- 1Department of Medical Oncology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 2Department of Basic and Clinical Medicine, Shanghai University of Medicine and Health Sciences, Shanghai, China
- 3International Center for Multimorbidity and Complexity in Medicine (ICMC), Universität Zürich, Zürich, Switzerland
- 4Department of General Surgery, Zhongshan Hospital, Fudan University, Shanghai, China
- 5Department of Public Health Sciences, University of Rochester School of Medicine and Dentistry, Rochester, NY, United States
- 6Wright State University Boonshoft School of Medicine, Dayton, OH, United States
- 7Center for Reproductive Medicine, School of Medicine, Renji Hospital, Shanghai Jiao Tong University, Shanghai, China
- 8Shanghai Key Laboratory for Assisted Reproduction and Reproductive Genetics, Shanghai, China
- 9Department of Obstetrics and Gynecology, The University of Colorado, Denver, CO, United States
Background: As cancer has become a major public health issue in China, fertility preservation remains limited despite the wide application of Assisted Reproductive Technology (ART) throughout the country.
Objective: This study aimed to identify gaps in knowledge and communication as well as referrals in the previous year regarding oncofertility among medical and surgical oncologists and breast cancer patients (BCPs) in Chinese academic settings to target areas of needed improvement.
Materials and Methods: A WeChat online questionnaire was designed, distributed, and compared between medical and surgical oncology specialists and reproductive age BCPs in academic teaching settings in Shanghai.
Results: Sixty-one medical and surgical oncologists and 125 BCPs responded to the survey. 63.3% of oncologists were familiar with the term “oncofertility” compared to 25.6% of BCPs (p < 0.001). Oncologists were more likely to correctly know the costs associated with treatment (59.0 vs. 32.0%, p < 0.001); patient did not have to be married to undergo oncofertility treatment (50.8 vs. 24.8%, p < 0.001). Both oncologists and BCPs were similarly unlikely to know when patients could utilize cryopreserved tissue in the future (37.7 vs. 22.2%, p = 0.056). While oncologists reported they discussed all oncofertility options (41.0%) and offered psychological counseling (98.4%), significantly fewer BCPs reported receiving information on all options and offered counseling (3.2%, p < 0.001 and 85.6%, p < 0.01). Knowledge of oncofertility was the most important predictor for providing and receiving counseling from oncologists [OR = 6.44 (95% CI = 1.59–26.1, p = 0.009] and BCPs (OR = 3.73 95% CI: = 1.36–10.2, p = 0.011). Overall, 57.4% of oncologists referred <10 patients and none referred more than 25 patients in the past year.
Conclusion: Data suggests a significant knowledge gap and ineffective communication/comprehension exists between academic Chinese oncologists and BCPs. Continued education and raised awareness are needed to optimize utilization of oncofertility services in China.
Introduction
Cancer incidence and mortality continue to increase world-wide, despite rather stable rates over the last decade in the Western countries (1). This positions cancer as one of the top global burden diseases (2). China takes a special position within the international oncological arena due to the large population and severe regional disparities in cancer epidemiology. The reported incidence of all cancers by the China National Cancer Center remains on the rise, with 3,929,000 in 2015 and 4,285,033 cases in 2018 (3). While cancer is mostly a disease of the elderly, there remains a large number of cancer types in young adults and adolescents, e.g., breast and colon cancer which includes nearly 400,000 reproductive age adults and 23,000 pre- and post-adolescents (4). Fortunately, mortality rates both globally and in China have decreased due to advancements in treatment regimens (1, 5), thus, leading to a rise in the number of cancer survivors in pre- and reproductive age (6, 7).
Cancer treatments have the potential to have highly detrimental effects on gamete function and many adolescent and young adult (AYA) cancer survivors face the prospect of infertility caused by the disease process and/or the cancer treatment itself (8–10). In survey based studies, over half (51.7%) of young cancer survivors describe parenthood as the “most important” issue in their life with many wishing to use their own oocytes and the risk of treatment-related infertility even affecting their decision making about undergoing recommended cancer treatments (11–13). Moreover, even for those who initially stated that fertility preservation was not that important to them, (14) has reported that the issue of fertility becomes increasingly important over time (15).
Novel approaches in preventative therapy and preserving the potential for future fertility for cancer patients have become standard including gamete, embryo, and ovarian tissue cryopreservation (OTC) (12, 16). As such, numerous international fertility preservation and restoration guidelines have been published by American Society of Clinical Oncology (ASCO) (17–19), American Society for Reproductive Medicine (ASRM) (20, 21), European Society for Medical Oncology (21–23), American Oncofertility Consortium (OC) (24, 25), International Society for Fertility Preservation (ISFP) (19, 26–28), National Comprehensive Cancer Network (NCCN), American Academy of Pediatrics (AAP), Association of Pediatric Hematology/Oncology Nurses (APHON) (18), and the German Fertility Preservation Network (FertiPROTEKT) (29) endorsing the requirements that all AYA are to be counseled about the gonadotoxic risk of anticancer therapy, with timely referrals to reproductive specialists in order to provide information, treatment options and follow up.
Despite the interest in parenthood expressed by many cancer patients, the number of patients who access fertility preservation remains relatively low (30). Patients' lack of awareness of treatment-related infertility, together with the time pressures and conflicting priorities of physicians are among the many factors which may hinder adequate oncologist-patient fertility discussions and timely referrals (31). In China, as cancer has become a major public health issue, fertility preservation remains limited despite the wide application of Assisted Reproductive Technology (ART) throughout the country for more than 30 years. Meanwhile, the services for ART are available for all oncology patients in China, albeit not fully covered by the national insurance. Females undergoing oncofertility treatment can in principle access their gametes at any point of time in the future (subject to specific limitations in terms of storage duration). Being married is not a requested in order to preserve fertility in cancer patients (as opposed to social freezing). Lack of oncofertility integration into the Chinese medical field, failure to use ovarian protective strategies such as gonadotropin-releasing hormone agonist treatment in certain populations and regulations that restrict donor oocytes and gestational surrogacy have been cited as reasons for its restricted use (20, 32, 33).
Marginal data exist on fertility preservation for cancer patients in China. Biskup et al. (19) recently reported the number of oncofertility cases performed in the past 5 years at one level 3 teaching hospital, which are the only facilities allowed to provide ART services in China, included just 270 semen and 14 cases of ovarian tissue cryopreservation, while oocyte and embryo cryopreservation were not performed. In comparison, USA long term storage facilities including California Cryobank and Fairfax Cryobank, Inc., performed 1,550 and 768 cases of semen cryopreservation for oncofertility, respectively, and REPROTECH LIMITED had requests for banking that increased over 200%. For females, between 2007 and 2017, 420 underwent OTC at National Physicians Cooperative (NPC) member institutions of the OC19.
More recently, the impetus to overcome these barriers and to enhance awareness of fertility preservation options for oncofertility patients in China has moved forward with the establishment of evolving guidelines and regulations. In 2017, the Chinese Society of Oncofertility was created, yet, as of 2018 there were no ART programs in Shanghai that were part of the OC-NPC. To further highlight the limited use of these services among Chinese reproductive health professionals, Ju et al. reported on the overall the lack of oncofertility knowledge in Fujan, a province in the Southeast part of China, where there is a relatively high incidence in breast cancer in young females (20). The authors highlighted the need for continued oncofertility education and training. Biskup et al. (19) additionally suggested that oncofertility utilization may be further limited due to mistaken provider beliefs and complex social and cultural attitudes among patients regarding fertility in general and fertility preservation.
