
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg. , 21 July 2021
Sec. Surgical Oncology
Volume 8 - 2021 | https://doi.org/10.3389/fsurg.2021.713171
 Feng Mao1
Feng Mao1 Zhenmin Huang2*
Zhenmin Huang2*Background: Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) is a promising approach for the management of peritoneal carcinomatosis, but is associated with significant morbidity and prolonged hospital stay. Herein, we review the impact of Enhanced recovery after surgery (ERAS) protocol on length of stay (LOS) and early complications in patients undergoing CRS and HIPEC for peritoneal carcinomatosis.
Methods: PubMed and Embase were searched for studies comparing ERAS protocol with control for CRS + HIPEC. Mean difference (MD) and risk ratios (RR) were calculated for LOS and complications respectively.
Results: Six retrospective studies were included. Meta-analysis indicated statistically significant reduction in LOS with ERAS (MD: −2.82 95% CI: −3.79, −1.85 I2 = 29% p < 0.00001). Our results demonstrated significantly reduced risk of Calvien Dindo grade III/IV complications with the use of ERAS protocol as compared to the control group (RR: 0.60 95% CI: 0.41, 0.87 I2 = 0% p = 0.007). Pooled analysis of limited studies demonstrated no statistically significant difference in the risk of reoperation (RR: 1.04 95% CI: 0.54, 2.03 I2 = 50% p = 0.90) readmission (RR: 0.55 95% CI: 0.21, 1.49 I2 = 0% p = 0.24), acute kidney injury (RR: 0.55 95% CI: 0.28, 1.10 I2 = 0% p = 0.09) or mortality (RR: 0.62 95% CI: 0.17, 2.26 I2 = 0% p = 0.46) between the study groups.
Conclusion: For CRS + HIPEC, ERAS is associated with significantly reduced LOS along with lower incidence of complications. Limited data suggest that use of ERAS protocol is not associated with increased readmission, reoperation, and mortality rates in these patients. There is a need for randomized controlled trials to corroborate the current evidence.
Peritoneal carcinomatoses are a heterogeneous group of disease which can either arise primarily from the peritoneum itself or metastasis of other tumors in the abdomen located at the colon, rectum, appendix, stomach or ovary (1). Indeed, the treatment plan varies with the disease histology and the extent of peritoneal involvement; but if left untreated, survival with this disease can be as low as 4 months (2).
Over the last two decades, cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have emerged as an encouraging treatment option for managing peritoneal carcinomatosis (3, 4). While the surgical component of the regimen aims to eliminate the perceivable tumor mass via peritonectomy and visceral resections, the HIPEC component eradicates the microscopic disease (4). Studies have shown that in selected patients, the combination of CRS + HIPEC can lead to significant improvement in survival as compared to CRS or HIPEC alone (5, 6). Historically, CRS + HIPEC was known to cause a high incidence of morbidity and mortality owing to the significant physiological insult associated with the treatment (7, 8). However, recent data suggest that the safety of CRS + HIPEC is similar to that of other high-risk oncological procedures and increased morbidity with this treatment is a misperception from early experience (9). Nevertheless, this relatively resource-intensive therapy is offered by limited healthcare setups worldwide with varying perioperative practices which can significantly influence early patient outcomes (10).
Over the years, there has been an effort in the surgical community to improve patient care by following standard perioperative protocols (11). One such popular guideline is the enhanced recovery after surgery (ERAS) protocol which was initially developed for colorectal surgeries (12). ERAS is a multimodal strategy that incorporates many evidence-based preoperative, perioperative, and postoperative guidelines to improve patient recovery by reducing hospital stay and complication rates (13). However, despite the benefits offered by the ERAS protocol, concerns have also been raised regarding the increased risk of readmissions and acute kidney injury (AKI) due to the stringent fluid management guideline associated with the protocol (14, 15). While researchers have used some components of ERAS like pre-habilitation or restrictive fluid therapy for patients undergoing CRS + HIPEC (16, 17), the impact of a comprehensive ERAS protocol on these patients is still unclear. In the past 3 years, some researchers have presented their experience of ERAS with CRS + HIPEC but with a limited sample size (18, 19). To the best of our knowledge, no review has been attempted to synthesize data from these studies to present the best available evidence regarding the impact of ERAS on CRS + HIPEC. Therefore, the purpose of this study was to compare outcomes of patients undergoing CRS + HIPEC with and without the use of ERAS protocol.
