
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg. , 17 December 2021
Sec. Reconstructive and Plastic Surgery
Volume 8 - 2021 | https://doi.org/10.3389/fsurg.2021.680930
Background: Homeopathic Arnica montana is used in surgery as prevention or treatment for the reduction of pain and other sequelae of surgery. Our aim was to perform a metaanalysis of clinical trials to assess efficacy of Arnica montana to reduce the inflammatory response after surgery.
Method: We conducted a systematic review and metaanalysis, following a predefined protocol, of all studies on the use of homeopathic Arnica montana in surgery. We included all randomized and nonrandomized studies comparing homeopathic Arnica to a placebo or to another active comparator and calculated two quantitative metaanalyses and appropriate sensitivity analyses. We used “Hegde's g,” an effect size estimator which is equivalent to a standardized mean difference corrected for small sample bias. The PROSPERO registration number is CRD42020131300.
Results: Twenty-three publications reported on 29 different comparisons. One study had to be excluded because no data could be extracted, leaving 28 comparisons. Eighteen comparisons used placebo, nine comparisons an active control, and in one case Arnica was compared to no treatment. The metaanalysis of the placebo-controlled trials yielded an overall effect size of Hedge's g = 0.18 (95% confidence interval −0.007/0.373; p = 0.059). Active comparator trials yielded a highly heterogeneous significant effect size of g = 0.26. This is mainly due to the large effect size of nonrandomized studies, which converges against zero in the randomized trials.
Conclusion: Homeopathic Arnica has a small effect size over and against placebo in preventing excessive hematoma and other sequelae of surgeries. The effect is comparable to that of anti-inflammatory substances.
Homeopathic Arnica montana has a reputation for stemming bleeding, mainly internal bleeding following internal lacerations, for instance from contusions, concussions, sprains, and other injuries (1–5). Hahnemann, the founder of Homeopathy, took this indication from European folk-medicine, where Arnica has been long in use for the treatment of sprains and lacerations. The principle of homeopathy is the law of similars, similia similibus curentur, let like be cured by like (6). Hahnemann developed an operationalization by using known toxicological data from poisonings with various plants and substances, and the symptoms that these substances produce when ingested by healthy volunteers, either crudely, or more often in potentized form (7). For this purpose, the substances are successively diluted and succussed (i.e.. vigorously shaken) in a series of dilutions, and the probability to find any molecule of the potentized substance rapidly converges to zero for homeopathic potencies beyond 24X or 12C. Hence, it is unclear how such potencies can at all be effective. However, reviews of pathogenetic trials in healthy volunteers document effects that are different from placebo, at least sometimes (8), and a recent meta-analysis shows that individualized homeopathy can be statistically separated from placebo (9). The bone of contention is of course the question as to how remedies without a known active ingredient could be at all effective. We plead ignorance here. But absence of knowledge is not knowledge of absence. It might well be possible, as has been often the case in medicine, that future theories or further research might unearth some new therapeutic principle. To understand whether this is at all a useful exercise, it is helpful to see whether the data to date would justify such efforts.
Arnica is used a lot for self-care (10, 11) and also in midwifery (12) and surgery (13). Meanwhile, some 30 studies have been conducted where homeopathic Arnica has been applied before or after surgery to improve wound healing, stop bleeding and swelling, and reduce pain. Studies of Arnica for the prevention of muscle-soreness after sportive activities such as marathon running are negative (14, 15), and an early systematic review was skeptical about the effectiveness of Arnica (16). A recent publication summarized the effects of Arnica as found in 20 placebo-controlled trials descriptively, and the authors stated that homeopathic Arnica “seems to have a mitigating effect on ecchymosis, most notably following rhinoplasty and facelifts/facial procedures” and claimed the importance to determine the efficacy of this possibly beneficial therapeutic intervention (17).
Hence, we set out to conduct a systematic review and meta-analysis of clinical trials asking whether homeopathically prepared Arnica is more effective than placebo, or at least as effective as active controls in surgery for the treatment of postoperative pain, prevention of swelling, edema, and other sequelae. We deliberately focused on studies that used Arnica only and excluded trials that used a combination of remedies.
