the human ileum and colon. J Cell Mol Med. (2020) 24:3399–406. doi: 10.1111/jcmm.15013
51. Schulman C, Duarte-Escalante O, Boyarsky S. The autonomic innervation of the ureter and ureterovesical junction. In: Lutzeyer W, Melchior H, editors. Urodynamics. Berlin: Springer-Verlag (1973). p. 90–7. doi: 10.1007/978-3-642-65640-8_14
52. Gockel I, Bohl JR, Eckardt VF, Junginger T. Reduction of interstitial cells of Cajal (ICC) associated with neuronal nitric oxide synthase (n-NOS) in patients with achalasia. Am J Gastroenterol. (2008) 103:856–64. doi: 10.1111/j.1572-0241.2007.01667.x
53. Wang H, Zhang Y, Liu W, Wu R, Chen X, Gu L, et al. Interstitial cells of Cajal reduce in number in recto-sigmoid Hirschsprung's disease and total colonic aganglionosis. Neurosci Lett. (2009) 451:208–11. doi: 10.1016/j.neulet.2009.01.015
54. Der T, Bercik P, Donnelly G, Jackson T, Berezin I, Collins SM, et al. Interstitial cells of Cajal and inflammation-induced motor dysfunction in the mouse small intestine. Gastroenterology. (2000) 119:1590–9. doi: 10.1053/gast.2000.20221
55. Chang IY, Glasgow NJ, Takayama I, Horiguchi K, Sanders KM, et al. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. (2001) 536:555. doi: 10.1111/j.1469-7793.2001.0555c.xd
56. Gomez-Pinilla PJ, Gibbons SJ, Sarr MG, Kendrick ML, Robert Shen K, Cima RR, et al. Changes in interstitial cells of cajal with age in the human stomach and colon. Neurogastroenterol Motil. (2011) 23:36–44. doi: 10.1111/j.1365-2982.2010.01590.x
57. Wang THH, Angeli TR, Ishida S, Du P, Gharibans A, Paskaranandavadivel N, et al. The influence of interstitial cells of Cajal loss and aging on slow wave conduction velocity in the human stomach. Physiol Rep. (2021) 8:e14659. doi: 10.14814/phy2.14659
58. Lammers W, Ahmad H, Arafat K. Spatial and temporal variations in pacemaking and conduction in the isolated renal pelvis. Am J Physiol. (1996) 270:F567–74. doi: 10.1152/ajprenal.1996.270.4.F567
59. Ekinci S, Ertunc M, Ciftci A, Senocak M, Buyukpamukcu N, Onur R. Evaluation of Pelvic contractility in ureteropelvic junction obstruction: an experimental study. Eur J Pediatric Surg. (2004) 14:93–9. doi: 10.1055/s-2004-815854
60. Mccloskey KD. Interstitial cells of Cajal in the urinary tract. Handb Exp Pharmacol. (2011) 2:233–54. doi: 10.1007/978-3-642-16499-6_11
Keywords: interstitial cells of Cajal, Cajal like cells, ureteropelvic junction obstruction, density, aging
Citation: Samaranayake UMJE, Mathangasinghe Y, Liyanage UA, de Silva MVC, Samarasinghe MC, Abeygunasekera S, Lamahewage AK and Malalasekera AP (2021) Variations in the Density and Distribution of Cajal Like Cells Associated With the Pathogenesis of Ureteropelvic Junction Obstruction: A Systematic Review and Meta-Analysis. Front. Surg. 8:721143. doi: 10.3389/fsurg.2021.721143
Received: 06 June 2021; Accepted: 30 June 2021;
Published: 28 July 2021.
Edited by:
Abdurrahman Onen, Onen Pediatric Urology Center, TurkeyReviewed by:
Roberto Iglesias Lopes, Hospital for Sick Children, CanadaAli Avanoglu, Ege University, Turkey
Copyright © 2021 Samaranayake, Mathangasinghe, Liyanage, de Silva, Samarasinghe, Abeygunasekera, Lamahewage and Malalasekera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Y. Mathangasinghe, eWFzaXRoQGFuYXQuY21iLmFjLmxr
 U. M. J. E. Samaranayake
U. M. J. E. Samaranayake Y. Mathangasinghe
Y. Mathangasinghe U. A. Liyanage2
U. A. Liyanage2