We performed this review in accordance with the recommendations of the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses) (20). It is, however, clarified that the protocol was not preregistered on any online database.
For clarity, we defined the Inclusion criteria based on the PICOS (Population, Intervention, Comparison, Outcome, and Study design) format as follows:
Population: Adult patients with peritoneal carcinomatosis undergoing CRS + HIPEC
Intervention: ERAS protocol
Comparison: Non-ERAS protocol (control)
Outcomes: Length of hospital stay (LOS) or complications
Study design: All types of studies
The following studies were excluded: (1) Studies without a control group. (2) Studies not reporting relevant outcome data (3) Studies only on CRS. (4) Review articles and non-English language studies. Where studies presented data from the same database with the same or overlapping study period, we included the study presenting larger sample size data.
With the help of a librarian, we searched the databases of PubMed and Embase to look for relevant studies. The databases were screened from inception to 15th February 2021. Two reviewers independently conducted the electronic search with the following keywords: “enhanced recovery”, “ERAS”, “fast recovery”, “accelerated rehabilitation”, “multimodal perioperative care”, “cytoreductive surgery”, and “hyperthermic intraperitoneal chemotherapy”. Supplementary Table 1 demonstrates the search strategy. Every search result was evaluated by the two reviewers independently, initially by their titles and abstracts and then by full texts of relevant publications. All full-texts were reviewed based on the inclusion and exclusion criteria and the article satisfying all the criteria was finally selected for this review. Any disagreements were resolved by discussion. To avoid any missed studies, the bibliography of included studies was hand searched for any additional references.
We prepared a data extraction form at the beginning of the review to extract relevant details from the studies. The final version of this template was approved by all the study investigators. Details of the first author, publication year, study type, HIPEC drugs, sample size, demographic details, peritoneal cancer index (PCI), site of the primary tumor, operative time, ERAS protocol, and outcomes were extracted. Data were extracted by two reviewers independent of each other. Any disagreements were resolved by discussion. Outcomes assessed via a meta-analysis were, LOS, complications [grade III/IV based on Calvien Dindo classification (21)], readmission rates, reoperation rates, acute kidney injury (AKI), and mortality.
The methodological quality of included studies was assessed using the Newcastle-Ottawa scale (NOS) (22). This too was carried out in duplicate and independently by two study investigators. Studies were awarded points for selection of study population, comparability, and outcomes. The maximum score which can be awarded is nine.
The software “Review Manager” [RevMan, version 5.3; Nordic Cochrane Centre (Cochrane Collaboration), Copenhagen, Denmark; 2014] was used for the meta-analyses. As LOS is a continuous variable, we extracted mean and standard deviation (SD) data from the studies and pooled it to calculate the mean difference (MD) with 95% confidence intervals (CI). Median, range, or interquartile range data were converted into mean and SD when required using the method of Wan et al. (23). For the remaining categorical variables, we calculated risk ratios (RR) with 95% CI. Since there was already methodological heterogeneity in the included studies, we preferred a random-effects model for the analysis. The I2 statistic was used to assess inter-study heterogeneity. I2 values of 25–50% represented low, values of 50–75% medium, and >75% represented substantial heterogeneity. Funnel plots were used to assess publication bias (24).
The flow-chart of the study is presented in Figure 1. The literature search revealed 383 records from both the databases combined. After deduplication, 125 unique records were screened of which 113 were excluded after title/abstract screening. From the remaining 12 articles, six were excluded after full-text analysis with reasons, and a total of six studies were included for this review (18, 19, 25–28).
