
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg. , 25 May 2021
Sec. Surgical Oncology
Volume 8 - 2021 | https://doi.org/10.3389/fsurg.2021.682348
 Guorong Yao1
Guorong Yao1 Xiaotian Jin2*†
Xiaotian Jin2*†Objective: The study aimed to assess if additional lymphadenectomy with primary staging surgery improves overall survival (OS) and disease-free survival (DFS) of early-stage ovarian cancer (ESOC).
Methods: PubMed and Embase databases were searched for any type of study comparing OS or DFS between lymphadenectomy and control groups for any type of ESOC. Adjusted hazard ratios (HR) were pooled in a random-effects model.
Results: Twelve studies were included. Meta-analysis indicated that lymphadenectomy is associated with significantly improved OS only for epithelial tumors (HR 0.75 95% CI 0.68, 0.82 I2 = 0% p < 0.00001) but not for malignant germ cell tumors (HR 1.31 95% CI 0.88, 1.94 I2 = 0% p = 0.18). Single studies indicated a tendency of improved OS with lymphadenectomy which was significant for ovarian carcinosarcoma but not for sex cord-stromal tumors. On meta-regression of all histological types, the percentage of patients with lymph node metastasis in the lymphadenectomy group was not found to influence the effect size. Meta-analysis also indicated that lymphadenectomy is associated with significantly improved DFS for epithelial tumors (HR 0.59 95% CI 0.45, 0.77 I2 = 0% p < 0.0001). Single studies on malignant germ cell and sex cord-stromal tumors failed to demonstrate any significant beneficial effect of lymphadenectomy on DFS.
Conclusions: Within the limitations of the review, lymphadenectomy may improve OS and DFS for epithelial ESOC. Scarce data suggest that lymphadenectomy is not associated with improved outcomes for malignant germ cell and sex cord-stromal tumors but may benefit ovarian carcinosarcoma. Large-scale RCTs and robust observational studies shall improve current evidence.
Ovarian cancer remains one of the most lethal gynecological malignancies worldwide. It is the second most common genital malignancy and the most common cause of genital cancer-related death in females (1). Ovarian cancer not only impacts survival but also leads to significant reduction in the overall quality of life and sexual functions in affected individuals (2). Importantly, only about 25% of these tumors are detected at an early stage with the majority of cases diagnosed with advanced disease (3). Indeed, distinguishing adnexal masses to recognize ovarian cancer has been a challenge for clinicians. Over the years, research has been directed toward the use of tumor markers like CA125, HE4, PRSS8, FOLR1, KLK6/7, GSTT1 and the use of transvaginal ultrasonography for early recognition of these tumors. Also different validated models like International Ovarian Tumor Analysis (IOTA) and the Assessment of Different NEoplasias in the AdneXa (ADNEX) model have been developed to aid in preoperative characterization of the adnexal pathology (4). Despite such advances, the survival with ovarian cancer remains low, ranging from 30 to 40% (5).
Surgical treatment remains the primary mode of management of ovarian cancer. However, as with any surgical intervention inherent complications exists (6, 7). Over the years, there has been a trend to use minimally invasive approaches for managing this disease. Laparoscopy is being increasingly used to assess the resectibility of ovarian cancer (8). The use of minimally invasive laparoscopic and robotic approaches for surgery has been suggested to improve perioperative outcomes with minimal impact on overall survival (9). A recent study by Lago et al. (10) has demonstrated improved psychological impact of minimally invasive surgery as compared to traditional laparotomy in patients with advance ovarian cancer. However, irrespective of the surgical approach, the quality and extent of the excision are known to be important factors influencing survival in these patients (11, 12).
Metastasis to the pelvic and para-aortic lymph nodes is frequently seen in case of ovarian cancer as compared to other gynecological tumors and sampling of these nodes is important for the staging of the disease (13). However, owing to the low rate of lymph node involvement in early-stage ovarian cancer (ESOC), sentinel lymph node technique has been recently suggested but is still under trial stage (10). For lymph node sampling in ESOC, a distinction should be made between sampling and complete pelvic and/or para-aortic dissection i.e., lymphadenectomy which is associated with significant surgical morbidity (14, 15). Studies indicate that around 6.1 to 29.6% of ESOC have occult lymph node metastasis (16, 17). Patients with positive nodes identified via staging lymphadenectomy are usually upgraded to an advanced stage and require adjunctive therapy for the residual disease (18). However, the recent ESMO-ESGO consensus conference for ovarian cancer has questioned the need for lymphadenectomy for all histological types of ESOC. Furthermore, it advocated that lymph node dissection for re-staging purposes can be omitted if patients' management is not affected by the nodal status (19).
Whether lymphadenectomy is beneficial for advanced as well as early-stage ovarian cancer (ESOC) has been a subject of intense research in the past two decades (15, 18, 20). To date, several meta-analyses have been published evaluating the role of lymphadenectomy for ovarian cancer (21–26). However, most of these studies have focused on advanced stage ovarian cancer and to the best of our knowledge, only three meta-analysis studies (24–26) have assessed the impact of lymphadenectomy on the outcomes of ESOC. However, these reviews were focused only on epithelial ESOC and could include only a limited number of studies in their meta-analysis. At this point, it is still unclear how does lymphadenectomy benefits patients with ESOC with different histological subtypes. Therefore, the purpose of this study was to systematically search the literature and conduct a meta-analysis to assess the impact of lymphadenectomy on the outcomes of ESOC.