Given that female fertility preservation is more complex than male preservation, and the high volume work-load of Chinese health-care providers, we objectively sought to assess knowledge, attitudes, and communication regarding oncofertility services among academic medical and surgical oncologist compared to BCPs. The aim was to identify gaps in knowledge and communication as a strategy to target areas of improvement.
Materials and Methods
Ethical Approval
Medical and surgical oncologists and BCP from five academic hospitals including Zhongshan Hospital, Fudan University; Changhai Hospital, Second Military Medical University; Medical College of Shanghai Jiaotong University; International Peace Maternal and Child Health Hospital, Medical College of Shanghai Jiaotong University; and Obstetrics and Gynecology Hospital, Fudan University, were surveyed between June 2019 and August 2019.
Study Protocol
All attending and resident physicians (n = 77) from each of the five medical and surgical oncology departments were invited to participate (80% response rate) in a 30-question online survey while BCPs were asked to complete a 20-question online survey. Using a Wenjuanxing (WJX) survey system, a link with the survey questions was created and distributed via the WeChat application directly to the respective departmental physicians. BCPs received the link to their survey either directly from their physician/nurse, and were asked to share the link among other BCPs. Surveys responses were automatically stored in an electronic, exportable database of WJX. Confidentiality was protected with unique identification numbers that were used on all data collection forms and when performing statistical analyses. Each participant read and signed an informed consent prior to taking the survey. The survey was anonymous and confidentiality was protected with unique identification numbers when performing statistical analysis.
Variables Studied
The surveys (attached in Appendices A, B) were translated from English to Mandarin and consisted of five domains for HCPs including demographics, knowledge, services offered, attitudes, and utilization; for BCPs, domains included: demographics, knowledge, and services discussed with oncology provider.
Statistical Analyses
Descriptive statistics were performed using means with standard deviations for continuous variables, and frequencies and percentages for categorical variables. Differences between medical and surgical oncologists, as well as between oncologists and patients, were compared using the 2-sample t-test, Chi-square test, or Fisher's exact test. A sum knowledge score was constructed based on the number of correctly answered questions regarding oncofertility knowledge (e.g., correctly knowing the estimated cost of oocyte/embryo cryopreservation). Univariate logistic regression models examined associations between each physician and patient characteristics and “knowing what oncofertility was” and “discussing oncofertility,” respectively. Variables significant at an alpha level of 0.2 were then entered into multiple logistic regression models. Odds ratios (ORs) with 95% confidence intervals (95% CIs) were used to describe the magnitude of associations. All statistical tests were two-sided, and p < 0.05 were considered statistically significant. Data management and statistical analyses were conducted in SAS version 9.4 (SAS Inc., Cary, NC).
Results
HCP and Patient Demographics
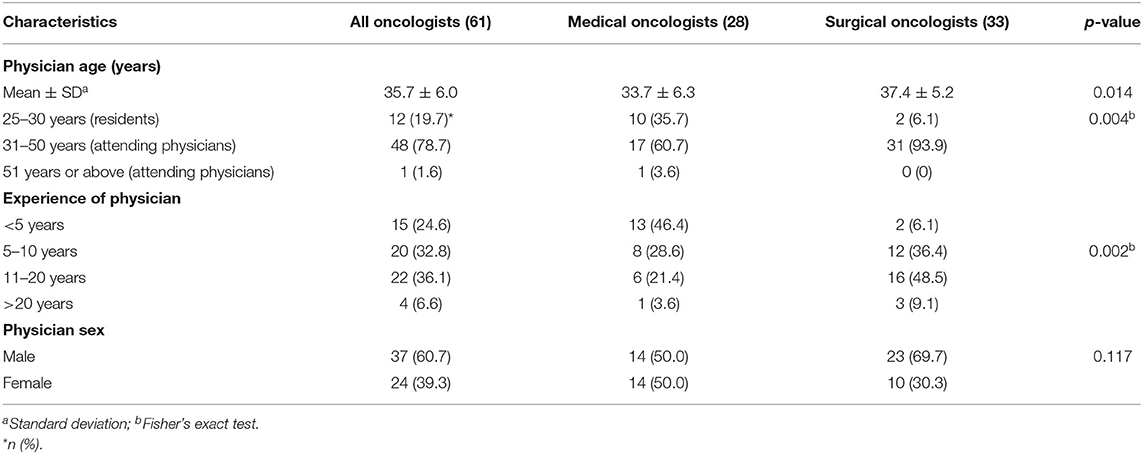
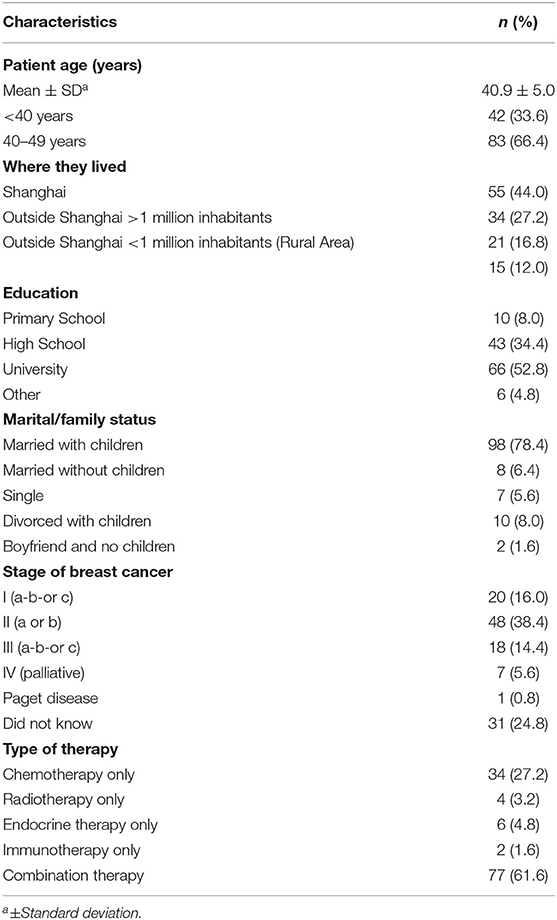
Medical and surgical oncologists and patient characteristics are displayed in Tables 1, 2. A total of 61 of oncologists responded including 28 medical oncologists and 33 surgical oncologists. The mean age of responding physicians were 35.7 years (range, 27–55 years); most were in practice for 11–20 years (36.1%); and 60.7% were male. Surgical oncologists were older and in practice longer than medical oncologists. One-hundred-twenty-five BCP respondents completed the survey with a mean age of 40.9 years, (range 23–49 years); 44.0% lived in Shanghai and 12.0% were from rural areas; 52.8% had a university education; 78.4% were married with children. Breast cancer stage at initial time of diagnosis included 16% Stage 1; 38.4% Stage II; 14.4% Stage III; 5.4 % Stage IV; and 24.8% did not know.