Details of included studies are presented in Table 1. No randomized controlled trials (RCTs) were available and all were retrospective studies. Mitomycin, cisplatin, and oxaliplatin were used for the chemotherapy in the included studies. The sample size of the ERAS arm varied from 15 to 81 patients while in the control group it varied from 11 to 105 patients. The NOS score of the studies varied from 5 to 6. None of the studies carried out baseline matching of the study groups. A detailed description of the ERAS protocol in the pre-operative, intra-operative, and post-operative periods for each of the included studies is presented in Table 2.
LOS was reported by all included studies. A meta-analysis of data of 278 patients in the ERAS group and 309 patients in the control group indicated a statistically significant reduction in LOS with the ERAS protocol (MD: −2.82 95% CI: −3.79, −1.85 I2 2 = 9% p < 0.00001) (Figure 2). There was no evidence of publication bias (Supplementary Figure 1). While data on complications were reported by all included studies, the majority (five studies) reported incidence of higher grade complications (Calvien Dindo grade III/IV) only. Hence, only such data could be pooled for the analysis. Our results demonstrated a significantly reduced risk of grade III/IV complications with the use of ERAS protocol as compared to the control group (RR: 0.60 95% CI: 0.41, 0.87 I2 0 = % p = 0.007) (Figure 3). There was no evidence of publication bias (Supplementary Figure 2).
Data on early reoperation and readmission rates were reported by four and two studies, respectively. Pooled analysis demonstrated no statistically significant difference in the risk of early reoperation (RR: 1.04 95% CI: 0.54, 2.03 I2 5 = 0% p = 0.90) or readmission (RR: 0.55 95% CI: 0.21, 1.49 I2 0 = % p = 0.24) between the two study groups (Figure 4). Only three studies reported data on the incidence of postoperative AKI. Meta-analysis failed to demonstrate any statistically significant difference between the ERAS and control groups (RR: 0.55 95% CI: 0.28, 1.10 I2 0 = % p = 0.09) (Figure 4). Similarly, on a pooled analysis of data from just two studies, we did not find any statistically significant difference in the risk of mortality between the two groups (RR: 0.62 95% CI: 0.17, 2.26 I2 0 = % p = 0.46) (Figure 4).
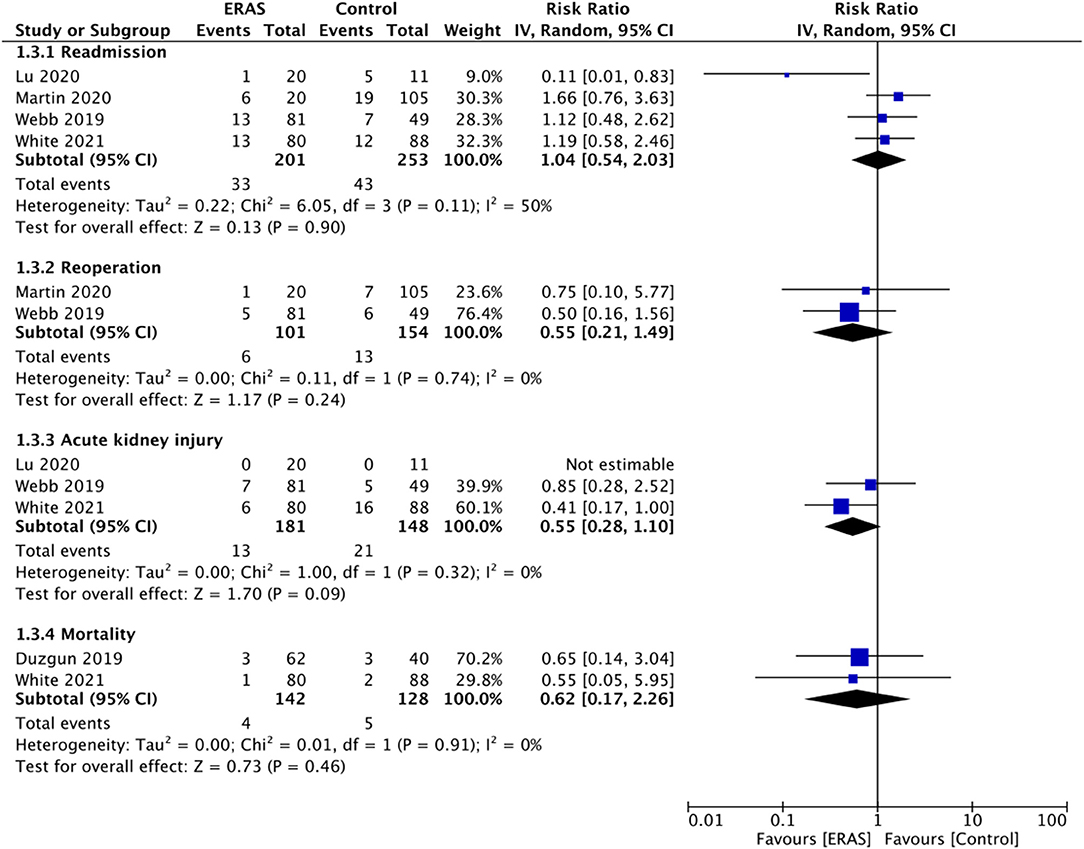
Figure 4. Meta-analysis for readmission, reoperation, AKI and mortality between ERAS and control groups.
The core principle of ERAS protocol is to standardized and optimize patient care to harmonize the exaggerated inflammatory surgical response which is associated with adverse patient outcomes (12). Specifically, the foundation of ERAS is built on several elements like patient education, nutritional screening, multimodal opioid-sparing anesthesia, controlled perioperative fluid management, early feeding and ambulation, early removal of catheters and drains, prevention of postoperative nausea and vomiting (PONV) along with other ancillary guidelines depending on the surgery type (13). Since its success with colorectal surgery, the program has been adopted in several surgical specialties with different guidelines according to the surgery type (29, 30). The ERAS society has also recently published recommendations to standardize the methodology of development of these guidelines (31).