The review was conducted as per the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses) (27). The review protocol was not registered on any of the online databases. The research questions for the review were: (1) Does lymphadenectomy improve the overall survival (OS) of patients with ESOC? (2) Does lymphadenectomy improve disease-free survival (DFS) of patients with ESOC?
We included the following studies in the systematic review:
(1) Any type of study [Randomized controlled trial (RCT), prospective or retrospective] conducted on patients with histologically confirmed ESOC. (2) Patients were to undergo staging surgery with or without lymphadenectomy (pelvic, para-aortic, or both). (3) Studies were to compare OS or DFS between lymphadenectomy and control groups and reported multivariable-adjusted outcomes. No restriction was placed on the histological type of ESOC.
Exclusion criteria were as follows: (1) Studies assessing outcomes of advanced ovarian cancer. (2) Studies on a mixed population of ovarian cancer and not reporting separate data for ESOC. (3) Studies with a total sample size of ≤20 patients. (4) Studies not reporting relevant data. (5) Non-comparative studies and review articles.
Articles related to the review were searched by two reviewers independently. With the help of a librarian, the databases of PubMed and Embase were searched to identify relevant publications. All databases were screened from inception to 15th February 2021. We used the following keywords for the literature search: “ovarian cancer,” “lymph node,” “lymphadenectomy,” “pelvic,” “para-aortic,” “dissection,” “resection,” and “survival.” Supplementary Table 1 demonstrates the search strategy. Every search result was evaluated by the two reviewers independently, initially by their titles and abstracts and then by full texts of relevant publications. All full-texts were reviewed based on the inclusion and exclusion criteria and the article satisfying all the criteria was finally selected for this review. Any disagreements were resolved by discussion. To avoid any missed studies, the bibliography of included studies was hand searched for any additional references.
We prepared a data extraction form at the beginning of the review to extract relevant details from the studies. The final version of this template was approved by all the study investigators. Data of study authors, year of publication, study type, location, study groups and definition, sample size, age of the sample, histological type, percentage of metastasis detected in lymphadenectomy group, factors adjusted for multivariable analysis, and outcome data were extracted. Data were extracted by two reviewers independent of each other. Any disagreements were resolved by discussion.
The methodological quality of included studies was assessed using the Newcastle-Ottawa scale (28). This too was carried out in duplicate and independently by two study investigators. Studies were awarded points for selection of study population, comparability, and outcomes. The maximum score which can be awarded is nine.
We used “Review Manager” (RevMan, version 5.3; Nordic Cochrane Centre [Cochrane Collaboration], Copenhagen, Denmark; 2014) for the meta-analyses. Adjusted hazard ratios (HR) or related effect sizes of the outcomes were extracted along with the 95% confidence intervals (CI). Data were pooled using the generic inverse function of the meta-analysis software. Sub-group analysis was performed for the histological type of ESOCA random-effects model was preferred for the meta-analysis. The I2 statistic was used to assess inter-study heterogeneity. I2 values of 25–50% represented low, values of 50–75% medium, and more than 75% represented substantial heterogeneity. As <10 studies were included per meta-analysis, funnel plots were not used to assess publication bias. Random-effects meta-regression analysis was performed to assess the influence of the percentage of lymph node metastasis in the lymphadenectomy on the pooled effect size. Open MetaAnalyst software was used for the meta-regression analysis (29).
The flow-chart of the study is presented in Figure 1. Three thousand five hundred and forty two unique articles were identified after the literature search. After reviewing them by the titles and abstracts, we excluded 3,504 studies due to non-relevance with the review topic. Of the 38 studies selected for full-text analysis, 24 were excluded with reasons and a total of 12 studies were included in the review (30–41). Details of included studies are presented in Table 1. Only one study was an RCT while all others were retrospective observational studies. Six studies (30, 31, 33, 34, 36, 41) were conducted on epithelial tumors, three on malignant germ-cell tumors (32, 39, 40), two on sex cord-stromal tumors (35, 38) and one was only on ovarian carcinosarcoma (37). The sample size in the lymphadenectomy arm varied from 40 to 8,489 patients while in the control arm it varied from 22 to 4,628 patients. The number of patients with positive lymph nodes in the lymphadenectomy group varied from 0.8 to 25.8%. The number of lymph nodes removed in the lymphadenectomy group differed across studies. Similarly, there were variations in the factors adjusted in the multivariable analysis and the follow-up duration across studies.
Nine studies reported data on OS. A meta-analysis of all studies irrespective of the histological type of ESOC indicated that lymphadenectomy is associated with significantly improved OS as compared to no lymphadenectomy (HR 0.78 95% CI 0.71, 0.86 I2 = 4% p < 0.00001) (Figure 2). On subgroup analysis, significant improvement in OS was noted only for epithelial tumors (HR 0.75 95% CI 0.68, 0.82 I2 = 0% p < 0.00001) but not for malignant germ cell tumors (HR 1.31 95% CI 0.88, 1.94 I2 = 0% p = 0.18). Analysis of single studies indicated a tendency of improved OS with lymphadenectomy which was significant for ovarian carcinosarcoma (HR 0.75 95% CI 0.57, 0.99 p = 0.04) but not for sex cord-stromal tumors (HR 0.80 95% CI 0.60, 1.07 p = 0.13) (Figure 2). On meta-regression, the percentage of patients with lymph node metastasis in the lymphadenectomy group was not found to influence the effect size (ß −0.003 95% CI −0.019, 0.013 p = 0.7) (Figure 3).