Oncofertility Knowledge
As depicted in Table 3 and Figure 1, oncologists, overall, knew “what oncofertility was” (63.3%); correctly knew the costs associated with oocyte/embryo cryopreservation (59.0%), the marital status requirements (50.8%), and to assess ovarian reserve with serum cycle day 2 or 3 FSH or AMH (80.3%). However, the majority incorrectly knew or did not know whether oncofertility treatments were covered by insurance (88.5%); and whether those undergoing fertility preservation could access gametes anytime in the future (i.e., required a partner by marriage) (62.3%). While medical oncologists tended to know “what oncofertilty was” (75.0 vs. 53.1%, p-NS), they were less likely to know how to test for ovarian reserve (64.3 vs. 93.9%, p = 0.009). Compared to oncologists, BCPs were less likely to know “what oncofertility was” (25.6%, p < 0.001); costs associated with treatment (32.0% [59.2% did not know], p < 0.001); marital status requirements (24.8%, p = 0.001); that they could access their cryopreserved tissue upon marriage (22.4%, p-NS); and know how to test for ovarian reserve (16.0%, p < 0.001). Compared to oncologists, they were more likely to know whether oncofertility treatment was covered by insurance (21.6 vs. 11.5%, p < 0.001).
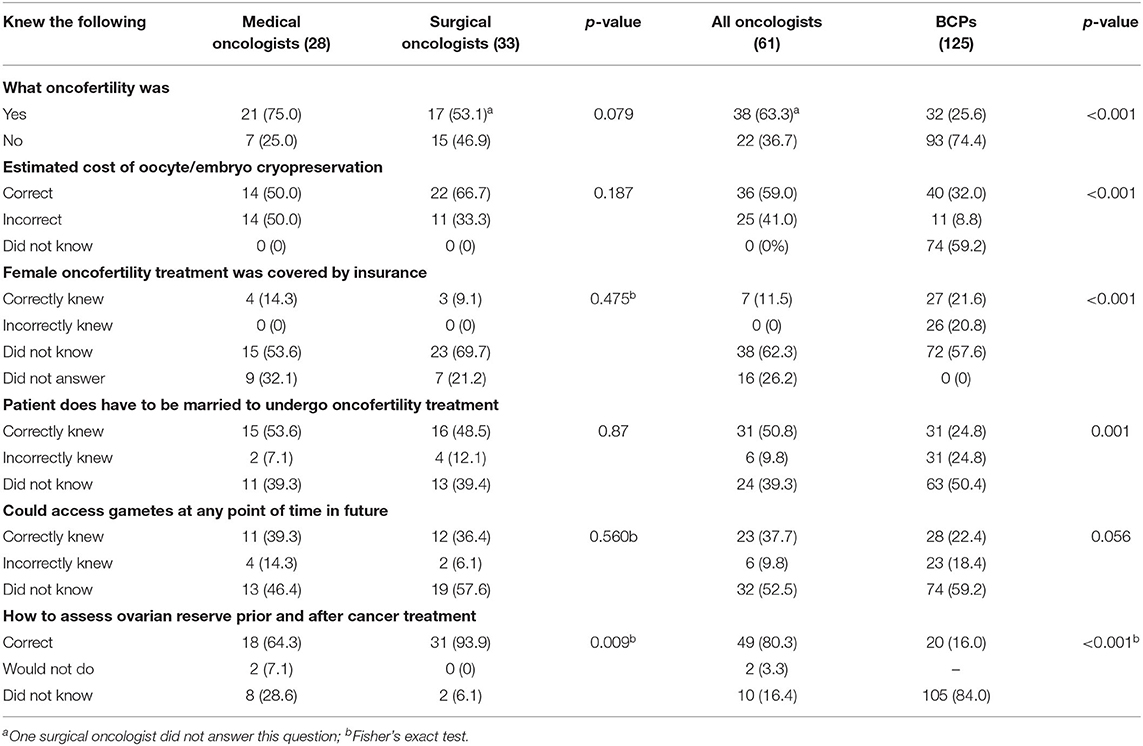
Table 3. Medical and surgical oncologists and breast cancer patients' knowledge related to oncofertility services.
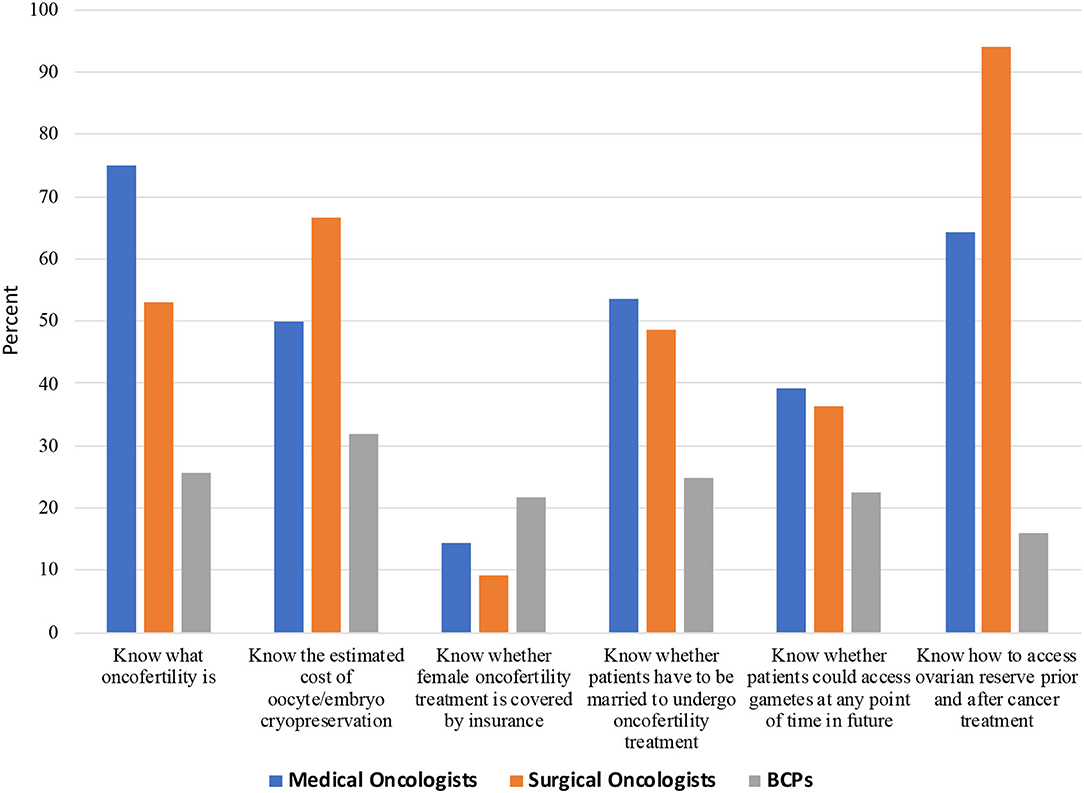
Figure 1. Knowledge of oncofertility among Chinese medical oncologists, surgical oncologists, and breast cancer patients (BCPs).
