
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Surg., 17 June 2021
Sec. Genitourinary Surgery and Interventions
Volume 8 - 2021 | https://doi.org/10.3389/fsurg.2021.687636
This article is part of the Research TopicRecent Advances in Bladder Cancer Diagnosis and TreatmentView all 10 articles
An adequate pelvic lymph node dissection (PLND) is an essential part of radical cystectomy for muscle invasive bladder cancer. However, the definition of what constitutes an adequate PLND is often shrouded in controversy. Various authors have defined different anatomic templates of PLND based on levels of pelvic lymph nodes. Some have suggested other surrogate markers of the adequacy of PLND, namely lymph node count and lymph node density. While individual studies have shown the efficacy and reliability of some of the above markers, none of them have been recommended forthright due to the absence of robust prospective data. The use of non-standardized nomenclature while referring to the above variables has made this matter more complex. Most of older data seems to favor use of extended template of PLND over the standard template. On the other hand, one recent randomized controlled trial (RCT) did not show any benefit of one template over the other in terms of survival benefit, but the study design allowed for a large margin of bias. Therefore, we conducted a systematic search of literature using EMBASE, Medline, and PubMed using PRISMA-P checklist for articles in English Language published over last 20 years. Out of 132 relevant articles, 47 articles were included in the final review. We have reviewed existing literature and guidelines and have attempted to provide a few suggestions toward a uniform nomenclature for the various anatomical descriptions and the extent of PLND done while doing a radical cystectomy. The results of another large RCT (SWOG S1011) are awaited and until we have a definitive evidence, we should adhere to these suggestions as much as possible and deal with each patient on a case to case basis.
Each year, more than 400,000 patients worldwide are diagnosed with bladder cancer of which ~30% are muscle invasive (1). Radical cystectomy (RC) with bilateral pelvic lymph node dissection (PLND) is the standard of care for recurrent high risk non muscle invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC) (2, 3). Preoperative cross-sectional imaging, with a sensitivity of about 52% for positive pelvic lymph nodes, often leads to significant under staging (4–6). A thorough bilateral PLND therefore increases the staging procedure's accuracy and provides a probable survival benefit in patients of MIBC irrespective of the nodal involvement (7, 8). Now the question arises as to how should one assess the adequacy of PLND? Whether it is the levels of pelvic lymph nodes removed, the anatomical template of dissection followed, the lymph node count or the lymph node density remains a bone of contention? Adding to this confusion, is the frequent use of non-standardized nomenclature in denoting the extents of PLND across various studies. Therefore, we attempted to review the existing literature related to the levels of pelvic lymph nodes and the various templates of PLND defined by different authors to bring clarity to this issue. We also suggest certain points which can bring a uniformity to this procedure and thus facilitate better reporting of the outcomes of PLND for MIBC.
The systematic literature search was done for relevant papers, by two authors RJ and APS, in various electronic databases as follows. The following keywords with the operators from the years 2001 to 2020 were used: (“lymph node*” OR standard OR extended OR lymphadenectomy) AND ((“bladder cancer” OR “bladder carcinoma” OR urothelial) AND cancer OR “urothelial carcinoma of the bladder”) AND radical AND cystectomy for searching EMBASE/Medline and the following keywords were used for searching PubMed - (lymphadenectomy) OR (Standard) OR (extended) OR (lymph node) AND ((bladder cancer) OR (urothelial cancer) OR (bladder carcinoma) OR (urothelial carcinoma)) AND (radical cystectomy). The conference abstracts, conference papers, conference review, erratum, and notes were removed and the search results were filtered to include the articles only in English Language.
Initial search yielded 4,604 articles from EMBASE and 202 results from PUBMED. After applying exclusion criteria, this narrowed the number of articles to 98 from Embase. After removing the duplicates a total of 132 articles (EMBASE+PUBMED) were assessed by two authors (APS and RJ) independently. The reference list was searched for more relevant articles. Total of 47 articles were selected for review of literature (Table 1). The selection process is outlined in Figure 1. The current narrative review is based on these 47 included articles.
The primary drainage site for the bladder consists of the external iliac, internal iliac, obturator, and presacral lymph nodes. The secondary drainage goes to the common iliac, para-aortic, inter-aortocaval, and para-caval lymph nodes (Figure 2) (9). Bilaterality of lymph nodal spread has been demonstrated in up to 39% of patients. It has been confirmed using single- photon emission computed tomography (SPECT) and an intraoperative γ-probe after injection with a technetium nano-colloid, which has also shown that up to 52% of nodes may lie outside the true pelvis (10–12). Leissner et al. (13) have identified 12 anatomical sites with variable probability of metastatic deposits, with the obturator groups being the commonest involved site. In this study, 6.9% patients had metastases in the regions above the common iliac bifurcation and 2.9% had metastases in the inter-aortocaval and precaval regions. Many subsequent studies have shown that up to 41% of positive lymph nodes lie above the bifurcation of the common iliac arteries (14). In 591 patients, Tarin et al. (15) reported lymph node involvement in 1194 patients (19%). Of these, seven patients (6%) had no positive lymph nodes within the true pelvis (skip lesions). Since skip lesions are known to be very rare, this phenomenon may be the result of missed positive lymph nodes in the true pelvis or of a specimen-labeling error. But a few things are clear. First, PLND should be bilateral since drainage is bilateral. Next, a limited PLND template has a small but significant chance of missing positive nodes lying outside the true pelvis. However, whether wider dissection necessarily translates into oncological advantage needs to be seen.