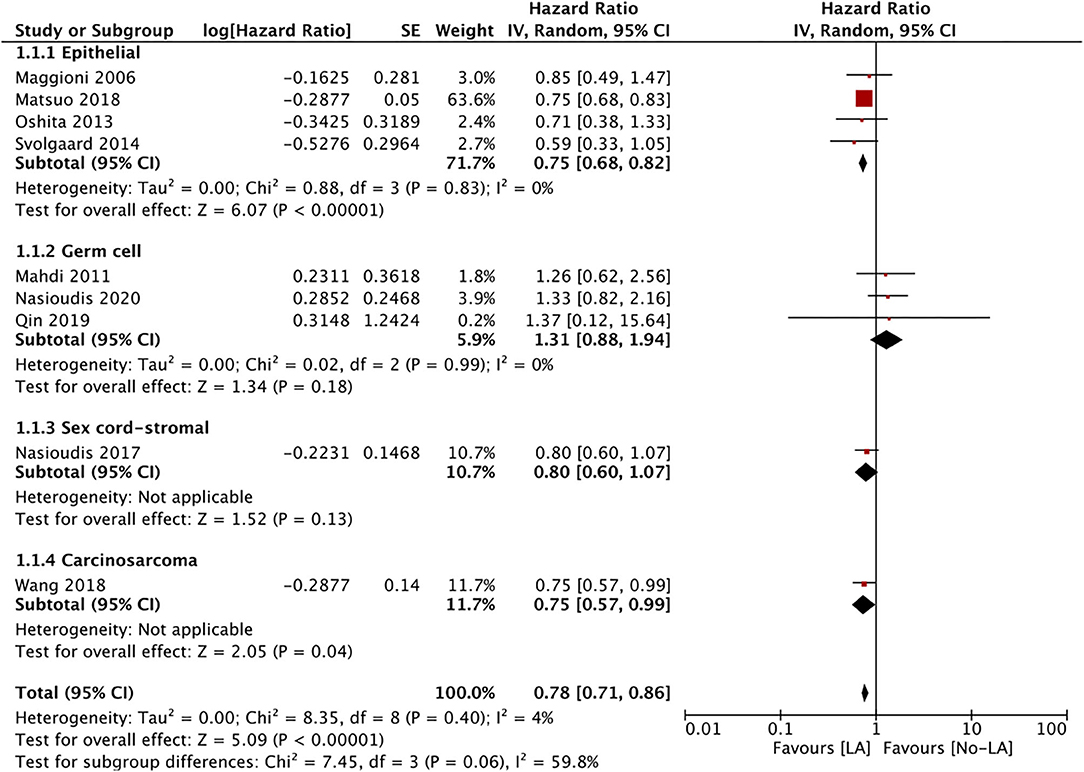
Figure 2. Meta-analysis of OS for ESOC between lymphadenectomy and control groups with sub-group analysis based on type of ESOC.
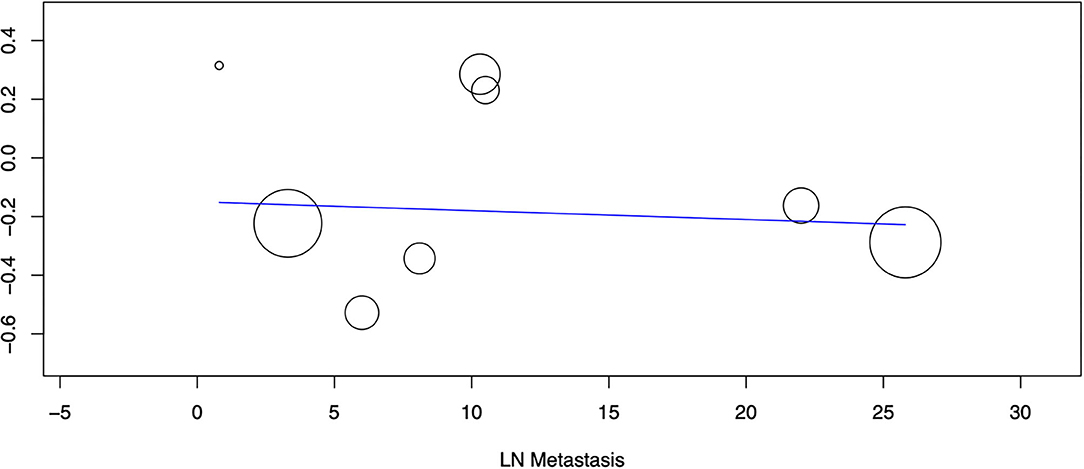
Figure 3. Meta-regression of the influence of percentage of lymph node metastasis (X-axis) on the pooled effect size of OS (Y-axis). Individual studies are represented by circles.