Attitudes, Services Offered, and Utilization
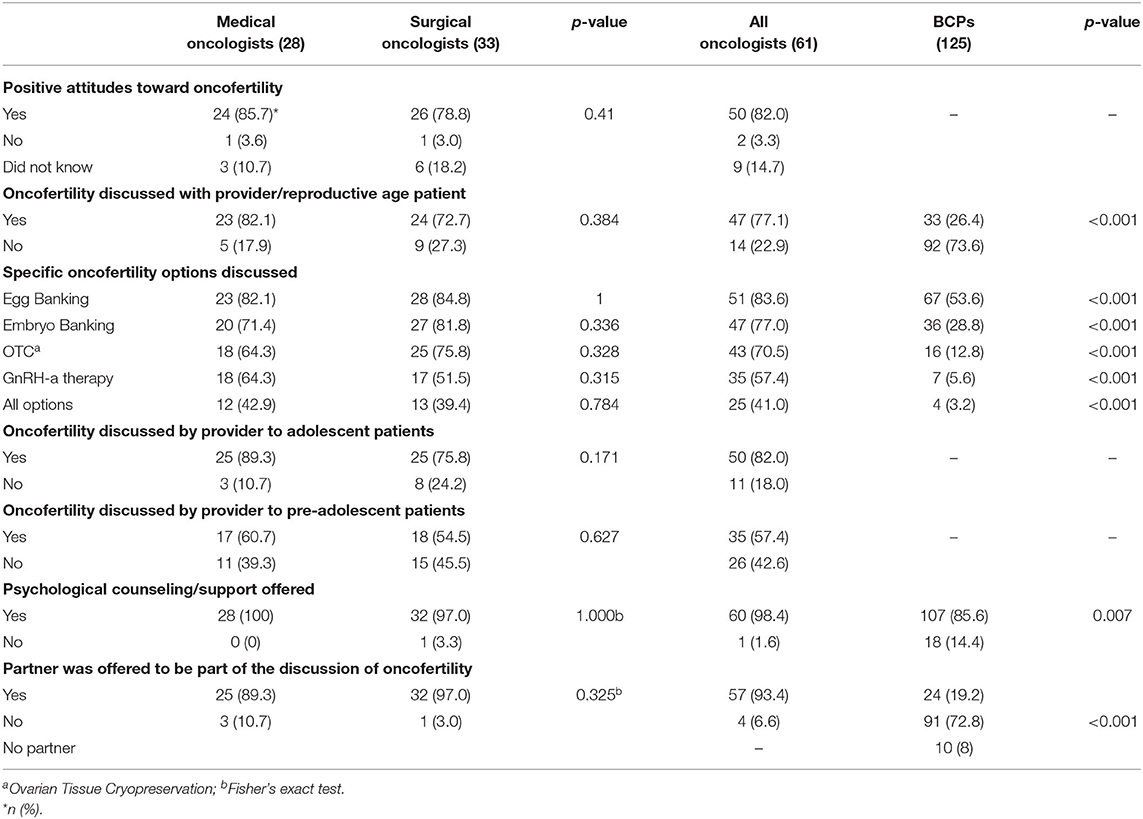
As shown in Table 4 and Figure 2, overall, 82.0% of oncologists had a positive attitude toward oncofertility treatment, but were less likely to discuss these options with pre-adolescent girls than adolescent and reproductive age women (57.4 vs. 82 and 77.1%, p < 0.001). Significantly more oncologists stated they provided information regarding oncofertility; offered psychological support/counseling; and allowed the partner (if applicable) to be part of the discussion (77.1, 98.4, and 93.4%, respectively) compared to 26.4; 85.6, and 19.2% of BCPs. No differences were noted between medical and surgical oncologists.
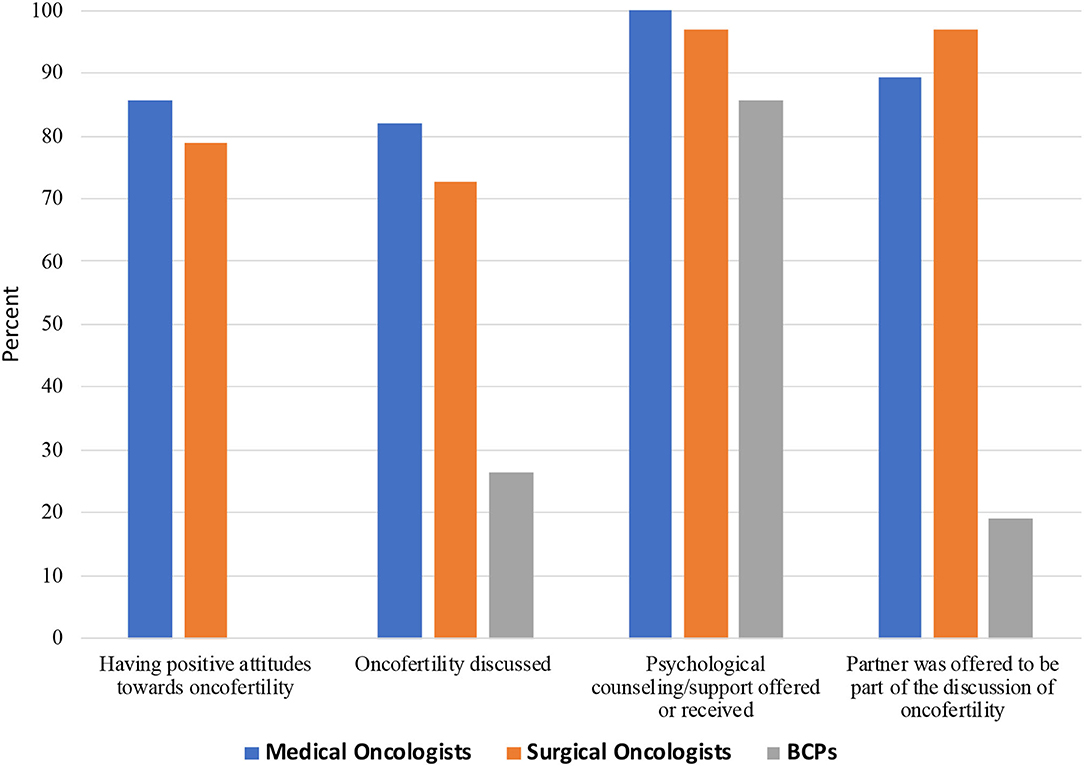
Figure 2. Attitudes and discussion of oncofertility treatment among Chinese medical oncologists, surgical oncologists, and breast cancer patients (BCPs).
Over the previous year, 64.3% of oncologists reported referring candidates for oocyte-embryo banking <10 times; 1.9% referred 10–25 times and 1.9%-more than 25 times, while 32.6% did not recall the number of referrals. Reasons given by the oncologists as to why BCPs were not utilizing oncofertility services included: patient's personal wishes (39.3% [24]), financial (39.3% [24]), unaware of options (70.5% [43]), cultural barriers (44.2% [27]), and did not know (4.9% [3]), as shown in Figure 3.