In their paper in 2004, Leissner et al. (13) proposed a 3-tier classification system for the extent of PLND during radical cystectomy (Figure 3). The anatomical sites and their boundaries have been described in Table 2. In the more contemporary studies, this system of denoting extent of PLND has been used sparingly compared to the anatomical templates discussed in the next section (16).
The EAU Working Group on MIBC proposed the following nomenclature for the anatomical templates used in PLND based on the recommendations of an expert panel: limited, standard, extended, and super-extended PLND. The definition of these terms has been rather inconsistent and studies have often termed anything less than an extended template as a limited template. Limited PLND typically includes dissection restricted to the bilateral obturator fossae (Figure 4A) (14). Boundaries of standard PLND include the common iliac bifurcation cranially and the inguinal ligament caudally. Laterally the boundaries are the genitofemoral nerve and medially it is the bladder wall. This template typically includes the distal common iliac, the external iliac, the obturator and the internal iliac lymph nodes bilaterally (Figure 4B) (14, 17). In addition to all the lymph nodes removed in the standard template, extended dissection entails removing the presacral nodes and all the nodes between the aortic and the common iliac bifurcations (Figure 4C) (14, 17). Super-extended PLND template involves removal of all the nodal tissue caudal to the base of the inferior mesenteric artery (Figure 4D) (14).
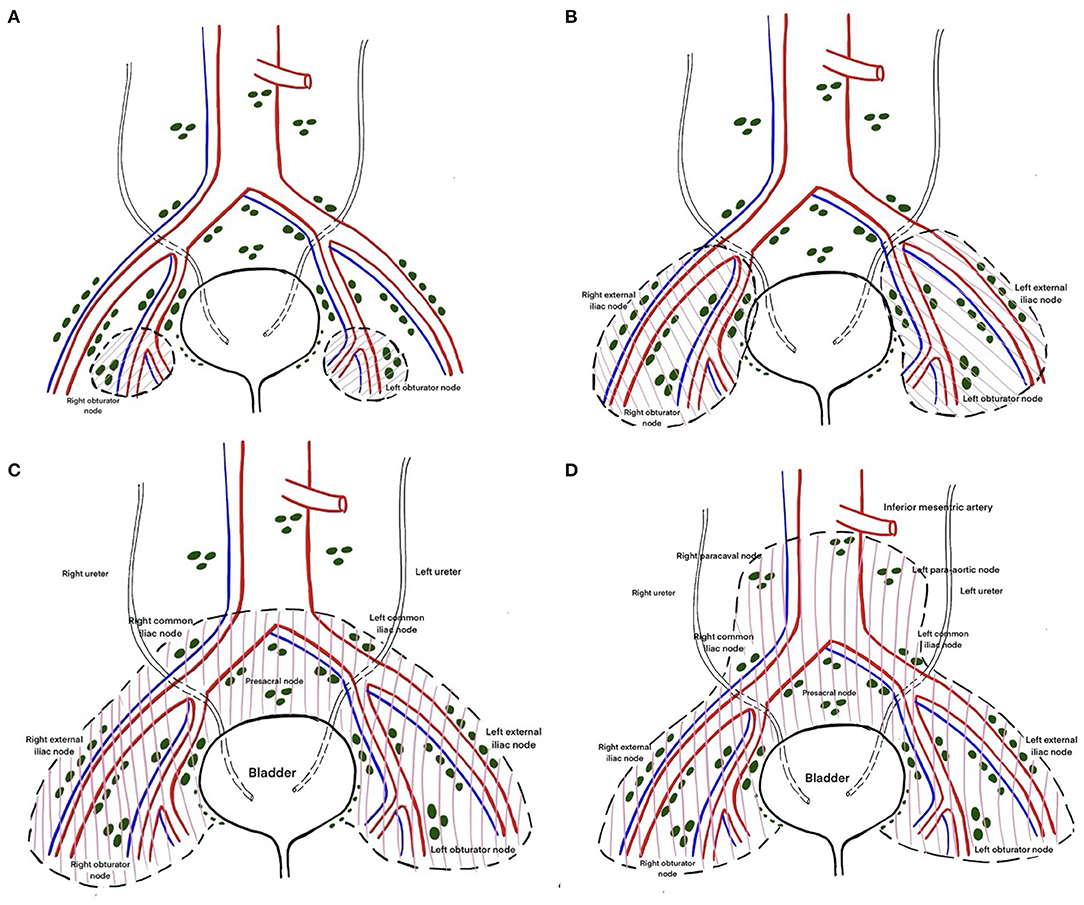
Figure 4. (A) Limited lymph node dissection including only bilateral obturator and perivesical lymph nodes. (B) Standard template of lymph node dissection including lymph nodes of the external and internal iliac groups, up to bifurcation of the common iliac artery. (C) Extended template of lymph node dissection including lymph nodes up to the aortic bifurcation. (D) Super extended lymph node dissection including lymph nodes above the aortic bifurcation below the origin of the inferior mesenteric artery.