We developed a protocol that was logged with the PROSPERO database before the commencement of the study (Registration number: CRD42020131300; available from https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020131300). One reviewer conducted the search using this predefined search strategy. We included all trials that produced a randomized or quasi-randomized comparison between homeopathic Arnica in any potency and dosage, and placebo or an active control in surgical procedures. Arnica tinctures without homeopathic processing into potencies were excluded. All references between January 1, 1980 and January 25, 2020 were eligible for screening. There were no language restrictions.
The present project was carried out as part of a general update of empirical evidence of homeopathic interventions. The search strategy of this general project aimed to identify controlled clinical investigations (randomized or non-randomized), employing one or more homeopathically processed substances on humans exhibiting a clinically relevant disease (treatment interventions) or on humans in danger of developing a disease (prophylactic or preventive interventions). The complete search strategy and classification methods of the literature overview is available in the framework-protocol of the project (18). The surgical studies eligible for this review were deducted from the literature overview. The deduction rules are available as a Supplementary Material (eligible studies). The flowchart for the eligible studies is represented in Figure 1.
The information was extracted into a spreadsheet mirroring the predefined variables by one reviewer (HW) and checked 100% by a second reviewer (KG). The extraction process was documented in a log (Supplementary Material 2), with all changes, conversions, and procedures. Discrepancies between the reviewers were discussed, the results logged, and the database adapted and saved under a new name, such that all changes can be followed.
We extracted the following formal information: publication type, time and country, type of homeopathy (preventive, individualized, or formulaic), type of dilution, diagnoses (ICD10 code), and also quantitative information such as: mean values and standard deviations of outcome criteria. Where a primary outcome was defined, we extracted the primary outcome. Very often multiple outcomes were stipulated and no primary outcome was mentioned. In that case all outcome-variables were extracted. If multiple outcomes were mentioned, the protocol stipulated that each outcome is converted into an effect size and averaged within a study, such that each study entered the analysis with one effect size. Sometimes studies had multiple arms (for instance Arnica vs. placebo vs. anti-inflammatory substance). Such studies were coded as two separate studies, one homeopathy vs. placebo and the other one as homeopathy vs. conventional treatment. Two analyses were calculated, one for studies that compared Arnica with placebo, and one for those that used a standard-treatment comparator.
We used two quality assessments, the Cochrane risk of bias tool (Version 1.0) and the Quality Assessment Tool for Quantitative Studies, developed by the Effective Public Health Practice Project (EPHPP) (19, 20). This latter summary coding was used as a moderator in the analysis.
Where data were only available in graphs, data were read off the graph by enlarging the display and reading the figures with a ruler. When only partial information was given, we converted the available information, for instance we used published formulae for converting median data into mean (21), or odds ratios (22). Sometimes data were given as categories. These were converted into quasi-continuous data such that mean and standard deviations could be calculated. As the majority of the studies used continuous outcome measures, we decided to use Hedge's g as the effect size measure because it corrects for the typically small study sizes in such trials. If decisions had to be made, they were all documented in the log and the more conservative option was adopted at all times. If only standard errors of the mean were given, or statistical results, such as p-values, the necessary data were derived from the standard statistical formulae. As a denominator to calculate Hedge's g, the pooled standard-deviation was used.
The statistical model was defined beforehand as a fixed model, if heterogeneity should turn out to be small. But we expected high heterogeneity from the outset and thus opted for a random effects model. Thus the analytical logic followed the rule “calculate a fixed effects model, if heterogeneity is high calculate a random effects model that incorporates heterogeneity, and conduct sensitivity analyses to study the source of heterogeneity” (1, 23). All calculations were done with comprehensive meta-analysis (24).
Our search strategy yielded 23 separate publications (25–47) which produced 29 distinct comparisons. One study was so badly documented (26) that no data could be extracted and was rejected, leaving 22 publications and 28 comparisons. In nine comparisons active controls were used, and anti-inflammatory drugs were used in all but one case, where Arnica was compared to Metronidazole, and in one case Arnica was compared to no treatment. Eighteen placebo-controlled trials were found. Three of the active comparator studies were controlled clinical trials, i.e. it was unclear whether they are randomized or a pseudo-randomization procedure, such as alternating patients, was used. One study was nonrandomized and patients could choose the treatment, all others (n = 24) were randomized trials. Descriptions of the studies are presented in Table 1 (included studies) and Supplementary Table (excluded studies: Supplementary Material 3).