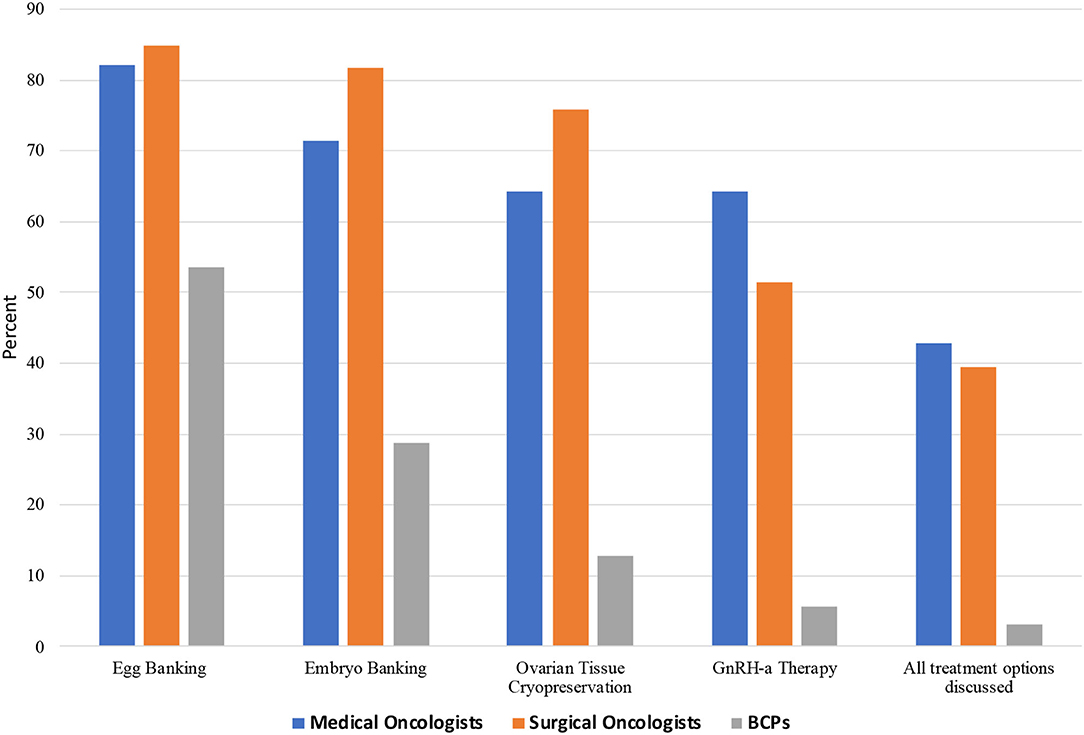
Figure 3. Specific oncofertility options discussed among Chinese medical oncologists, surgical oncologists, and breast cancer patients (BCPs).
Factors Associated With Knowledge (Awareness) and Service Offered (or Received) of Oncofertility
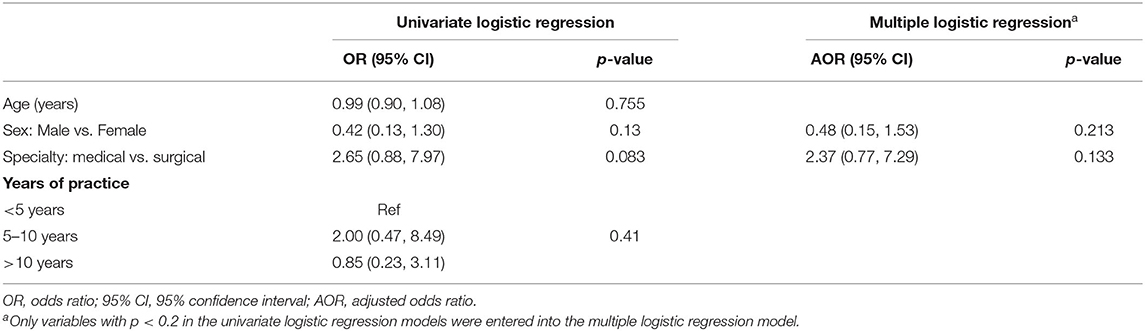
Table 5 shows factors associated with “knowing what oncofertility was” among oncologists. Female oncologists, compared to male, and medical oncologists, compared to surgical oncologists, were more likely to know what oncofertility was in both univariate and multiple logistic regression models, although the differences were not significant.
Table 6 shows factors associated with “discussing oncofertility with pre-adolescent, adolescent patients and reproductive-aged patients” among oncologists. In univariate models, “knowing what oncofertility was” was associated with 7.08 times the odds (95% CI = 1.87, 26.9) of discussing oncofertility with patients, with each 1-score increase in oncofertility knowledge associated with 81% greater odds of discussion (95% CI = −9%, 261%). A positive attitude toward oncofertility was associated with 3.8 times the odds of discussion (95% CI = 0.95, 15.2). When entered into the multiple logistic regression model, effect sizes attenuated but the direction of associations persisted.
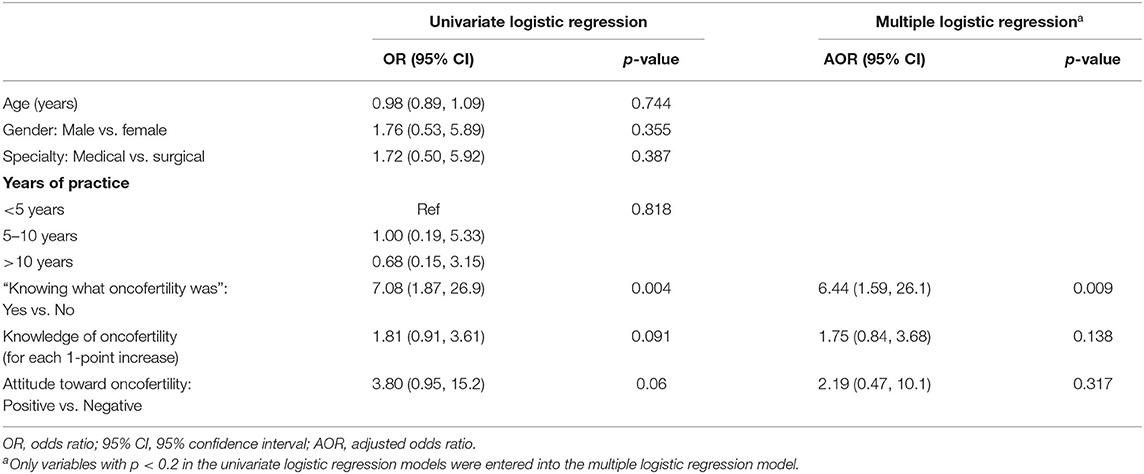
Table 6. Factors associated with “discussing oncofertility” with reproductive-aged patients among oncologists (n = 61).
Table 7 shows factors associated with “knowing what oncofertility was” among BCPs. Univariate models suggested that higher education levels and lower stage of breast cancer were associated with greater likelihood of “knowing what oncofertility was”. Although not statistically significant, patients of younger age, living in Shanghai, and without children tend to be more aware of oncofertility. In multiple logistic regression, only the stage of breast cancer was marginally associated with awareness (p = 0.058).

Table 7. Factors associated with knowing “what oncofertility was” among breast cancer patients (n = 125).
Table 8 shows factors associated with discussing oncofertility with oncologists among BCPs. Univariate models indicated that patients of younger age, living in Shanghai, of more education, without children, with lower stage of breast cancer, knowing what oncofertility was and having more knowledge of oncofertility, were more likely to discuss oncofertility with their oncologists. Multiple logistic regression indicated that knowing what oncofertility was (OR = 3.73, 95% CI = 1.36, 10.2) and greater knowledge of oncofertility (for 1-score increase, OR = 1.69, 95% CI = 1.14, 2.51), were associated with greater odds of discussing oncofertility with oncologists.