Only five studies reported data on DFS. Meta-analysis of all studies irrespective of the histological type of ESOC indicated that lymphadenectomy is associated with significantly improved DFS as compared to no lymphadenectomy (HR 0.62 95% CI 0.50, 0.77 I2 = 0% p < 0.0001) (Figure 4). Data on epithelial tumors was reported by three studies and subgroup analysis indicated significantly improved DFS with lymphadenectomy (HR 0.62 95% CI 0.50, 0.78 I2 = 0% p < 0.0001). Single studies on malignant germ cell (HR 0.58 95% CI 0.15, 2.24 p = 0.43)and sex cord-stromal tumors (HR 0.40 95% CI 0.04, 3.70 p = 0.42) failed to demonstrate any significant beneficial effect of lymphadenectomy on DFS (Figure 4).
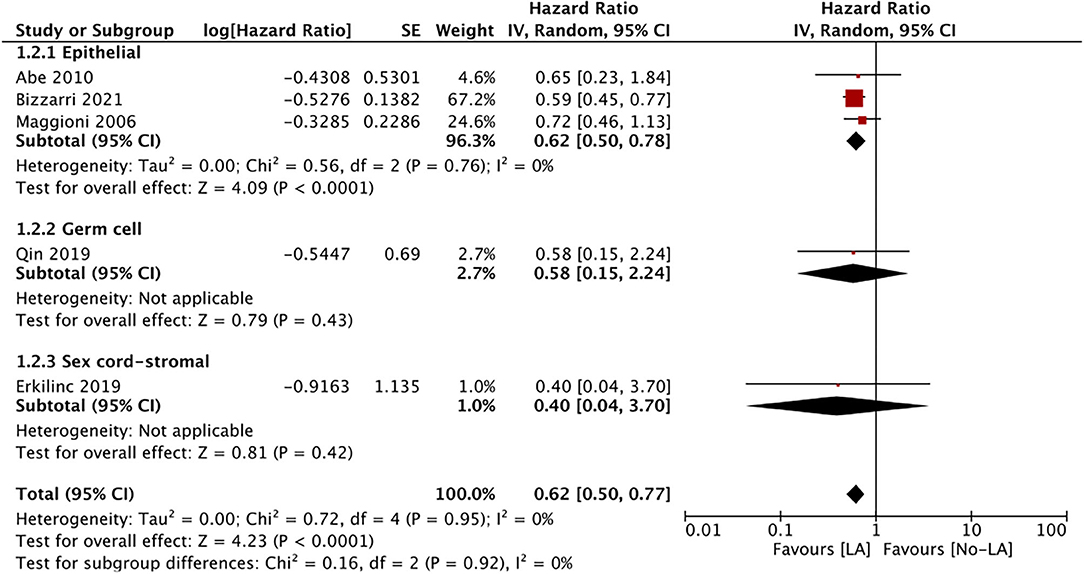
Figure 4. Meta-analysis of DFS for ESOC between lymphadenectomy and control groups with sub-group analysis based on type of ESOC.
This systematic review and meta-analysis aimed to assess if lymphadenectomy improves OS and DFS in the case of ESOC. Our results, mainly from retrospective observation studies, indicate that lymphadenectomy significantly increases OS and DFS in ESOC; however, the results also depend on the tumor histology.
The recently conducted LION trial comparing lymphadenectomy vs. no-lymphadenectomy has demonstrated that lymphadenectomy has no significant beneficial effect on OS or DFS in cases of advanced ovarian cancer. On the contrary, serious complications like early mortality and repeat laparotomy were significantly increased in the lymphadenectomy group (42). Indeed, a significant limitation of retroperitoneal lymphadenectomy is the potential for several intraoperative and postoperative complications, like hemorrhage, sepsis, vascular injury, lymphocysts, intestinal or chylous fistula, lower limb edema, pulmonary embolism, repeat laparotomy and post-operative mortality (43). Therefore, it is important to clarify the role of this procedure especially in ESOC. To date, only one RCT has evaluated the role of lymphadenectomy for ESOC. Like the LION trial, Maggioni et al. (30) in their study too did not report any significant difference in OS and DFS between the study groups albeit with the overall effect size in favor of the lymphadenectomy group (OS, HR:0.85; DFS, HR:0.72). Indeed, high-quality rigorously conducted RCTs provide the best available evidence to guide clinical practice. However, when such evidence is scarce, pooled data from real-world observation studies is the second-best option. The RCT of Maggioni et al. (30) had its own set of limitations, like the small sample size and a low number of outcome events which hinder the applicability of their results. Data from our meta-analysis thus presents the best available evidence, to date, on the role of lymphadenectomy for ESOC. In contrast to the results of the lone RCT (30), our analysis demonstrated a significantly improved OS and DFS in ESOC patients undergoing lymphadenectomy as compared to the control group. The results, however, varied with the histological subtype.