Limited PLND has been shown to miss about 50% of positive lymph nodes in muscle invasive bladder cancer (12). The rationale behind performing a standard template PLND is that 90–95% of all node positive patients can be identified (13, 18). Dhar et al. (19) retrospectively compared limited PLND to extended PLND in 658 patients across 2 centers. It is important to point out here that what the authors describe as a limited PLND in their report is actually a standard template PLND we have described earlier. This discrepancy is an example of how a non-standardized system of nomenclature is fraught with confusion. In this study, 26% node positive patients were identified in the extended template cohort compared to 13% in the limited PLND cohort. Five-years recurrence free survival (RFS) was 23 vs. 57% (p < 0.0001), and overall survival (OS) was 26 vs. 46% (p = 0.0021), in favor of the extended LND group. For node positive patients the 5-year relapse-free survival and overall survival were both 7% for a limited dissection compared with 35 and 34% for patients undergoing extended LND, respectively (p < 0.0001). The authors concluded that standard PLND is associated with suboptimal staging and poorer outcomes for node positive and node negative disease with comparable pT stage and a higher rate of local progression, as summarized in Table 2.
In a meta-analysis by Li et al. (20), a greater extent of LND during RC had statistically significant advantages in OS, CSS and RFS, corresponding to reduced risks of 28, 34, and 36%, respectively. Bi et al. (21) showed that extended PLND was associated with improved RFS (HR = 0.66, 95% CI: 0.56–0.78, and p < 0.001) (21). The benefits of extended PLND were present in node-negative disease (HR = 0.68, 95% CI: 0.51–0.90, and p = 0.007), node-positive disease (HR = 0.58, 95% CI: 0.47–0.72, and p < 0.001), and pT3–4 disease (HR = 0.61, 95% CI: 0.52–0.73, and p < 0.001). Similar results were obtained from other meta-analyses (22, 23). A source of bias in the above studies was that the demographics of the patient populations were different and PLND was performed at the clinician's discretion. In a recent systematic review, the influence of PLND on perioperative and oncologic outcomes in patients undergoing RC for MIBC was assessed (7). Due to large heterogeneity between the studies the original meta-analysis planned by the authors was not possible. But the final results showed that any PLND is better than no PLND and extended template might improve oncologic outcomes compared to standard PLND. However, the benefit of super-extended templates is unlikely when compared to extended template PLND.
Several groups published their results comparing extended PLND to super-extended PLND as mentioned in Table 2. Zehnder et al. (24) showed that super-extended PLND was not associated with a significantly improved 5-year RFS or OS even when stratified by node positivity. Møller et al. (25) did a similar comparison in 578 patients and reported no significant differences in RFS in the extended and super-extended groups. A trend toward increased RFS was seen in the >pT3 group in the super-extended cohort but was statistically insignificant. The younger age of patients in the super-extended cohort was also a potential source of bias. In light of the above evidence, super extended PLND appears to have no oncological benefit over extended PLND. This is probably because of the fact that metastatic spread beyond the anatomical pelvis could increase the risk of metastatic disease and further nodal deposits beyond the boundaries of the super-extended template.
Holmer et al. (26) analyzed 69 and 101 patients under-going limited PLND (perivesical and obturator nodes) and standard PLND (limited regions plus the internal, external and common iliac nodes, and presacral nodes), respectively. They found no significant difference in DSS between the two groups. However, patients with pT3-4a disease were more common in the standard PLND group than in the limited one. Multivariate analysis revealed that there was significantly improved survival in the patients who underwent standard PLND, as shown in Table 3. Simone et al. (27) supported that extended PLND has both staging and therapeutic roles reporting better oncologic outcomes of patients who underwent extended PLND than standard PLND. They showed that patients who underwent an extended PLND had a significant improvement of disease-free survival (DFS) (HR = 1.96, 95% CI: 1.56–2.47, and P < 0.001) and CSS (HR = 1.76, 95% CI: 1.36–2.99, and P < 0.001) probabilities compared to s-PLND. Thus, they described a therapeutic result of extending PLND from the iliac bifurcation up to the aortic bifurcation. In a study by Abdi et al., extended PLND appeared to reduce the risk of local recurrence, but was not an independent predictor of overall survival (28). Extended PLND was associated with greater blood loss than s-PLND, but not with other perioperative complications. In contrast, Hugen et al. (29) compared standard and extended lymph node dissection and found no difference in 5-year recurrence free survival when stratified by node yield using the Kaplan–Meier method (P = 0.138). Table 3 summarizes the conclusion drawn by various studies on the adequacy of pelvic lymph node dissection extent.