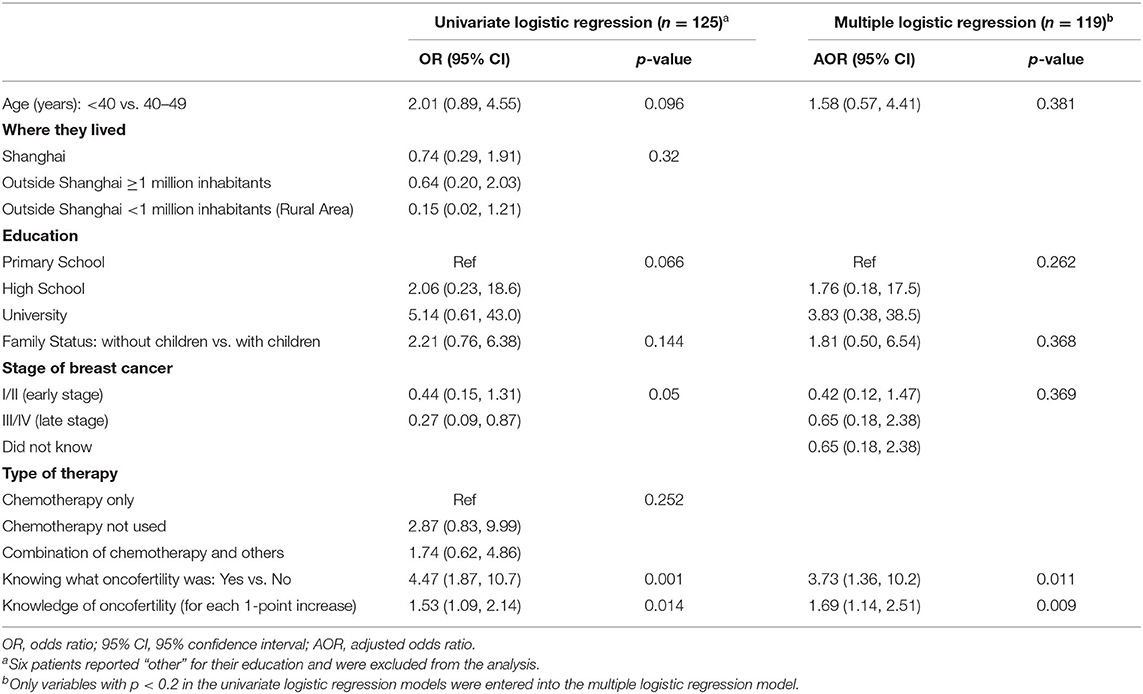
Table 8. Factors associated with receiving oncofertility discussion from their oncologist among breast cancer patients (n = 125).
Discussion
Our study revealed that slightly less than two-thirds of academic Chinese medical and surgical oncologists knew what the term oncofertility meant, though more than three quarters discussed at least some type of fertility preservation option. The majority of responding oncologists felt positively regarding offering these services, extended psychological support/counseling to their patients and encouraged partners to be part of the discussion. This contrasts with BCPs who were significantly less likely to know and discuss oncofertility with their oncologist and be offered/extended support services. Providers and BCPs' “knowing what oncofertility was” and level of knowledge were significantly associated with discussing fertility preservation options.
Since ASCO and ASRM published clinical practice guidelines in 2006 and with updates in 2012 adding oocyte cryopreservation as a standard practice for adults and children (11, 16, 34), there has been a world-wide increase in awareness for oncofertility referrals. This appears to be particular to BCPs who seemingly prefer to receive fertility-related information from reproductive endocrinology infertility specialists (35). However, fertility preservation remains one of the most under-prescribed and least implemented services in patients with cancer (35). This is particularly apparent for female fertility preservation, which is significantly more complex than male fertility preservation given the time required for ovarian stimulation and oocyte retrieval. In fact, data suggest that males are five times more likely to make arrangements for fertility preservation (36). Recently, oncofertility models of care including the EUropean REcommendations for female FERtility preservation (EU-REFER), which was a joint collaboration between oncologists and fertility specialists, has attempted to provide clinicians, and particularly oncologists, with a comprehensive standard reference when dealing with female cancer patients (37). Recommendations have included effective communication by oncologists, decision aids, age-appropriate care, referral pathways, documentation, training, supportive care during treatment, reproductive care after cancer treatment, psychosocial support, and ethical practice. Working as part of a multidisciplinary team, it is the hope that it will ensure that patients are less likely to miss out on receiving time-critical fertility information, which is potentially crucial to their chances of having children.
Despite the increasing awareness for oncofertility referrals, numerous previous qualitative studies have reported low actual rates of referrals (38–43). Several recent large population-based retrospective studies suggest that while referral rates for fertility assessment and treatment have increased not only for reproductive-aged cancer patients, pre-adolescent and adolescent patients, the overall referral and utilization of these services still remains remarkably low. This ranges from 4% for BCP [(35) and 1.7–3% in those with any cancer diagnosis in reproductive-aged women in the United States (44)]. This contrasts with European data which reveal slightly higher rates of referral (9%), though this still falls far from desired international guidelines (41, 45). Moreover, referrals have been inversely correlated with patient demographics, prognosis, advancing reproductive age, prior parity, and advanced disease. Patients' lack of awareness of oncofertility services together with the time pressures and conflicting priorities of physicians, reimbursement, and collaborative multi-disciplinary approaches continue to hinder adequate oncologist-patient fertility discussions and timely referrals appears to be a world-wide phenomenon (30, 37).
In China, models of care, referred to as multidisciplinary team (MDT) models, are slowly gaining traction and becoming increasingly prominent over the past 2 decades (46). Given the country's ever-increasing health care demands, imbalanced medical resource distribution, inadequate health insurance, and unsatisfactory implementation of disease management guidelines, it is clear that individual professional or discipline knowledge no longer is sufficient to cope with complicated medical conditions. Thus, the use of multidisciplinary collaborative guidance documents, such as systemic specifications of disease diagnosis and treatment, consultation systems, and protocols related to multi- disciplinary comprehensive management for difficult and severe diseases including cancer or critical diseases are being implemented (46). The goal is to diagnosis as soon as possible and treat patients in a timely and effective manner. With MDT's becoming more in-bread in the minds of physician leaders, models like EU-REFER model of care may serve as a template to enhance the awareness, create stream-line processes, and increase the utilization of fertility sparing options among health care professionals and AYA cancer patients (47–51). Since the inception of our current study, five Shanghai ART programs have since become part of the OC-NPC, though much work remains to be done. It is also crucial to educate physicians and patients about the requirements to enjoy oncofertility services such as the insurance coverage and the fact that—in contrast to elective fertility services—the patient does not have to be married.
Several limitations of this study should be acknowledged. First, the cross-sectional nature of this study precludes the ability to make conclusions on causation. For example, although more knowledge of oncofertility could be associated with a greater likelihood of discussing oncofertility with oncologists, BCPs who received discussion of oncofertility from their oncologist may be more likely to know what oncofertility was. Second, the relatively small sample size of oncologists and BCP respondents reduced statistical power to examine predictors of oncofertility knowledge and discussion. Third, since oncologists and BCPs were recruited from academic hospitals in Shanghai, the generalizability of our findings may be limited. Nevertheless, even in academic hospitals in Shanghai where medical education is relatively advanced in China, we found low referral rates for oncofertility among oncologists, as well as a large gap in the knowledge and service of oncofertility between oncologists and BCPs, which is concerning. In spite of these limitations, this study is valuable in providing support for continued study in this area. It provides useful insights into the knowledge gap and ineffective communication and comprehension that exists between Chinese oncologists and BCPs. Continued education and raised awareness are needed to optimize utilization of oncofertility services in China.