Table 2 gives the results of the main meta-analyses and sensitivity analyses, and Figure 2 presents the main analysis as forest plot. The overall analysis of all comparisons with placebo yielded a small effect size just missing formal significance of Hedge's g = 0.18 (z = 1.89; p = 0.059), which was chosen, because the fixed effects model produced a very heterogeneous summary (I2 = 63.2; g = 0,13; z = 2.29; p = 0.02).
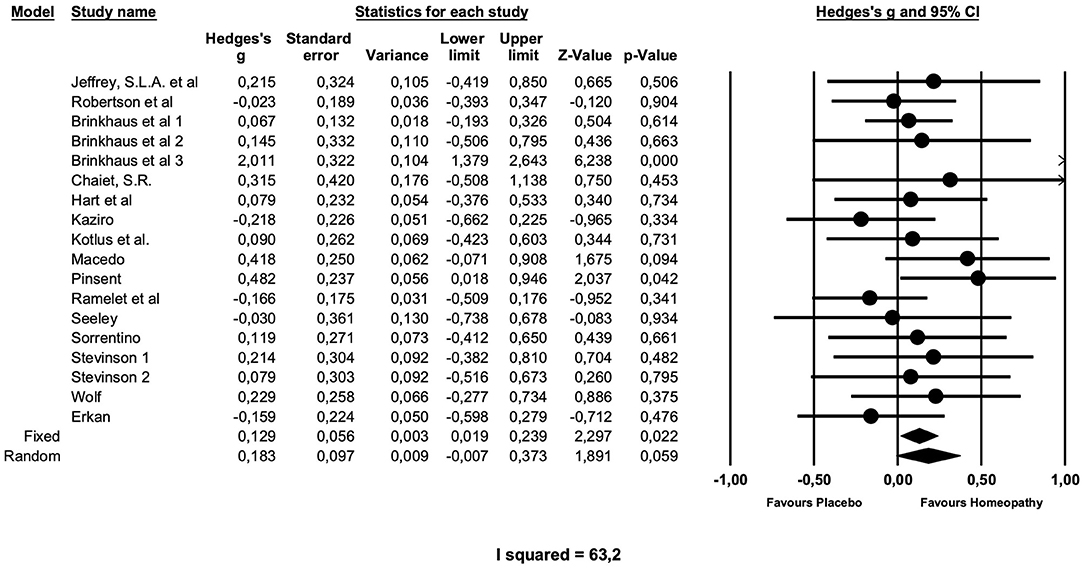
Figure 2. Forest plot of main meta-analysis of placebo-controlled trials of arnica in postoperative complications.
A sensitivity analysis showed that the 16 preventive trials produced an effect size of similar magnitude, which is not significant (g = 0.20; z = 1.9; p = 0.06). The two treatment studies, both of low quality (31, 40), showed no effect (g = 0.04; z = 0.8; p = 0.8) over placebo.
Studies with higher potencies produced a smaller effect size (g = 0.18; z = 1.81 p = 0.07) than the single study with a low potency (g = 0.21), which is, however, not significant.
Interestingly, studies of higher quality, as determined by the Cochrane risk of bias and the quality assessment tool for quantitative studies by Thomas et al., produced a higher effect size, compared with studies of low to moderate quality. The latter (n = 10) had a smaller Effect size of g = 0.09, which is not significant and not heterogeneous. High quality studies (n = 8) have an effect size of g = 0.29, which just misses formal significance (p = 0.057).
Metaregressions exploring whether year of study or study size have any influence on effect sizes were not significant, nor was a meta-regression on duration of study (data not shown).
Publication bias does not seem to account for the positive effect of homeopathy vs. placebo. Eggers regression test is not significant (intercept 2.07; confidence interval −0.6/4.77). Duwal and Tweedie's trim and fill method does not impute missing studies to the left of the mean, i.e,. favoring placebo, only to the right of the mean. This is not a realistic assumption as it would mean that seven studies favoring homeopathy over placebo would have gone unpublished, raising the effect size to g = 0.39. The funnel plot does not exhibit a distortion (Figure 3).