The above literature supporting extended PLND was somewhat challenged by the findings of the LEA trial, which is the first prospective randomized phase III trial comparing standard PLND with super-extended PLND (31). Extended LND (n = 198) failed to show a significant advantage over standard LND (n = 203) for RFS [5-year RFS 65 vs. 59%; hazard ratio 0.84; 95% confidence interval (CI): 0.58–1.22], cancer-specific survival (CSS) (5-year CSS 76 vs. 65%; HR 0.70; 95% CI 0.46–1.07) and OS (5-year OS 59 vs. 50%; HR 0.78; 95% CI: 0.57–1.07). However, a significant number of confounding factors are evident in this study. None of the patients received neoadjuvant chemotherapy. The relatively high percentage (14%) of pT1 disease could have limited the results' strength since more extensive PLND usually benefits those with more advanced disease. Post-operative chemotherapy was given at the discretion of the physician. Also, the relatively high percentage of positive surgical margins, 8.9% in the limited and 8.6% in the extended arm, raise questions about the adequacy of RC and if this could have influenced the OS and RFS. Lastly, this study was not powered to demonstrate the non-inferiority of standard PLND to super-extended PLND.
However, this trial has given us some valuable pointers. Firstly, 11% patients had positive lymph nodes located outside the standard PLND template in the super-extended group. If these patients had undergone a standard PLND, 2% would have had a false diagnosis of node negative disease. Also, of the total number of identified lymph nodes, 35% were solely in the super-extended template and would have been missed in a standard PLND. So, by using a super-extended, the authors identified 2% more patients as node positive and resected 35% more positive LNs, which would have been left behind using a standard PLND, with a limited increase in morbidity. In addition, the survival curves show some clinically significant but statistically insignificant divergence in respect to all the 3 endpoints and longer follow up is necessary. The results of the SWOG S1011 trial are awaited (32). This trial has a similar study design to the LEA trial, but it excludes patients with pT1 disease. Also 56% of patients in this trial received neoadjuvant therapy, hence it is more representative of the real world scenario. The accrual is complete and the estimated completion date is August 2022. Table 4 compares chief characteristics of both of these trials.
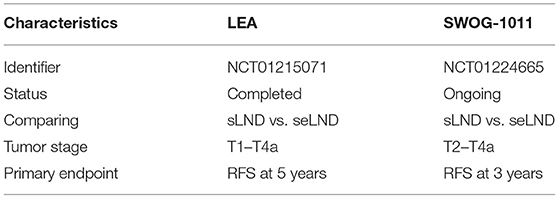
Table 4. Comparison of the LEA and SWOG-1011 trial (30).
Lymph node count from a dissected specimen is influenced by various factors like method of lymph node submission (en-bloc vs. separate packets and the number of packets sent), surgical technique, and variability in the pathologic practices and reporting standards, along with inter individual variability in retrieving lymph nodes from the same template (33). Lymph node density refers to the ratio of positive lymph nodes on histopathology to the total number of nodes removed (29). Extra-nodal extension of tumor in an involved lymph node refers to the growth of a nodal cancer metastasis beyond the confines of the capsule of a lymph node into the adjacent tissues (34).
Multiple authors have reported the decreased probability of cancer death with increased number of lymph nodes harvested (35–39). The mechanism of this benefit is probably the removal of undetected micro-metastases, particularly in the setting of neoadjuvant or adjuvant therapies (40). Confounding factors like patient, surgeon, or institutional factors might also contribute to improved outcomes (41). Capitanio et al. (42) evaluated the probability of detecting node positive disease in a multi-institutional cohort of 731 patients based on total number of lymph nodes removed. 23.8% patients had positive nodes. Using receiver operating characteristic (ROC) curves, the authors predicted a 75% chance of identifying one or more lymph node metastases if 25 nodes were removed which improved to 90% with 45 nodes and decreased to 50% if 15–25 nodes were removed. So, a 25-node minimum was a reasonable cut-off to adequately stage and detect lymph node metastasis. In a retrospective analysis by Koppie et al. (43) from the MSKCC group, multivariate analysis showed that that increased lymph node counts did not correlate with increased survival above a count of 23. However, none of the authors could identify a continuous group of lymph nodes to be an independent predictor of cancer specific survival.
Increased lymph node density >20% decreases OS to <10% (41, 44–46). Extra-nodal extension of tumor is also a bad prognostic factor. A meta-analysis of 1,893 patients showed that it considerably correlated with reduced RFS (HR = 1.56, 95% CI: 1.13–2.14) and cancer-specific survival (HR = 1.60, 95% CI: 1.29–1.99) but not OS (HR = 1.47, 95% CI: 0.71–3.05) (47). Figure 5 is a bar diagram representing minimum lymph node yield to be predictive of outcome of urinary bladder cancer as stated in different studies.