Epithelial sub-type accounts for >85% of all cases of ovarian cancer (44). These tumors are also further classified into serous, endometrioid, clear cell, and mucinous carcinoma with differences in etiology, morphology, molecular biology, and prognosis (45). Due to limited data, we were unable to discern evidence on the role of lymphadenectomy in this subtype of epithelial ovarian cancer. All-inclusive, our analysis demonstrated a significantly improved OS as well as DFS with lymphadenectomy in cases of epithelial ESOC. This is in contrast with the past review of lymphadenectomy in ESOC by Chiyoda et al. (25) which reported improved OS but no difference in DFS. An increase in the sample size of the current analysis contributed to this difference. Another important point of consideration is the role of adjuvant chemotherapy, which is thought to improve outcomes for epithelial ESOC (46). However, many of the included studies lacked information on the use of adjuvant chemotherapy in the study groups. In the study of Maggioni et al. (30), there was bias in the use of chemotherapy as 90% lymph node-positive patients received chemotherapy compared to 56% node-negative patients. This may have contributed to the lack of difference in outcomes of the RCT. Oshita et al. (33) have shown that adjuvant chemotherapy may improve outcomes only in the non-lymphadenectomy group with no effect in patients with complete lymphadenectomy. Based on these results, it has been suggested that micro-metastasis in the lymph nodes in epithelial ESOC can be eliminated either via complete lymphadenectomy or adjuvant chemotherapy, and patients undergoing lymphadenectomy can avoid adjuvant chemotherapy (25, 33). Contrastingly, in the study of Bizzarri et al. (41) all patients in the lymphadenectomy and control group received adjuvant chemotherapy and lymphadenectomy was still associated with better DFS but not in OS as compared to no lymphadenectomy. Thus, given the significant heterogeneity in the studies for adjuvant therapy, strong conclusions cannot be derived and there is a need for large-scale RCTs to confirm the benefits of lymphadenectomy for epithelial ESOC.
Malignant ovarian germ cell tumors are a less common sub-type and are usually seen in a younger age group (47). These tumors have an excellent prognosis with >95% 5-year survival rates if the tumor is confined to the ovary (brown). Treatment guidelines for these tumors are extrapolated from epithelial sub-type and the role of lymphadenectomy is not very clear (40). Our analysis indicated that lymphadenectomy does not offer any survival advantage in malignant germ cell tumors. The difference in this outcome as compared to the epithelial sub-type can be attributed to the higher chemosensitivity of these tumors (48). Recent studies have demonstrated that postoperative chemotherapy is effective in managing these tumors with complete cure (49, 50). However, comprehensive staging is important in patients who cannot undergo adjuvant chemotherapy (49). In the absence of both, the significance of careful radiographic evaluation of lymph nodes, the need for re-operation for staging, and surveillance for grade 1 tumors have been suggested (40, 51). Given that these tumors affect the pediatric and adolescent age group, lesser invasive surgery would be beneficial and can avoid the complications associated with lymphadenectomy (47). However, our results should be interpreted with caution as only three studies were available for the OS analysis and only one study reported no difference in DFS with lymphadenectomy.
Similarly, limited data were available for sex-cord stromal tumors with only two studies; each indicating no difference in OS or DFS with lymphadenectomy. This is not surprising as these tumors are rare and similar to malignant germ cell tumors, their management is based on experience with epithelial tumors (47). The lack of difference in outcomes with lymphadenectomy can be due to the indolent nature of these tumors with rare lymph node metastasis as the disease is usually confined to the ovary (52). Lastly, ovarian carcinosarcoma is a rare variant of ovarian cancer also known as the mesodermal mixed tumor as it contains both epithelial and sarcomatous components (53). Optimal treatment is still not established but surgery and chemotherapy have shown good results (54). The tumor is known to be aggressive and associated with poor survival as compared to epithelial ovarian cancer (55). The lone study in an analysis indicated significantly better OS with lymphadenectomy with this tumor subtype.
The limitations of our review need to be specified. Foremost, except for epithelial tumors, data for other histological sub-types was limited. Several studies had to be excluded from our analysis as adjusted outcomes were not reported. Future studies should include reporting of adjusted HRs to allow clear delineation of outcomes. Secondly, the majority of the studies were retrospective in nature and would have been influenced by selection bias. An effort was made to minimize this by using only adjusted outcomes for the analysis. However, there was significant heterogeneity in the studies for outcomes adjusted with many not presenting data for adjuvant therapy. This may have skewed our results. Thirdly, the quality of lymphadenectomy could not be assessed and the number of lymph nodes removed was variable in the included studies. This is an important confounder that needs to be clarified in future studies. Fourthly, the definition of lymphadenectomy was not coherent across the included studies. Some studies performed both pelvic and para-aortic lymphadenectomy while in others only one technique was performed. Furthermore, some studies did not define lymphadenectomy per se in their cohorts. This could have been a major source of bias influencing the outcomes of this review. Lastly, an important component of any analysis on a surgical technique is its impact on complications. While we comprehensively discussed the role of lymphadenectomy on OS and DFS for ESOC, due to lack of data, no analysis on lymphadenectomy-related complications was possible. Future studies should report detailed data on the incidence of complications in order to better understand the role of lymphadenectomy for these tumors.
Nevertheless, the strengths include that our study is the first review focusing on all sub-types of ESOC. To minimize bias, only adjusted outcomes were pooled. A meta-regression was conducted to assess if the positivity of lymph nodes in the study group influences outcomes.