The effect size for Arnica vs. comparators other than placebo, most of them active comparator studies, is highly heterogeneous and therefore a random effects model is used. This is non-significant with g = 0.28.
We tried to identify the source of this heterogeneity. If the single study, which compared against no-treatment (but presumably allowed conventional pain medication), is removed from the analysis, the overall result remains unchanged (sensitivity analysis 4, Table 2).
The type of comparator is one differentiating factor. The two studies that use standard care as a comparator have a larger and significant effect size, which is homogeneous of g = 0.5 (p = 0.03). Since they are at the same time part of the non-randomized study set this is likely the more important moderator (sensitivity analysis 6, Table 2), and non-randomized studies exhibit a significant effect size of g = 0.49.
The way how homeopathy is used, preventive or as a formulaic treatment, changes the results (sensitivity analysis 7, Table 2). The preventive use produces a non-significant effect size of g = 0.23, therapeutic use a higher effect size of g = 0.35, which is, however not significant.
High quality studies produce a higher effect size than lower quality studies (sensitivity analysis 8, Table 2). However, these effect sizes are not significant.
The comparison of potencies does not clarify heterogeneity, as both groups of studies, with high and low potencies, have similar, non-significant effect sizes (high potencies: g = 0.15; low potencies: g = 0.22; sensitivity analysis 9, Table 2; one study with undeclared potency had a high and significant effect size of g = 1.1).
The metaregression of study year on effect size is significant with a significant slope of 0.03 (z = 2.1, p = 0.04; maximum likelihood estimation, Figure 4; only clearly designated active comparator trials used, which are at the same as the randomized studies). The metaregression of duration of study on the effect size is not significant (data not shown).
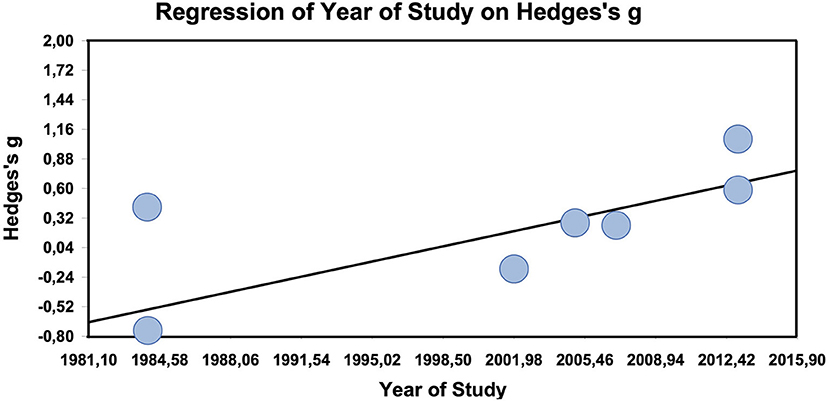
Figure 4. Meta-regression of year of study on effect size in other-than-placebo-controlled trials (n = 7).
In this metaanalysis of studies testing homeopathic Arnica as a medication to prevent and treat postoperative pain, bleeding, swelling, and discomfort after surgery, we found a small effect size of g = 0.18 in 18 placebo-controlled trials that missed conventional significance by a small margin, and a non-zero, not significant effect size in active comparator studies, which is likely inflated, because non-randomized studies of lower quality contribute with a large effect size.
In comparison with a recently published systematic review on perioperative Arnica (tinctures and homeopathic potencies), our analysis adds the quantitative cumulative effects of homeopathic Arnica compared the placebo to the descriptive results and summarizes for the first time the clinical effects of homeopathic Arnica compared with an active agent (17).
The outcomes used to document this effect in trials were varied and ranged from pain, which was documented in most of the studies, to swelling, edema, discomfort, or physical measures of range of movement. The studies were most often of smaller surgical interventions, such as carpal tunnel surgery, oral-facial, or dental surgery. The heterogeneity is high in these studies, and clearly limits the results, though it can only be partially explained according to our analysis. Studies that are of better quality have a higher effect size. The findings of sensitivity analyses are in themselves not very robust, as they are based on only a few studies or are not significant. Preventive usage has a lower effect size.