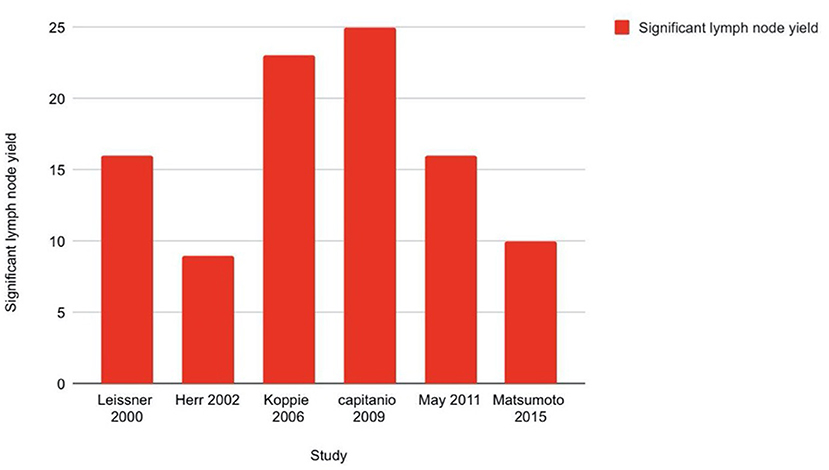
Figure 5. Bar diagram showing minimum lymph node yield to be predictive of outcome of urinary bladder cancer.
Prior to the publication of the LEA trial, majority of the retrospective and prospective literature were in favor of extended lymphadenectomy providing a survival advantage over standard lymphadenectomy. Many authors used factors like lymph node number, lymph density, extra nodal extension, etc., as markers of adequacy of lymphadenectomy and many studies showed significant correlation between number of lymph nodes dissected, lymph node density and oncological outcomes after radical cystectomy. Since extended lymphadenectomy was thought to harvest more lymph nodes and provide a better idea about lymph density it was recommended over and above standard dissection in these studies. Now, whether template is more important or number of harvested lymph nodes? A meticulous dissection with emphasis on skeletonization of the pelvic vessels is more appropriate than relying on the absolute lymph node count. There is a reasonable amount of consensus on this and that is why contemporary studies comparing various types of pelvic lymphadenectomy in bladder cancer make use of fixed anatomical templates rather than absolute number of lymph nodes.
Both the EAU and AUA guidelines recommend PLND during RC. However, they are guarded in elaborating the extent of lymphadenectomy required. The EAU guidelines state that “extended LND might have a therapeutic benefit compared to less extensive PLND, but due to bias, no firm conclusions can be drawn” (48). The NCCN guidelines recommend that bilateral PLND should be performed, with a minimum of common iliac, internal iliac, and obturator nodes excised (49). It also states that a more extensive PLND, which may include the common iliac and the lower para-aortic and paracaval nodes may be associated with better survival and lower local recurrence rates. Still, it stops short of recommending this type of PLND. The AUA guidelines state that a bilateral PLND should be performed in every surgery with a curative intent (grade B). The standard PLND template with a minimum total lymph node count of 12 should be included (50). Even though one prospective randomized study has been published, the lack of robust clinical data on the various extents of PLND probably limits the scope of recommendations in the above guidelines.
None of the surrogate markers of adequacy of PLND, namely anatomical template, lymph node number or lymph node density have been recommended forthright due to the lack of robust prospective data. In even the most recent meta-analysis on this topic, most of the included studies were retrospective. The differences in use of neoadjuvant chemotherapy worldwide also make it difficult to compare the studies without introducing bias. The extent of lymphadenectomy was left at the surgeon's discretion in most retrospective studies, thereby lacking randomization. Therefore, all of these issues should be accounted for before attempting to compare the different extents of PLND, reported in different studies. Therefore, to facilitate the above, based on this review, we wish to emphasize upon and recommend the following points:
- Level of pelvic lymph nodes are used to denote specific anatomical locations of the pelvic lymph nodes. The extent of pelvic lymphadenectomy would be denoted by whether just one, two, or all three levels of lymph nodes have been dissected out during PLND.
- Anatomical templates of dissection have been defined in the preceding sections and this nomenclature should be strictly followed in all future trials to bring uniformity and facilitate comparison.
- Whenever possible, we should adhere to guidelines and try to do an extended PLND, especially whenever there is visible lymphadenopathy on exploration and imaging.
- Super-extended PLND appears to have no survival benefit over extended PLND and its use should be decided on a case to case basis.
- PLND should be deemed adequate if an anatomical template is followed and thorough dissection is done rather than relying on the number of harvested lymph nodes or the lymph node density. Anatomical recommendations of a particular template are favorable and generalizable to a wider clinical community. This obviates the reliance on histopathologist for lymph node identification and subsequent prognostication.