Owing to the historically high rates of mortality and morbidity of CRS + HIPEC (7, 8), hesitation exists amongst clinicians to completely utilize the program for these patients despite reports of improved outcomes with singular elements of ERAS protocol. Hendrix et al. (16) in a sample size of 169 CRS + HIPEC patients have demonstrated that intraoperative restrictive fluid therapy with standard monitoring is associated with reduced LOS and grade III/IV complications. Similarly, Osseis et al. (17) have shown that clear preoperative education significantly reduced LOS, time to first ambulation, and patient satisfaction in CRS + HIPEC patients. Indeed, goal-directed intraoperative fluids as part of the ERAS program were used by all included studies in this review while patient education was utilized by five of the six studies. Important to note is that detailed ERAS guidelines specific to CRS + HIPEC have been made available only recently much after the conduct of the included studies (32, 33). Hubner et al. (32, 33) in August 2020 provided several preoperative, intraoperative, and postoperative recommendations for CRS + HIPEC, focusing on the core tenants of the ERAS program mentioned earlier. However, of the 72 items on the list, direct evidence was available only for eight items and the rest were extrapolated from other related colorectal and abdominal surgical procedures. Indeed, the paucity of evidence is a major limiting factor in the application of several elements of the ERAS program to CRS + HIPEC patients.
On a detailed analysis of the ERAS protocol, it can be noted that there was variation in the elements of the ERAS across the six included studies. This was expected owing to the different participating centers and timelines of the studies; and it is has been a factor of heterogeneity in other meta-analysis studies on ERAS programs as well (13, 34). Nevertheless, several core principles were utilized by majority studies like preoperative education, nutrition optimization, goal-directed fluid management, use of regional blocks or multimodal anesthesia, early removal of tubes and drains, and postoperative pain control. Indeed, our meta-analysis indicated that the application of ERAS protocol significantly reduced LOS for CRS + HIPEC patients. Individually, all of the included studies demonstrated a significantly reduced LOS, except for Siddharthan et al. (19). The lack of difference in this study could be attributed to its small sample size. Our results concur with other meta-analysis studies on the ERAS program. Tan et al. (35) on breast reconstruction, Malczak et al. (34) on bariatric surgery, Zhuang et al. (13) on colorectal surgery; have all demonstrated that implementation of ERAS leads to significantly reduced LOS.