To conclude, within the limitations of the review, lymphadenectomy may improve OS and DFS for epithelial ESOC. Scarce data suggest that lymphadenectomy is not associated with improved outcomes for malignant germ cell and sex cord-stromal tumors but may benefit ovarian carcinosarcoma. Large-scale RCTs and robust observational studies shall improve current evidence.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the Corresponding author.
GY designed the study. Both the authors were involved in data acquisition, analysis, synthesis, wrote, edited, and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.682348/full#supplementary-material
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
2. Cianci S, Tarascio M, Rosati A, Caruso S, Uccella S, Cosentino F, et al. Sexual function and quality of life of patients affected by ovarian cancer. Minerva Med. (2019) 110:320–9. doi: 10.23736/S0026-4806.19.06080-4
3. Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. (2018) 68:284–96. doi: 10.3322/caac.21456
4. Giampaolino P, della Corte L, Foreste V, Vitale SG, Chiofalo B, Cianci S, et al. Unraveling a difficult diagnosis: the tricks for early recognition of ovarian cancer. Minerva Med. (2019) 110:279–91. doi: 10.23736/S0026-4806.19.06086-5
5. Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol. (2012) 55:3–23. doi: 10.1097/GRF.0b013e31824b4611
6. Cosentino F, Turco LC, Fagotti A, Cianci S, Gallotta V, Rosati A, et al. Arterial-enteric fistula after pelvic lymphadenectomy in secondary cytoreductive surgery for recurrent ovarian cancer. J Obstet Gynaecol. (2019) 39:1049–56. doi: 10.1080/01443615.2019.1586856
7. Scaletta G, Quagliozzi L, Cianci S, Vargiu V, Mele MC, Scambia G, et al. Management of postoperative chylous ascites after surgery for ovarian cancer: a single-institution experience. Updates Surg. (2019) 71:729–34. doi: 10.1007/s13304-019-00656-x
8. Gueli Alletti S, Capozzi VA, Rosati A, de Blasis I, Cianci S, Vizzielli G, et al. Laparoscopy vs. laparotomy for advanced ovarian cancer: a systematic review of the literature. Minerva Med. (2019) 110:341–57. doi: 10.23736/S0026-4806.19.06132-9
9. Uccella S, Franchi MP, Cianci S, Zorzato PC, Bertoli F, Alletti SG, et al. Laparotomy vs. minimally invasive surgery for ovarian cancer recurrence: a systematic review. Gland Surg. (2020) 9:1130–9. doi: 10.21037/gs-20-353
10. Lago V, Bello P, Matute L, Padilla-Iserte P, Marina T, Agudelo M, et al. Sentinel lymph node technique in apparent early ovarian cancer: laparoscopic technique. J Minim Invasive Gynecol. (2020) 27:1019–20. doi: 10.1016/j.jmig.2019.09.790
11. Chang SJ, Bristow RE, Chi DS, Cliby WA. Role of aggressive surgical cytoreduction in advanced ovarian cancer. J Gynecol Oncol. (2015) 26:336–42. doi: 10.3802/jgo.2015.26.4.336
12. Azaïs H, Vignion-Dewalle A-S, Carrier M, Augustin J, Da Maïa E, Penel A, et al. Microscopic Peritoneal Residual Disease after Complete Macroscopic Cytoreductive Surgery for Advanced High Grade Serous Ovarian Cancer. J Clin Med. (2020) 10:41. doi: 10.3390/jcm10010041
13. Kleppe M, Kraima AC, Kruitwagen RFPM, Van Gorp T, Smit NN, Van Munsteren JC, et al. Understanding lymphatic drainage pathways of the ovaries to predict sites for sentinel nodes in ovarian cancer. Int J Gynecol Cancer. (2015) 25:1405–14. doi: 10.1097/IGC.0000000000000514
14. Warwick J, Vardaki E, Fattizzi N, McNeish I, Jeyarajah A, Oram D, et al. Defining the surgical management of suspected early-stage ovarian cancer by estimating patient numbers through alternative management strategies. BJOG An Int J Obstet Gynaecol. (2009) 116:1225–41. doi: 10.1111/j.1471-0528.2009.02213.x
15. Benedetti Panici P, Giannini A, Fischetti M, Lecce F, Di Donato V. Lymphadenectomy in ovarian cancer: is it still justified? Curr Oncol Rep. (2020) 22:22. doi: 10.1007/s11912-020-0883-2
16. Powless CA, Aletti GD, Bakkum-Gamez JN, Cliby WA. Risk factors for lymph node metastasis in apparent early-stage epithelial ovarian cancer: implications for surgical staging. Gynecol Oncol. (2011) 122:536–40. doi: 10.1016/j.ygyno.2011.05.001
17. Kleppe M, Wang T, Van Gorp T, Slangen BFM, Kruse AJ, Kruitwagen RFPM. Lymph node metastasis in stages i and II ovarian cancer: a review. Gynecol Oncol. (2011) 123:610–4. doi: 10.1016/j.ygyno.2011.09.013