Clinical heterogeneity due to the variety of surgical procedures may have contributed to the wide range of outcome measures. As our analysis model did not separate different types of outcome measures, one might argue that this has conflated effects. This could be true, as effect sizes within studies vary quite a lot. However, since there was no uniform best set of measures that would have been available in all studies, we deemed it better to adopt a conservative strategy of synthesis of all information rather than lose a lot of information for the sake of consistency. Even pain measures varied widely, from real visual analog scale, to nurse-reported ratings, and to area under the curve measures of all pain data. Thus, it would have been possible, but impractical to analyze certain outcome types separately and would likely not have changed the overall result considerably. Thus, our attempt to rather include all information and estimate a robust effect is an analytical decision that might have erred toward the conservative side. If only those studies that used placebo-controls and VAS measures of pain are considered descriptively, then the effect of Arnica can be quantified as lying between a reduction of 5 and 9 mm visual analoque scale (VAS) pain rating.
The active comparator trials produced a small, but non-significant and heterogeneous effect size. In the active comparator trials, the only clear moderator is whether studies were randomized or not. If they were, the effect size converges against zero, i.e., there is no difference between homeopathy and active treatment. Thus, homeopathy seems to work as well as anti-inflammatory substances, which makes sense, as these substances also have an effect over and against placebo (48, 49). Interestingly, the metaregression suggests that in active comparator studies effect sizes against the comparators tend to increase significantly by 0.03 points per year.
The effect size of the overall effect is just missing conventional significance and is small. This lack of formal significance is due to the high heterogeneity of the trials. Sensitivity analyses, see below, can clarify some of this heterogeneity. From a homeopathic point of view, a part of this might be due to the fact that Arnica is only partially indicated in surgical wounds, despite its popularity. The established homeopathic indication is blunt trauma, lacerated, internal wounds, and bleeding from such wounds (2–5). Surgical wounds with clear cuts, where tissues have not been “violently stretched” (5) are a different indication, but due to the popularity of Arnica and because it seems such an easy indication to study, a lot of studies investigated Arnica in surgery without profound homeopathic reasoning. Our analysis shows that it is possible to use Arnica effectively in surgery, but not necessarily optimal. The variation in effect sizes is large. In some cases Arnica might be counter-indicated, especially if used in a preventive mode, as it might facilitate bleeding (11) and diminish fibrinogen (50). Only two trials were included in the sensitivity analysis of therapeutic use of Arnica with placebo-controlled trials. This might explain, why this effect is near-zero and non-significant. The majority of placebo controlled trials were preventive.
It might be worthwhile, therefore, to study other remedies for surgery, such as Staphisagria or Bellis perennis, either alone or in combination. As we included only pure Arnica studies, some studies were not included that used Arnica in combination with other remedies, which might have had clinically more interesting results (51–53). We propose to analyze these separately.
The studies that had been conducted so far were rather small. The largest study included 237 patients, and only four studies had more than 100 participants. Considering the small overall effect size, the individual power of studies was small, which is part of the reason why this intervention is debated. However, a metaregression of study size on effect size did not reveal a significant slope, although the slope was slightly negative. Thus, study size in itself does not have any influence on effect size estimation.
Publication bias is an unlikely explanation for our findings, as the analytical methods of publication bias used point out that either an unrealistically large number of studies would have gone unpublished or studies favoring homeopathy were unpublished, none of which is a reasonable assumption.
Active comparator trials compared Arnica against standard anti-inflammatories, such as ibuprofen, paracetamol, or diclofenac. The overall effect size is positive but not significant, favoring Arnica. This is mainly due to the large effect sizes of non-randomized trials, and hence likely overestimated. However, looking only at the randomized trials presents a non-significant small negative effect size. This suggests that overall Arnica and antiinflammatories are largely comparable.
The effect size is clinically small. Arnica in itself might be a suboptimal homeopathic indication for bleeding and swelling after surgery. Thus, an analysis summarizing studies with other remedies or combinations might be warranted. However, considering that Arnica is very cheap and an easy intervention, one might consider it worthwhile in cases where patients ask for it. Since the effect size is larger, when used therapeutically, and because of the underlying rationale, we would recommend using it only therapeutically, not preventively.
We conclude that homeopathic Arnica produces a small effect size across a series of 18 placebo-controlled trials, which just misses significance, and it is equal in effectiveness to conventional non-steroidal anti-inflammatory drugs in the treatment of postoperative pain, swelling, and functional limitations.