Conclusions
In conclusion, this study reveals that most of the surveyed Chinese medical and surgical oncologists have a positive attitude toward oncofertility services; however, a lack of fertility preservation knowledge for both health-care providers and patients exists, which may hinder patient referrals. These findings emphasize the need for the standardization of oncofertility education and training as well as the need for a rapid and effective navigation mechanism between oncologists, cancer patients, and reproductive health specialists.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
EB, ZX, SL, and HZ contributed to the study design, recruitment-analysis, and manuscript writing. JZ and RL contributed to manuscript writing (figures/tables) and study's statistical analysis. YS and YL contributed to the study recruitment practices and manuscript review. LA contributed to the study design, analysis, and manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
EB was supported by Krebsliga Schweiz, BIL KFS 4261-08-2017.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Dr. Hadley Kelly and Dr. Elena Laura Georgescu Margarint, for their support during the submission process.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.681614/full#supplementary-material
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
2. Global Burden of Disease Cancer Collaboration Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study [published correction appears in JAMA Oncol. 6:444] [published correction appears in JAMA Oncol. (2020) 6:789] [published correction appears in JAMA Oncol. (2021) 7:466]. JAMA Oncol. (2019) 5:1749–68. doi: 10.1001/jamaoncol.2019.2996
3. Feng R-M, Zong Y-N, Cao S-M, Xu R-H. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics?. Cancer Commun (Lond). (2019) 39:22. doi: 10.1186/s40880-019-0368-6
4. Gupta S, Harper A, Ruan Y, Barr R, Frazier AL, Ferlay J, et al. International trends in the incidence of cancer among adolescents and young adults. J Natl Cancer Inst. (2020) 112:1105–17. doi: 10.1093/jnci/djaa007
5. Hashim D, Boffetta P, La Vecchia C, Rota M, Bertuccio P, Malvezzi M, et al. The global decrease in cancer mortality: trends and disparities. Ann Oncol. (2016) 27:926–33. doi: 10.1093/annonc/mdw027
6. Tang M, Webber K. Fertility and pregnancy in cancer survivors. Obstet Med. (2018) 11:110–5. doi: 10.1177/1753495X18757816
7. Miller KD, Nogueira L, Mariotto AB, Rowland J, Yabroff Robin K, Alfano Catherine M, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. (2019) 69:363–85. doi: 10.3322/caac.21565
8. Dohle GR. Male infertility in cancer patients: review of the literature. Int J Urol. (2010) 17:327–31. doi: 10.1111/j.1442-2042.2010.02484.x
9. Schover LR, van der Kaaij M, van Dorst E, Creutzberg C, Huyghe E, Kiserud CE. Sexual dysfunction and infertility as late effects of cancer treatment. EJC Suppl. (2014) 12:41–53. doi: 10.1016/j.ejcsup.2014.03.004
10. Carter J, Chi DS, Brown CL, Abu-Rustum NR, Sonoda Y, Aghajania C, et al. Cancer-related infertility in survivorship. Int J Gynecol Cancer. (2010) 20:2–8. doi: 10.1111/IGC.0b013e3181bf7d3f
11. Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. (2018) 36:1994–2001. doi: 10.1200/JCO.2018.78.1914
12. Del-Pozo-Lérida S, Salvador C, Martínez-Soler F, Tortosa A, Perucho M, Giménez-Bonafé P. Preservation of fertility in patients with cancer (Review). Oncol Rep. (2019) 41:2607–14. doi: 10.3892/or.2019.7063
13. Levine J, Canada A, Stern CJ. Fertility preservation in adolescents and young adults with cancer. J Clin Oncol. (2010) 28:4831–41. doi: 10.1200/JCO.2009.22.8312
14. Thewes B, Meiser B, Taylor A, Phillips KA, Pendlebury S, Capp A, et al. Fertility- and menopause-related information needs of younger women with a diagnosis of early breast cancer. J Clin Oncol. (2005) 23: 5155–65. doi: 10.1200/JCO.2005.07.773
15. Wallace WH. (2011). Oncofertility and preservation of reproductive capacity in children and young adults. Cancer. 117(10 Suppl.):2301–10. doi: 10.1002/cncr.26045
16. Ethics Committee of the American Society for Reproductive Medicine. Electronic address: QVNSTSYjeDAwMDQwO2Fzcm0ub3Jn. Fertility preservation and reproduction in patients facing gonadotoxic therapies: an Ethics Committee opinion. Fertil Steril. (2018) 110:380–6. doi: 10.1016/j.fertnstert.2018.05.034
17. Takae S, Lee JR, Mahajan N, Wiweko B, Sukcharoen N, Novero V, et al. Fertility preservation for child and adolescent cancer patients in Asian Countries [published correction appears in Front Endocrinol (Lausanne). 11:241]. Front Endocrinol (Lausanne). (2019) 10:655. doi: 10.3389/fendo.2019.00655
18. Smith BM, Duncan FE, Ataman L, Smith K, Quinn Qwendolyn P, Chang J, et al. The National Physicians Cooperative: transforming fertility management in the cancer setting and beyond. Future Oncol. (2018) 14:3059–72. doi: 10.2217/fon-2018-0278
19. Biskup E, Lu Y, Sun Y, Trolice M, Appiah L, Lindheim S. The Great Wall: the symbol of a path to great progress in oncofertility services. Fertil Steril. (2019) 1:1.
20. Ju K, Kopp M, Wang Y, Yuan G, Zheng W, Ataman Lauren M, et al. A survey study of attitude and knowledge regarding female fertility preservation among reproductive health professionals in Fujian, China. J Adolesc Young Adult Oncol. (2019) 8:67–73. doi: 10.1089/jayao.2018.0065
21. Woodruff TK. From the bench to bedside to babies: translational medicine made possible by funding multidisciplinary team science. J Assist Reprod Genet. (2013) 30:1249–53. doi: 10.1007/s10815-013-0082-2
22. Lambertini M, Peccatori FA, Demeestere I, Amant F, Wyns C, Stukenborg J-B, et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. (2020) 31:1664–78. doi: 10.1016/j.annonc.2020.09.006
23. Woodruff TK. Oncofertility: a grand collaboration between reproductive medicine and oncology. Reproduction. (2015) 150:S1–S10. doi: 10.1530/REP-15-0163
24. Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. (2009) 360:902–11. doi: 10.1056/NEJMra0801454
25. Woodruff TK. The emergence of a new interdiscipline: oncofertility. Cancer Treat Res. (2007) 138:3–11. doi: 10.1007/978-0-387-72293-1_1
26. Ataman LM, Rodrigues JK, Marinho RM, Caetano J, Chehin M, Alves da Motta E, et al. Creating a global community of practice for oncofertility. J Glob Oncol. (2016) 2:83–96. doi: 10.1200/JGO.2015.000307
27. Shen MJ, Badr H. Incorporating partners and spouses in oncofertility communication. In: Woodruff TK, Clayman ML, Waimey KE, editors. Oncofertility Communication: Sharing Information and Building Relationships across Disciplines. New York, NY: Springer New York (2014). p. 73–85.