18. Harter P, Heitz F, Ataseven B, Schneider S, Baert T, Prader S, et al. How to manage lymph nodes in ovarian cancer. Cancer. (2019) 125:4573–7. doi: 10.1002/cncr.32514
19. Colombo N, Sessa C, Du Bois A, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. (2019) 30:672–705. doi: 10.1093/annonc/mdz062
20. Salcedo-Hernández RA. Can lymphadenectomy be omitted in advanced ovarian cancer? A brief review. Chin Clin Oncol. (2020) 9:46. doi: 10.21037/cco-20-35
21. Kim HS, Ju W, Jee BC, Kim YB, Park NH, Song YS, et al. Systematic lymphadenectomy for survival in epithelial ovarian cancer: a meta-analysis. Int J Gynecol Cancer. (2010) 20:520–8. doi: 10.1111/IGC.0b013e3181d6de1d
22. Lin Q, Liu W, Xu S, Li J, Tong J. The value of systematic lymphadenectomy during debulking surgery in the treatment of ovarian cancer: a meta-analysis of randomized controlled trials. J Ovarian Res. (2020) 13:56. doi: 10.1186/s13048-020-00653-4
23. Xu D, Xue J, Rozan R, Li L. The role systematic lymphadenectomy plays in determining the survival outcome for advanced ovarian cancer patients: a metaanalysis. Ann Cardiothorac Surg. (2020) 9:912–20. doi: 10.21037/apm.2020.04.01
24. Gao J, Yang X, Zhang Y. Systematic lymphadenectomy in the treatment of epithelial ovarian cancer: a meta-analysis of multiple epidemiology studies. Jpn J Clin Oncol. (2015) 45:49–60. doi: 10.1093/jjco/hyu175
25. Chiyoda T, Sakurai M, Satoh T, Nagase S, Mikami M, Katabuchi H, et al. Lymphadenectomy for primary ovarian cancer: a systematic review and meta-analysis. J Gynecol Oncol. (2020) 31:1–3. doi: 10.3802/jgo.2020.31.e67
26. Zhou J, Shan G, Chen Y. The effect of lymphadenectomy on survival and recurrence in patients with ovarian cancer: a systematic review and meta-analysis. Jpn J Clin Oncol. (2016) 46:718–26. doi: 10.1093/jjco/hyw068
27. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
28. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed October 30, 2020).
29. Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. (2009) 9:80. doi: 10.1186/1471-2288-9-80
30. Maggioni A, Panici PB, Dell'Anna T, Landoni F, Lissoni A, Pellegrino A, et al. Randomised study of systematic lymphadenectomy in patients with epithelial ovarian cancer macroscopically confined to the pelvis. Br J Cancer. (2006) 95:699–704. doi: 10.1038/sj.bjc.6603323
31. Abe A, Furumoto H, Irahara M, Ino H, Kamada M, Naka O, et al. The impact of systematic para-aortic and pelvic lymphadenectomy on survival in patients with optimally debulked ovarian cancer. J Obstet Gynaecol Res. (2010) 36:1023–30. doi: 10.1111/j.1447-0756.2010.01274.x
32. Mahdi H, Swensen RE, Hanna R, Kumar S, Ali-Fehmi R, Semaan A, et al. Prognostic impact of lymphadenectomy in clinically early stage malignant germ cell tumour of the ovary. Br J Cancer. (2011) 105:493–7. doi: 10.1038/bjc.2011.267
33. Oshita T, Itamochi H, Nishimura R, Numa F, Takehara K, Hiura M, et al. Clinical impact of systematic pelvic and para-aortic lymphadenectomy for pT1 and pT2 ovarian cancer: a retrospective survey by the Sankai gynecology study group. Int J Clin Oncol. (2013) 18:1107–13. doi: 10.1007/s10147-012-0483-8
34. Svolgaard O, Lidegaard Ø, Nielsen MLS, Nedergaard L, Mosgaard BJ, Lidang M, et al. Lymphadenectomy in surgical stage i epithelial ovarian cancer. Acta Obstet Gynecol Scand. (2014) 93:256–60. doi: 10.1111/aogs.12322
35. Nasioudis D, Kanninen TT, Holcomb K, Sisti G, Witkin SS. Prevalence of lymph node metastasis and prognostic significance of lymphadenectomy in apparent early-stage malignant ovarian sex cord-stromal tumors. Gynecol Oncol. (2017) 145:243–7. doi: 10.1016/j.ygyno.2017.03.005
36. Matsuo K, Machida H, Mariani A, Mandelbaum RS, Glaser GE, Gostout BS, et al. Adequate pelvic lymphadenectomy and survival of women with early-stage epithelial ovarian cancer. J Gynecol Oncol. (2018) 29:e69. doi: 10.3802/jgo.2018.29.e69
37. Wang WP, Li N, Zhang YY, Gao YT, Sun YC, Ge L, et al. Prognostic significance of lymph node metastasis and lymphadenectomy in early-stage ovarian carcinosarcoma. Cancer Manag Res. (2018) 10:1959–68. doi: 10.2147/CMAR.S166524
38. Erkilinç S, Taylan E, Karataşli V, Uzaldi I, Karadeniz T, Gökçü M, et al. Does lymphadenectomy effect postoperative surgical morbidity and survival in patients with adult granulosa cell tumor of ovary? J Obstet Gynaecol Res. (2019) 45:1019–25. doi: 10.1111/jog.13928