RJ and NS have contributed equally to writing and manuscript preparation of this work. RJ and APS have conceived the idea if the review and have done the search strategy and selection of the articles and have contributed equally to the process of review and critical analysis. GC and AS have helped equally in the selection of articles for review and in final revision and manuscript preparation. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Funt SA, Rosenberg JE. Systemic, perioperative management of muscle-invasive bladder cancer and future horizons. Nat Rev Clin Oncol. (2017) 14:221–34. doi: 10.1038/nrclinonc.2016.188
2. Buscarini M, Josephson DY, Stein JP. Lymphadenectomy in bladder cancer: a review. Urol Int. (2007) 79:191–9. doi: 10.1159/000107949
3. Sung HH, Lerner SP. Utility of lymphadenectomy in bladder cancer: where do we stand? Curr Opin Urol. (2020) 30:407–14. doi: 10.1097/MOU.0000000000000750
4. Papalia R, Simone G, Grasso R, Augelli R, Faiella E, Guaglianone S, et al. Diffusion-weighted magnetic resonance imaging in patients selected for radical cystectomy: detection rate of pelvic lymph node metastases. BJU Int. (2012) 109:1031–6. doi: 10.1111/j.1464-410X.2011.10446.x
5. Crozier J, Papa N, Perera M, Ngo B, Bolton D, Sengupta S, et al. Comparative sensitivity and specificity of imaging modalities in staging bladder cancer prior to radical cystectomy: a systematic review and meta-analysis. World J Urol. (2019) 37:667–90. doi: 10.1007/s00345-018-2439-8
6. Jeong IG, Hong S, You D, Hong JH, Ahn H, Kim CS. FDG PET-CT for lymph node staging of bladder cancer: a prospective study of patients with extended pelvic lymphadenectomy. Ann Surg Oncol. (2015) 22:3150–6. doi: 10.1245/s10434-015-4369-7
7. Bruins HM, Veskimae E, Hernandez V, Imamura M, Neuberger MM, Dahm P, et al. The impact of the extent of lymphadenectomy on oncologic outcomes in patients undergoing radical cystectomy for bladder cancer: a systematic review. Eur Urol. (2014) 66:1065–77. doi: 10.1016/j.eururo.2014.05.031
8. Suttmann H, Kamradt J, Lehmann J, Stöckle M. Improving the prognosis of patients after radical cystectomy. Part I: the role of lymph node dissection. BJU Int. (2007) 100:1221–4. doi: 10.1111/j.1464-410X.2007.07114.x
9. Cattaneo F, Motterle G, Zattoni F, Morlacco A, Dal Moro F. The role of lymph node dissection in the treatment of bladder cancer. Front Surg. (2018) 5:62. doi: 10.3389/fsurg.2018.00062
10. Abol-Enein H, El-Baz M, Abd El-Hameed MA, Abdel-Latif M, Ghoneim MA. Lymph node involvement in patients with bladder cancer treated with radical cystectomy: a patho-anatomical study-a single center experience. J Urol. (2004) 172:1818–21. doi: 10.1097/01.ju.0000140457.83695.a7
11. Bochner BH, Cho D, Herr HW, Donat M, Kattan MW, Dalbagni G. Prospectively packaged lymph node dissections with radical cystectomy: evaluation of node count variability and node mapping. J Urol. (2004) 172(4 Pt 1):1286–90. doi: 10.1097/01.ju.0000137817.56888.d1
12. Roth B, Wissmeyer MP, Zehnder P, Birkhäuser FD, Thalmann GN, Krause TM, et al. A new multimodality technique accurately maps the primary lymphatic landing sites of the bladder. Eur Urol. (2010) 57:205–11. doi: 10.1016/j.eururo.2009.10.026
13. Leissner J, Ghoneim MA, Abol-Enein H, Thüroff JW, Franzaring L, Fisch M, et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer:: results of a prospective multicenter study. J Urol. (2004) 171:139–44. doi: 10.1097/01.ju.0000102302.26806.fb
14. Perera M, McGrath S, Sengupta S, Crozier J, Bolton D, Lawrentschuk N. Pelvic lymph node dissection during radical cystectomy for muscle-invasive bladder cancer. Nat Rev Urol. (2018) 15:686–92. doi: 10.1038/s41585-018-0066-1
15. Tarin TV, Power NE, Ehdaie B, Sfakianos JP, Silberstein JL, Savage CJ, et al. Lymph node-positive bladder cancer treated with radical cystectomy and lymphadenectomy: effect of the level of node positivity. Eur Urol. (2012) 61:1025–30. doi: 10.1016/j.eururo.2012.01.049
16. Hwang EC, Sathianathen NJ, Imamura M, Kuntz GM, Risk MC, Dahm P. Extended versus standard lymph node dissection for urothelial carcinoma of the bladder in patients undergoing radical cystectomy. Cochrane Database Syst Rev. (2019) 5:CD013336. doi: 10.1002/14651858.CD013336
17. Sundi D, Svatek RS, Nielsen ME, Schoenberg MP, Bivalacqua TJ. Extent of pelvic lymph node dissection during radical cystectomy: is bigger better? Rev Urol. (2014) 16:159–66. doi: 10.3909/riu0626
18. Dorin RP, Daneshmand S, Eisenberg MS, Chandrasoma S, Cai J, Miranda G, et al. Lymph node dissection technique is more important than lymph node count in identifying nodal metastases in radical cystectomy patients: a comparative mapping study. Eur Urol. (2011) 60:946–52. doi: 10.1016/j.eururo.2011.07.012
19. Dhar NB, Klein EA, Reuther AM, Thalmann GN, Madersbacher S, Studer UE. Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. J Urol. (2008) 179:873–8. doi: 10.1016/j.juro.2007.10.076