According to Alyami et al. (36), the rate of major complications after CRS + HIPEC based on Calvien Dindo classification can be as high as 25%. In our analysis, the rates of grade III/IV complications in the control group were close to this figure at 26.9%. However, in the ERAS group, the incidence was 13.9% with a statistically significant 40% reduced risk of complications. Owing to the different ERAS elements used in the studies, it is difficult to delineate which intervention or interventions might have contributed to these results. CRS + HIPEC procedure is associated with large hemodynamic changes due to surgical, chemical, and hyperthermic trauma and inadequate volume resuscitation can lead to hemodynamic instability, hypoperfusion, and nephrotoxicity while excessive fluids can cause overload, tissue edema, and a higher risk of major complications (16). The use of goal-directed fluid therapy may therefore be an important contributor to the reduced incidence of complications (16). Furthermore, nutrition support and other preoperative measures like physiotherapy, adequate pre-anesthetic screening for comorbidities like diabetes can also contribute to a reduced risk of infectious complications with ERAS (28).
There have been concerns with the use of the ERAS program which include risk of readmission and AKI (14, 15). Increased early readmission rates with ERAS are, however, not universal with research also indicating a reduced risk of readmission with the program (37). Important contributors to early readmission with abdominal surgical procedures are postoperative ileus and infectious complications (27). However, Francis et al. (38) have demonstrated that poor compliance to ERAS elements is an independent predictor of early readmissions. While the majority of studies in our review did not report percentage compliance with the ERAS protocol, analysis of limited data revealed that application of ERAS does not increase early readmission, reoperation, or mortality in patients undergoing CRS + HIPEC. The scarce data also limited our ability to assess the rates of postoperative ileus and infectious complications.
The chemotherapeutic agents used with HIPEC especially cisplatin can lead to significant renal injury and there are apprehensions that restricted use of fluids in the perioperative period may exacerbate it (16, 39). However, studies assessing restrictive fluid therapy for CRS + HIPEC have failed to demonstrate such association (16, 40). In our meta-analysis, only limited studies reported the impact of ERAS on AKI. Although the results were non-significant, in view of the scarce data, it is difficult to comment on the actual association between ERAS and AKI in patients undergoing CRS + HIPEC. It is important to note that restriction of fluid therapy can be difficult in patient undergoing CRS + HIPEC. Fleres et al. (41) have demonstrated that cisplatin levels with HIPEC remain high up to the 4th post-operative day and return to preoperative levels only by the 7th post-operative day. The authors noted that hyperhydration along with infusion of colloids is of particular importance in the first four days after the procedure. In this context, application of ERAS can be difficult to apply in all patients undergoing CRS + HIPEC and may be restricted to patients with low peritoneal cancer index (PCI) undergoing minor resection.
The results of our study should be interpreted with the following limitations. Foremost, only six studies were available for review mostly with a small sample size. Furthermore, all were retrospective studies and prone to bias. Most importantly, none of the studies conducted baseline matching and this could have skewed our outcomes. The ERAS and control protocols were not parallelly followed in the included studies. The different periods of the study groups could have been associated with other changes in hospital practices which may have influenced outcomes. Secondly, data for all variables were not universally reported by the included studies. Some of the outcome variables were analyzed with just two or three studies which restricted the power of our analysis. We also could not analyze the impact of ERAS on several important variables like pain scores, post-operative ileus, hospitalization costs, etc. Thirdly, there was methodological heterogeneity across studies in the ERAS elements used. The compliance for these elements was not known in most studies and this may have impacted outcomes.
Nevertheless, our study is the first systematic review and meta-analysis comparing ERAS with no-ERAS protocols for patients undergoing CRS + HIPEC. The pooled analysis of six studies overcomes the limitation of a small sample size of individual studies and presents the largest dataset on the impact of ERAS on CRS + HIPEC.