The data analyzed in this study is subject to the following licenses/restrictions:The data was taken from the results of published clinical studies. The data-set is available from the authors on request. Requests to access these datasets should be directed to Katharina Gaertner, a2F0aGFyaW5hLmdhZXJ0bmVyQHVuaS13aC5kZQ==.
The study was performed according to Swiss law. As a research project analysing using literature data only, the analyses do not require ethical approval. Before any data-analyses were carried out, the project was registered with the international prospective register of systematic review (PROSPERO) on April 28th 2020. All consecutive procedures were logged in a protocol (procedural minutes).
Literature search and screening procedures was done by KG. Data extraction and quality assessments were performed by KG and HW. HW executed calculations and drafted large parts of the manuscript. All authors complemented the manuscript and approved the final version.
This investigation was funded by Förderverein komplementärmedizinische Forschung, Arlesheim, Switzerland. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
KG is a medical doctor with special education in homeopathy.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.680930/full#supplementary-material
1. Cooper H, Hedges LV. In: Cooper H, Hedges LV, editors. Handbook of Research Synthesis. New York: Russell Sage Foundation. (1994).
3. Boericke W. Homöopathische Mittel und ihre Wirkungen. Materia Medica und Repertorium Leer: Grundlagen & Praxis. (1973).
5. Hahnemann S. Reine Arzneimittellehre. Unveränderter Nachdruck der Ausgabe letzter Hand. Dresden und Leipzig 1827. Bd. 1-6. Heidelberg: Haug; (1991).
6. Hahnemann S. Organon der Heilkunst. Standard edition of 6th ed. Schmidt JM, editor. Heidelberg: Haug; (1996/1999). p. 388.
7. Walach H. The pillar of homoeopathy: remedy provings in a scientific framework. Br Homoeopathic J. (1997) 86:219–24. doi: 10.1016/S0007-0785(97)80048-8
8. Walach H, Teut M. Scientific proving of ultra high dilutions on humans. Homeopathy. (2015) 104:322–7. doi: 10.1016/j.homp.2015.08.008
9. Mathie RT, Lloyd SM, Legg LA, Clausen J, Moss S, Davidson JR, et al. Randomised placebo-controlled trials of individualised homeopathic treatment: systematic review and meta-analysis. Syst Rev. (2014) 3:142. doi: 10.1186/2046-4053-3-142
10. Blumenthal M, Goldberg A J B. Herbal medicine: expanded Commission E monographs. Newton, MA: Integrative Medicine Communications. (2000).
11. Kouzi SA, Nuzum DS. Arnica for bruising and swelling. Am J Health Syst Pharm. (2007) 64:2434–43. doi: 10.2146/ajhp070155
12. Hofmeyr GJ, Piccioni V, Blauhof P. Postpartum homoeopathic arnica montana: a potency-finding pilot study. Br J Clin Pract. (1990)44:619–21.
13. Iannitti T, Morales-Medina JC, Bellavite P, Rottigni V, Palmieri B. Effectiveness and safety of arnica montana in post-surgical setting, pain and inflammation. Am J Ther. (2016) 23:e184–97. doi: 10.1097/MJT.0000000000000036
14. Vickers AJ, Fisher P, Smith C, Wyllie SE, Rees R. Homeopathic Arnica 30X is ineffective for muscle soreness after longdistance running: a randomized, double-blind, placebo-controlled trial. Clin J Pain. (1998) 14:227–31. doi: 10.1097/00002508-199809000-00009
15. Tveiten D, Bruset S, Borchgrevink C, Norseth J. Effects of the homoeopathic remedy Arnica D30 on marathon runners: a randomized, double-blind study during the 1995 Oslo Marathon. Complement Ther Med. (1998) 6:71–4. doi: 10.1016/S0965-2299(98)80078-2
16. Ernst E, Pittler MH. Efficacy of homeopathic arnica. A systematic review of placebo-controlled clinical trials. Arch Surg. (1998) 133:1187–90. doi: 10.1001/archsurg.133.11.1187
17. Knackstedt R, Gatherwright J. Perioperative homeopathic arnica and bromelain current results and future directions. Ann Plast Surg. (2020) 84:E10–E5. doi: 10.1097/SAP.0000000000002043
18. Gaertner K, Walach H, Baumgartner S, Frass M. Update of empirical evidence: frame-work protocol for the systematic evaluation of homeopathic intervention studies (HOMIS) in humans. Version 1.0 (2020). Available online at: https://zenodo.org/record/4066778#.X3subC2w2-U.