20. Li F, Hong X, Hou L, Lin F, Chen P, Pang S, et al. A greater number of dissected lymph nodes is associated with more favorable outcomes in bladder cancer treated by radical cystectomy: a meta-analysis. Oncotarget. (2016) 7:61284. doi: 10.18632/oncotarget.11343
21. Bi L, Huang H, Fan X, Li K, Xu K, Jiang C, et al. Extended vs non-extended pelvic lymph node dissection and their influence on recurrence-free survival in patients undergoing radical cystectomy for bladder cancer: a systematic review and meta-analysis of comparative studies. BJU Int. (2014) 113:E39–48. doi: 10.1111/bju.12371
22. Mandel P, Tilki D, Eslick GD. Extent of lymph node dissection and recurrence-free survival after radical cystectomy: a meta-analysis. Urol Oncol. (2014) 32:1184–90. doi: 10.1016/j.urolonc.2014.01.017
23. Wang YC, Wu J, Dai B, Shen YJ, Ma CG, Ye DW, et al. Extended versus non-extended lymphadenectomy during radical cystectomy for patients with bladder cancer: a meta-analysis of the effect on long-term and short-term outcomes. World J Surg Oncol. (2019) 17:1–9. doi: 10.1186/s12957-019-1759-5
24. Zehnder P, Studer UE, Skinner EC, Dorin RP, Cai J, Roth B, et al. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: a comparative study. J Urol. (2011) 186:1261–8. doi: 10.1016/j.juro.2011.06.004
25. Møller MK, Høyer S, Jensen JB. Extended versus superextended lymph-node dissection in radical cystectomy: subgroup analysis of possible recurrence-free survival benefit. Scand J Urol. (2016) 50:175–80. doi: 10.3109/21681805.2015.1132473
26. Holmer M, Bendahl PO, Davidsson T, Gudjonsson S, Månsson W, Liedberg F. Extended lymphnode dissection in patients with urothelial cell carcinoma of thebladder: can it make a difference? World J Urol. (2009) 27:521–6. doi: 10.1007/s00345-008-0366-9
27. Simone G, Papalia R, Ferriero M, Guaglianone S, Castelli E, Collura D, et al. Stage-specific impact of extended versus standard pelvic lymph node dissection in radical cystectomy. Int J Urol. (2013) 20:390–7. doi: 10.1111/j.1442-2042.2012.03148.x
28. Abdi H, Pourmalek F, Gleave ME, So AI, Black PC. Balancing risk and benefit of extended pelvic lymph node dissection in patients undergoing radical cystectomy. World J Urol. (2016) 34:41–8. doi: 10.1007/s00345-015-1734-x
29. Hugen CM, Polcari AJ, Fitzgerald MP, Dauw C, Flanigan RC, Quek ML. Risk factors for recurrence following radical cystectomy for pathologic node negative bladder cancer. J Surg Oncol. (2010) 102:334–7. doi: 10.1002/jso.21642
30. Muilwijk T, Akand M, Gevaert T, Joniau S. No survival difference between super extended and standard lymph node dissection at radical cystectomy: what can we learn from the first prospective randomized phase III trial? Transl Androl Urol. (2019) 8(Suppl 1):S112–S5. doi: 10.21037/tau.2018.12.09
31. Gschwend JE, Heck MM, Lehmann J, Rübben H, Albers P, Wolff JM, et al. Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: survival results from a prospective, randomized trial. Eur Urol. (2019) 75:604–11. doi: 10.1016/j.eururo.2018.09.047
32. Lerner SP, Svatek RS. What is the standard of care for pelvic lymphadenectomy performed at the time of radical cystectomy? Eur Urol. (2019) 75:612. doi: 10.1016/j.eururo.2018.12.028
33. Josephson DY, Stein JP. The extent of lymphadenectomy at the time of radical cystectomy for bladder cancer and its impact on prognosis and survival. Sci World J. (2005) 5:403859. doi: 10.1100/tsw.2005.115
34. Boström PJ, Jensen JB, Jerlström T, Arum CJ, Gudjonsson S, Ettala O, et al. Clinical markers of morbidity, mortality and survival in bladder cancer patients treated with radical cystectomy. A systematic review. Scand J Urol. (2020) 54:267–76. doi: 10.1080/21681805.2020.1773527
35. Chou R, Selph SS, Buckley DI, Gustafson KS, Griffin JC, Grusing SE, et al. Treatment of muscle-invasive bladder cancer: a systematic review. Cancer. (2016) 122:842–51. doi: 10.1002/cncr.29843
36. May M, Herrmann E, Bolenz C, Brookman-May S, Tiemann A, Moritz R, et al. Association between the number of dissected lymph nodes during pelvic lymphadenectomy and cancer-specific survival in patients with lymph node-negative urothelial carcinoma of the bladder undergoing radical cystectomy. Ann Surg Oncol. (2011) 18:2018–25. doi: 10.1245/s10434-010-1538-6
37. Morgan TM, Barocas DA, Penson DF, Chang SS, Ni S, Clark PE, et al. Lymph node yield at radical cystectomy predicts mortality in node-negative and not node-positive patients. Urology. (2012) 80:632–40. doi: 10.1016/j.urology.2012.03.070
38. Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. (2002) 167:1295–8. doi: 10.1016/S0022-5347(05)65284-6
39. VAN Bruwaene S, Costello AJ, VAN Poppel H. Prognosis of node-positive bladder cancer in 2016. Minerva Urol Nefrol. (2016) 68:125–37.