For patients undergoing CRS + HIPEC, our results indicate that ERAS is associated with significantly reduced LOS along with lower incidence of complications. Limited data suggest that use of ERAS protocol is not associated with increased readmission, reoperation, and mortality rates in these patients. There is a need for RCTs to corroborate the current evidence. Future studies should focus on the incidence of AKI with ERAS in patients undergoing CRS + HIPEC. Trials should also be conducted on a highly selected group of patients with low PCI and minor resections in order to segregate the efficacy of ERAS in this cohort.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
FM designed the study. Both the authors were involved in data acquisition, analysis, and synthesis. Both authors wrote, edited and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.713171/full#supplementary-material
Supplementary Figure 1. Funnel plot for the meta-analysis of LOS.
Supplementary Figure 2. Funnel plot for the meta-analysis of grade III/IV complications.
1. Klos D, Riško J, Loveček M, Skalický P, Svobodová I, Krejčí D, et al. Trends in peritoneal surface malignancies: evidence from a Czech nationwide population-based study. World J Surg Oncol. (2019) 17:182. doi: 10.1186/s12957-019-1731-4
2. Thomassen I, Van Gestel YR, Van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. (2014) 134:622–8. doi: 10.1002/ijc.28373
3. Brind'Amour A, Dubé P, Tremblay JF, Soucisse ML, Mack L, Bouchard-Fortier A, et al. Canadian guidelines on the management of colorectal peritoneal metastases. Curr Oncol. (2020) 27:621–631. doi: 10.3747/co.27.6919
4. Yano H. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy for peritoneal metastasis from colorectal cancer. Clin Colon Rectal Surg. (2020) 33:372–6. doi: 10.1055/s-0040-1714242
5. Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. (2010) 116:3756–62. doi: 10.1002/cncr.25116
6. Bonnot PE, Piessen G, Kepenekian V, Decullier E, Pocard M, Meunier B, et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): a propensity score analysis. J Clini Oncol (American Society of Clinical Oncology). (2019) 37:2028–40. doi: 10.1200/JCO.18.01688
7. Jacquet P, Stephens AD, Averbach AM, Chang D, Ettinghausen SE, Dalton RR, et al. Analysis of morbidity and mortality in 60 patients with peritoneal carcinomatosis treated by cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy. Cancer. (1996) 77:2622–9. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2622::AID-CNCR28>3.0.CO;2-T
8. Kecmanovic DM, Pavlov MJ, Ceranic MS, Sepetkovski A V, Kovacevic PA, Stamenkovic AB. Treatment of peritoneal carcinomatosis from colorectal cancer by cytoreductive surgery and hyperthermic perioperative intraperitoneal chemotherapy. Eur J Surg Oncol. (2005) 31:147–52. doi: 10.1016/j.ejso.2004.09.021
9. Foster JM, Sleightholm R, Patel A, Shostrom V, Hall B, Neilsen B, et al. Morbidity and mortality rates following cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy compared with other high-risk surgical oncology procedures. JAMA Netw open. (2019) 2:e186847. doi: 10.1001/jamanetworkopen.2018.6847