28. Massarotti C, Scaruffi P, Lambertini M, Sozzi F, Remorgida V, Anserini P. Beyond fertility preservation: role of the oncofertility unit in the reproductive and gynecological follow-up of young cancer patients. Hum Reprod. (2019) 34:1462–9. doi: 10.1093/humrep/dez108
29. von Wolff M, Andersen CY, Woodruff TK, Nawroth F. FertiPROTEKT, Oncofertility Consortium and the Danish Fertility-Preservation Networks - what can we learn from their experiences?. Clin Med Insights Reprod Health. (2019) 13:1179558119845865. doi: 10.1177/1179558119845865
30. Linkeviciute A, Boniolo G, Chiavari L, Peccatori FA. Fertility preservation in cancer patients: the global framework. Cancer Treat Rev. (2014) 40:1019–27. doi: 10.1016/j.ctrv.2014.06.001
31. Flink DM, Sheeder J, Kondapalli LA. A review of the oncology patient's challenges for utilizing fertility preservation services. J Adolesc Young Adult Oncol. (2017) 6:31–44. doi: 10.1089/jayao.2015.0065
32. Qiao J, Xia XLH. Fertility preservation and assisted reproduction issues of patients after treatment of gynecological malignancies. Chinese J Pract Gynecol Obstet. (2014) 30:729–31.
33. Munhoz RR, Pereira AA, Sasse AD, Hoff P, Traina T, Hudis C, et al. Gonadotropin-releasing hormone agonists for ovarian function preservation in premenopausal women undergoing chemotherapy for early-stage breast cancer: a systematic review and meta-analysis. JAMA Oncol. (2016) 2:65–73. doi: 10.1001/jamaoncol.2015.3251
34. Lambertini M, Di Maio M, Pagani O, Curigliano G, Poggio F, Del Mastro L, et al. The BCY3/BCC 2017 survey on physicians' knowledge, attitudes and practice towards fertility and pregnancy-related issues in young breast cancer patients. Breast. (2018) 42:41–9. doi: 10.1016/j.breast.2018.08.099
35. Korkidakis A, Lajkosz K, Green M, Strobino D, Velez MP. Patterns of referral for fertility preservation among female adolescents and young adults with breast cancer: a population-based study [published correction appears in J Adolesc Young Adult Oncol. (2020) 9:546–7]. J Adolesc Young Adult Oncol. (2019) 8:197–204. doi: 10.1089/jayao.2018.0102
36. Keegan T, Harlan LC, Smith AW, Shnorhavorian M, Lynch C, Prasad P, et al. Fertility preservation knowledge, counseling, and actions among adolescent and young adult patients with cancer: a population-based study. Cancer. (2015) 121:3499–506. doi: 10.1002/cncr.29328
37. Dolmans MM, Lambertini M, Macklon KT, Santos TA, Ruiz-Casado A, Borini A, et al. EUropean REcommendations for female FERtility preservation (EU-REFER): a joint collaboration between oncologists and fertility specialists. Crit Rev Oncol Hematol. (2019) 138:233–40. doi: 10.1016/j.critrevonc.2019.03.010
38. Shimizu C, Kato T, Tamura N, Bando H, Asada Y, Mizota Y, et al. Perception and needs of reproductive specialists with regard to fertility preservation of young breast cancer patients. Int J Clin Oncol. (2015) 20:82–9. doi: 10.1007/s10147-014-0676-4
39. Louwé LA, ter Kuile MM, Hilders CG, jenninga E, Tiemessen SM, Peters A, et al. Oncologists' practice and attitudes regarding fertility preservation in female cancer patients: a pilot study in the Netherlands. J Psychosom Obstet Gynaecol. (2013) 34:129–32. doi: 10.3109/0167482X.2013.821977
40. Rabah DM, El-Nimr N, Rafe BA, Arafa MA. Fertility cryopreservation for female cancer patients: attitudes and clinical practices of oncologists in Riyadh, Saudi Arabia. J Reprod Med. (2012) 57:431–4.
41. Bastings L, Baysal O, Beerendonk CC, Braat DD, Nelen WL. Referral for fertility preservation counselling in female cancer patients. Hum Reprod. (2014) 29:2228–37. doi: 10.1093/humrep/deu186
42. Goodwin T, Elizabeth Oosterhuis B, Kiernan M, Hudson MM, Dahl GV. Attitudes and practices of pediatric oncology providers regarding fertility issues. Pediatr Blood Cancer. (2007) 48:80–5. doi: 10.1002/pbc.20814
43. Besse D, Bellavia M, de Ziegler D, Wunder D. Fertility and cancer: psychological support in young women who contemplate emergency assisted reproductive technologies (ART) prior to chemo- and/or radiation-therapy. Swiss Med Wkly. (2010) 140:w13075. doi: 10.4414/smw.2010.13075
44. Hariton E, Bortoletto P, Cardozo ER, Hochberg EP, Sabatini ME. The role of oncofertility clinics in facilitating access to reproductive specialists. J Patient Exp. (2016) 3:131–6. doi: 10.1177/2374373516685960
45. Logan S, Perz J, Ussher JM, Peate M, Anazodo A. A systematic review of patient oncofertility support needs in reproductive cancer patients aged 14 to 45 years of age. Psychooncology. (2018) 27:401–9. doi: 10.1002/pon.4502
46. Song P, Wu Q, Huang Y. Multidisciplinary team and team oncology medicine research and development in China. Biosci Trends. (2010) 4:151–60.
47. Coccia PF, Pappo AS, Altman J, Bahtia S, Borinstein S, Flynn J, et al. Adolescent and young adult oncology, version 2.2014. J Natl Compr Canc Netw. (2014) 12:21–32. doi: 10.6004/jnccn.2014.0004
48. Moore HC, Unger JM, Phillips KA, Boyle F, Hitre E, Porter D, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. (2015) 372:923–32. doi: 10.1056/NEJMoa1413204
49. Pearson H. History of pediatric hematology oncology. Pediatr Res. (2002) 52:979–92. doi: 10.1203/00006450-200212000-00026
50. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
Keywords: oncofertility, fertility preservation, breast cancer, breast cancer survivors, oncology, oncologist
Citation: Biskup E, Xin Z, Li R, Zucal JP, Lu Y, Sun Y, Appiah LC, Lindheim SR and Zhang H (2021) Oncofertility Knowledge and Communication: Comparison Between Medical and Surgical Oncologists and Breast Cancer Patients in Academic Chinese Centers. Front. Surg. 8:681614. doi: 10.3389/fsurg.2021.681614
Received: 07 April 2021; Accepted: 30 July 2021;
Published: 07 September 2021.
Edited by:
Lei Huang, Shanghai Jiao Tong University School of Medicine, ChinaReviewed by:
Luca Arecco, San Martino Hospital (IRCCS), ItalyOzgur Tanriverdi, Muğla University, Turkey
Copyright © 2021 Biskup, Xin, Li, Zucal, Lu, Sun, Appiah, Lindheim and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongwei Zhang, emhhbmcuaG9uZ3dlaSYjeDAwMDQwO3pzLWhvc3BpdGFsLnNoLmNu; Steven R. Lindheim, ZG9jbGFsYWxpbmRoZWltJiN4MDAwNDA7Z21haWwuY29t
†These authors have contributed equally to this work
 Ewelina Biskup1,2,3†
Ewelina Biskup1,2,3† Yun Sun
Yun Sun Leslie Coker Appiah
Leslie Coker Appiah Steven R. Lindheim
Steven R. Lindheim