39. Qin B, Xu W, Li Y. The impact of lymphadenectomy on prognosis and survival of clinically apparent early-stage malignant ovarian germ cell tumors. Jpn J Clin Oncol. (2020) 50:282–7. doi: 10.1093/jjco/hyz180
40. Nasioudis D, Ko EM, Haggerty AF, Cory L, Giuntoli RL, Burger RA, et al. Performance of lymphadenectomy for apparent early stage malignant ovarian germ cell tumors in the era of platinum-based chemotherapy. Gynecol Oncol. (2020) 157:613–8. doi: 10.1016/j.ygyno.2020.04.047
41. Bizzarri N, du Bois A, Fruscio R, De Felice F, De Iaco P, Casarin J, et al. Is there any therapeutic role of pelvic and para-aortic lymphadenectomy in apparent early stage epithelial ovarian cancer? Gynecol Oncol. (2021) 160:56–63. doi: 10.1016/j.ygyno.2020.10.028
42. Harter P, Sehouli J, Lorusso D, Reuss A, Vergote I, Marth C, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N Engl J Med. (2019) 380:822–32. doi: 10.1056/NEJMoa1808424
43. Ockerman A, Bornstein MM, Leung YY, Li SKY, Politis C, Jacobs R. Incidence of bleeding after minor oral surgery in patients on dual antiplatelet therapy: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. (2020) 49:90–8. doi: 10.1016/j.ijom.2019.06.002
44. Kossaï M, Leary A, Scoazec JY, Genestie C. Ovarian cancer: a heterogeneous disease. Pathobiology. (2018) 85:41–9. doi: 10.1159/000479006
45. Peres LC, Cushing-Haugen KL, Anglesio M, Wicklund K, Bentley R, Berchuck A, et al. Histotype classification of ovarian carcinoma: a comparison of approaches. Gynecol Oncol. (2018) 151:53–60. doi: 10.1016/j.ygyno.2018.08.016
46. Falcetta FS, Medeiros LR, Edelweiss MI, Pohlmann PR, Stein AT, Rosa DD. Adjuvant platinum-based chemotherapy for early stage cervical cancer. Cochrane Database Syst Rev. (2016) 11:CD005342. doi: 10.1002/14651858.CD005342.pub4
47. Brown J, Friedlander M, Backes FJ, Harter P, O'Connor DM, De La Motte Rouge T, et al. Gynecologic cancer intergroup (GCIG) consensus review for ovarian germ cell tumors. Int J Gynecol Cancer. (2014) 24:S48–54. doi: 10.1097/IGC.0000000000000223
48. Liu Q, Ding X, Yang J, Cao D, Shen K, Lang J, et al. The significance of comprehensive staging surgery in malignant ovarian germ cell tumors. Gynecol Oncol. (2013) 131:551–4. doi: 10.1016/j.ygyno.2013.08.016
49. Li J, Wu X. Current strategy for the treatment of ovarian germ cell tumors: role of extensive surgery. Curr Treat Options Oncol. (2016) 17:44. doi: 10.1007/s11864-016-0416-2
50. Wang D, Zhu S, Jia C, Cao D, Wu M, Shen K, et al. Role of staging surgery and adjuvant chemotherapy in adult patients with apparent stage i pure immature ovarian teratoma after fertility-sparing surgery. Int J Gynecol Cancer. (2020) 30:664–9. doi: 10.1136/ijgc-2019-001116
51. Morgan RJ, Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Behbakht K, Chen LM, et al. Ovarian cancer, version 1.2016: clinical practice guidelines in oncology. JNCCN J Natl Compr Cancer Netw. (2016) 14:1134–63. doi: 10.6004/jnccn.2016.0122
52. Kleppe M, Amkreutz LCM, Van Gorp T, Slangen BFM, Kruse AJ, Kruitwagen RFPM. Lymph-node metastasis in stage i and II sex cord stromal and malignant germ cell tumours of the ovary: a systematic review. Gynecol Oncol. (2014) 133:124–7. doi: 10.1016/j.ygyno.2014.01.011
53. Nizam A, Bustamante B, Shan W, Shih KK, Whyte JS, Sakaris A, et al. Overall survival and adjuvant therapy in women with ovarian carcinosarcoma: a single-institution experience. Diagnostics. (2019) 9:200. doi: 10.3390/diagnostics9040200
54. Menczer J. Review of recommended treatment of uterine carcinosarcoma. Curr Treat Options Oncol. (2015) 16:53. doi: 10.1007/s11864-015-0370-4
Keywords: ovarian cancer, lymph node, surgery, survival, pelvic nodes, para-aortic nodes
Citation: Yao G and Jin X (2021) Impact of Lymphadenectomy on Outcomes of Early-Stage Ovarian Cancer: A Systematic Review and Meta-Analysis. Front. Surg. 8:682348. doi: 10.3389/fsurg.2021.682348
Received: 18 March 2021; Accepted: 29 April 2021;
Published: 25 May 2021.
Edited by:
Stefano Cianci, University of Messina, ItalyReviewed by:
Vito Andrea Capozzi, University Hospital of Parma, ItalyCopyright © 2021 Yao and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaotian Jin, anh0NzA3MDcwQDE2My5jb20=
†Present address: Xiaotian Jin, Huzhou Maternity & Child Health Care Hospital, Huzhou, China
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.