10. Maciver AH, Al-Sukhni E, Esquivel J, Skitzki JJ, Kane JM, Francescutti VA. Current delivery of hyperthermic intraperitoneal chemotherapy with cytoreductive surgery (CS/HIPEC) and perioperative practices: an international survey of high-volume surgeons. Ann Surg Oncol. (2017) 24:923–30. doi: 10.1245/s10434-016-5692-3
11. Grocott MPW, Plumb JOM, Edwards M, Fecher-Jones I, Levett DZH. Re-designing the pathway to surgery: better care and added value. Perioper Med. (2017) 6:9. doi: 10.1186/s13741-017-0065-4
12. Chestovich PJ, Lin AY, Yoo J. Fast-track pathways in colorectal surgery. Surg Clin North Am. (2013) 93:21–32. doi: 10.1016/j.suc.2012.09.003
13. Zhuang C Le, Ye XZ, Zhang XD, Chen BC, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: A metaanalysis of randomized controlled trials. Dis Colon Rectum. (2013) 56:667–78. doi: 10.1097/DCR.0b013e3182812842
14. Martin TD, Lorenz T, Ferraro J, Chagin K, Lampman RM, Emery KL, et al. Newly implemented enhanced recovery pathway positively impacts hospital length of stay. Surg Endosc. (2016) 30:4019–28. doi: 10.1007/s00464-015-4714-8
15. Marcotte JH, Patel K, Desai R, Gaughan JP, Rattigan D, Cahill KW, et al. Acute kidney injury following implementation of an enhanced recovery after surgery (ERAS) protocol in colorectal surgery. Int J Colorectal Dis. (2018) 33:1259–67. doi: 10.1007/s00384-018-3084-9
16. Hendrix RJ, Damle A, Williams C, Harris A, Spanakis S, Lambert DH, et al. Restrictive intraoperative fluid therapy is associated with decreased morbidity and length of stay following hyperthermic intraperitoneal chemoperfusion. Ann Surg Oncol. (2019) 26:490–6. doi: 10.1245/s10434-018-07092-y
17. Osseis M, Weyrech J, Gayat E, Dagois S, Lo Dico R, Pocard M, et al. Epidural analgesia combined with a comprehensive physiotherapy program after Cytoreductive Surgery and HIPEC is associated with enhanced post-operative recovery and reduces intensive care unit stay: a retrospective study of 124 patients. Eur J Surg Oncol. (2016) 42:1938–43. doi: 10.1016/j.ejso.2016.06.390
18. Lu PW, Fields AC, Shabat G, Bleday R, Goldberg JE, Irani J, et al. Cytoreductive Surgery and HIPEC in an Enhanced Recovery After Surgery Program: a Feasibility Study. J Surg Res. (2020) 247:59–65. doi: 10.1016/j.jss.2019.10.042
19. Siddharthan R, Dewey E, Billingsley K, Gilbert E, Tsikitis VL. Feasibility and benefits of an enhanced recovery after surgery protocol for patients undergoing cytoreductive surgery and heated intraperitoneal chemotharpy: a single institution experience. Am J Surg. (2020) 219:1073–5. doi: 10.1016/j.amjsurg.2019.06.019
20. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
21. Clavien PA, Barkun J, De Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2
22. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed October 30, 2020)
23. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) s 14:135. doi: 10.1186/1471-2288-14-135
24. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions. Version 6. Cochrane. Chichester: John Wiley & Sons (2019). doi: 10.1002/9781119536604
25. White B, Dahdaleh F, Naffouje SA, Kothari N, Berg J, Wiemann W, et al. Impact of enhanced recovery after surgery on postoperative outcomes for patients undergoing Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. (2021). doi: 10.1245/s10434-020-09476-5. [Epub ahead of print].