40. Matsumoto R, Takada N, Abe T, Minami K, Harabayashi T, Nagamori S, et al. Prospective mapping of lymph node metastasis in Japanese patients undergoing radical cystectomy for bladder cancer: characteristics of micrometastasis. Jpn J Clin Oncol. (2015) 45:874–80. doi: 10.1093/jjco/hyv091
41. Cha EK, Donahue TF, Bochner BH. Radical transurethral resection alone, robotic or partial cystectomy, or extended lymphadenectomy: can we select patients with muscle invasion for less or more surgery? Urol Clin. (2015) 42:189–99. doi: 10.1016/j.ucl.2015.02.003
42. Capitanio U, Suardi N, Shariat SF, Lotan Y, Palapattu GS, Bastian PJ, et al. Assessing the minimum number of lymph nodes needed at radical cystectomy in patients with bladder cancer. BJU Int. (2009) 103:1359–62. doi: 10.1111/j.1464-410X.2008.08212.x
43. Koppie TM, Vickers AJ, Vora K, Dalbagni G, Bochner BH. Standardization of pelvic lymphadenectomy performed at radical cystectomy: can we establish a minimum number of lymph nodes that should be removed? Cancer. (2006) 107:2368–74. doi: 10.1002/cncr.22250
44. Ku JH, Kang M, Kim HS, Jeong CW, Kwak C, Kim HH. Lymph node density as a prognostic variable in node-positive bladder cancer: a meta-analysis. BMC Cancer. (2015) 15:447. doi: 10.1186/s12885-015-1448-x
45. Lee EK, Herr HW, Dickstein RJ, Kassouf W, Munsell MF, Grossman HB, et al. Lymph node density for patient counselling about prognosis and for designing clinical trials of adjuvant therapies after radical cystectomy. BJU Int. (2012) 110:E590–5. doi: 10.1111/j.1464-410X.2012.11325.x
46. Kondo T, Tanabe K. Role of lymphadenectomy in the management of urothelial carcinoma of the bladder and the upper urinary tract. Int J Urol. (2012) 19:710–21. doi: 10.1111/j.1442-2042.2012.03009.x
47. Ahn TS, Kim HS, Jeong CW, Kwak C, Kim HH, Ku JH. Extracapsular extension of pelvic lymph node metastasis is an independent prognostic factor in bladder cancer: a systematic review and meta-analysis. Ann Surg Oncol. (2015) 22:3745–50. doi: 10.1245/s10434-014-4359-1
48. EAU Guidelines. Edn. Presented at the EAU Annual Congress Amsterdam. (2020). ISBN 978-94-92671-07-3.
49. Bladder Cancer. NCCN Clinical Practice Guidelines in Oncology, Version 4. (2020). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf
Keywords: bladder cancer, pelvic lymphadenectomy, extended lymphadenectomy, pelvic lymph node dissection, super extended pelvic lymphadenectomy
Citation: Jena R, Shrivastava N, Sharma AP, Choudhary GR and Srivastava A (2021) The Adequacy of Pelvic Lymphadenectomy During Radical Cystectomy for Carcinoma Urinary Bladder: A Narrative Review of Literature. Front. Surg. 8:687636. doi: 10.3389/fsurg.2021.687636
Received: 29 March 2021; Accepted: 19 May 2021;
Published: 17 June 2021.
Edited by:
Daniele Castellani, Polytechnic University of Le Marche, ItalyReviewed by:
Hsiang Ying Lee, Kaohsiung Municipal Ta-Tung Hospital, TaiwanCopyright © 2021 Jena, Shrivastava, Sharma, Choudhary and Srivastava. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rahul Jena, amVuYS5yYWh1bEBnbWFpbC5jb20=
†These authors share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.