26. Webb C, Day R, Velazco CS, Pockaj BA, Gray RJ, Stucky CC, et al. Implementation of an enhanced recovery after surgery (eras) program is associated with improved outcomes in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. (2020) 27:303–12. doi: 10.1245/s10434-019-07900-z
27. Martin RC, Marshall BM, Philips P, Egger M, McMasters KM, Scoggins CR. Enhanced recovery after surgery is safe for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Am J Surg. (2020) 220:1428–32. doi: 10.1016/j.amjsurg.2020.08.041
28. Duzgun O. Evaluation of enhanced recovery after following a surgical protocol for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Med Arch (Sarajevo, Bosnia Herzegovina). (2019) 73:331–7. doi: 10.5455/medarh.2019.73.331-337
29. Chakravarthy V, Yokoi H, Manlapaz MR, Krishnaney AA. Enhanced Recovery in Spine Surgery and Perioperative Pain Management. Neurosurg Clin N Am. (2020) 31:81–91. doi: 10.1016/j.nec.2019.08.010
30. Low DE, Allum W, De Manzoni G, Ferri L, Immanuel A, Kuppusamy MK, et al. Guidelines for perioperative care in esophagectomy: enhanced recovery after surgery (ERAS®) society recommendations. World J Surg. (2019) 43:299–330. doi: 10.1007/s00268-018-4786-4
31. Brindle M, Nelson G, Lobo DN, Ljungqvist O, Gustafsson UO. Recommendations from the ERAS® Society for standards for the development of enhanced recovery after surgery guidelines. BJS Open. (2020) 4:157–63. doi: 10.1002/bjs5.50238
32. Hübner M, Kusamura S, Villeneuve L, Al-Niaimi A, Alyami M, Balonov K, et al. Guidelines for Perioperative Care in Cytoreductive Surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced recovery after surgery (ERAS®) Society Recommendations—Part II: Post-operative management and special considerations. Eur J Surg Oncol. (2020) 46:2311–23. doi: 10.1016/j.ejso.2020.08.006
33. Hübner M, Kusamura S, Villeneuve L, Al-Niaimi A, Alyami M, Balonov K, et al. Guidelines for Perioperative Care in Cytoreductive Surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced recovery after surgery (ERAS®) Society Recommendations — Part I: Preoperative and intraoperative management. Eur J Surg Oncol. (2020) 46:2292–310. doi: 10.1016/j.ejso.2020.07.041
34. Małczak P, Pisarska M, Piotr M, Wysocki M, Budzyński A, Pedziwiatr M. Enhanced recovery after bariatric surgery: systematic review and meta-analysis. Obes Surg. (2017) 27:226–35. doi: 10.1007/s11695-016-2438-z
35. Tan YZ, Lu X, Luo J, Huang ZD, Deng QF, Shen XF, et al. Enhanced recovery after surgery for breast reconstruction: pooled meta-analysis of 10 observational studies involving 1,838 patients. Front Oncol. (2019) 9:675. doi: 10.3389/fonc.2019.00675
36. Alyami M, Kim BJ, Villeneuve L, Vaudoyer D, Képénékian V, Bakrin N, et al. Ninety-day post-operative morbidity and mortality using the National Cancer Institute's common terminology criteria for adverse events better describe post-operative outcome after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Hyperth. (2018) 34:532–7. doi: 10.1080/02656736.2017.1367846
37. Lawrence JK, Keller DS, Samia H, Ermlich B, Brady KM, Nobel T, et al. Discharge within 24 to 72 hours of colorectal surgery is associated with low readmission rates when using enhanced recovery pathways. J Am Coll Surg. (2013) 216:390–4. doi: 10.1016/j.jamcollsurg.2012.12.014
38. Francis NK, Mason J, Salib E, Allanby L, Messenger D, Allison AS, et al. Factors predicting 30-day readmission after laparoscopic colorectal cancer surgery within an enhanced recovery programme. Color Dis. (2015) 17:O148–54. doi: 10.1111/codi.13002
39. Manohar S, Leung N. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. (2018) 31:15–25. doi: 10.1007/s40620-017-0392-z
40. Eng OS, Dumitra S, O'Leary M, Raoof M, Wakabayashi M, Dellinger TH, et al. Association of fluid administration with morbidity in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. JAMA Surg. (2017) 152:1156–60. doi: 10.1001/jamasurg.2017.2865
Keywords: ERAS, peritoneal neoplasm, abdominal malignancy, cytoreductive surgery, HIPEC
Citation: Mao F and Huang Z (2021) Enhanced Recovery After Surgery for Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Systematic Review and Meta-Analysis. Front. Surg. 8:713171. doi: 10.3389/fsurg.2021.713171
Received: 21 May 2021; Accepted: 21 June 2021;
Published: 21 July 2021.
Edited by:
Stefano Restaino, Ospedale Santa Maria della Misericordia di Udine, ItalyReviewed by:
Victor Lopez-Lopez, University of Murcia, SpainCopyright © 2021 Mao and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenmin Huang, aHVhbmd6aGVubWluMTIxNUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.