19. Thomas BHC D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evidence Nurs. (2004) 1:176–84. doi: 10.1111/j.1524-475X.2004.04006.x
21. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
22. Chinn S A. simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. (2000) 19:3127–31. doi: 10.1002/1097-0258(20001130)19:22<3127::AID-SIM784>3.0.CO;2-M
24. Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-Analysis Version 2.0. (2011).
25. Brinkhaus B, Wilkens JM, Ludtke R, Hunger J, Witt CM, Willich SN. Homeopathic arnica therapy in patients receiving knee surgery: results of three randomised double-blind trials. Complement Ther Med. (2006) 14:237–46. doi: 10.1016/j.ctim.2006.04.004
26. Camacho C LS, Melo M, Pedraza C, Vanegas S, Benítez G, Palencia R, Revelo I, (eds). Effectiveness of homeopathic medicine Arnica 7CH versus Naproxen on post operative extraction of third molar including pain relief. In: 63rd Congress of the Liga Medicorum Homoeopathica Internationalis. Oostende, Belgium: Liga Medicorum Homoeopathica Internationalis. (2008).
27. Chaiet SR, Marcus BC. Perioperative Arnica montana for reduction of ecchymosis in rhinoplasty surgery. Ann Plast Surg. (2016) 76:477–82. doi: 10.1097/SAP.0000000000000312
28. Erkan E, Parpar K, Develi T, Gündogar M, Gürler G. The efficacy of homeopathic Arnica montana 200 CH on dental surgical treatments: a double-blind, placebo-controlled study. Eur Res J. 5:793–99 (2018). doi: 10.18621/eurj.417262
29. González Sánchez AM GHJ, Rivaflecha GF, Morales Alcolea Y. Efectividad de remedios homeopáticos en niños operados de estrabismo. Medisan. (2014) 18:1310–6. Available online at: http://scielo.sld.cu/pdf/san/v18n10/san011810.pdf
30. Hart O MM, Lewith G, Miller J. Double-blind, placebo-controlled, randomized clinical trial of homoeopathic arnica C30 for pain and infection after total abdominal hysterectomy. J R Soc Med. (1997) 90:73–8. doi: 10.1177/014107689709000205
31. Jeffrey S BH. Use of Arnica to relieve pain after carpal-tunnel release surgery. Altern Ther Health Med. (2002) 8:66–8.
32. Karow JH, Abt HP, Frohling M, Ackermann H. Efficacy of Arnica montana D4 for healing of wounds after Hallux valgus surgery compared to diclofenac. J Altern Complement Med. (2008) 14:17–25. doi: 10.1089/acm.2007.0560
33. Kaziro GSN. Metronidazol (Fagyl) and arnica montana in the prevention of post-surgical complications, a comparative placebo controlled clinical trial. Br J Oral Maxillofac Surg. (1984) 22:42–9. doi: 10.1016/0266-4356(84)90007-X
34. Kotlus BS, Heringer DM, Dryden RM. Evaluation of homeopathic Arnica montana for ecchymosis after upper blepharoplasty: a placebo-controlled, randomized, double-blind study. Ophthal Plast Reconstr Surg. (2010) 26:395–7. doi: 10.1097/IOP.0b013e3181cd93be
35. Macedo S CJ, Ferreira L, Dos Santos-Pinto R. Effect of Arnica montana 6 cH on edema, mouth opening and pain in patients submitted to extraction of impacted third molars. Ärztezeitschrift für Naturheilverfahren. (2005) 46:381–7.
36. Pinsent RJFHB GPI, Ives G, Davey RW, Jonas S. Does Arnica reduce pain and bleeding after dental extractions? Brit Hom Res. (1986) 15:3–11.
37. Pöllmann L HG. Zur Gabe von Arnika, Planta tota D3, bei kieferchirurgischen Eingriffen. Erfahrungsheilkunde. (1993) 7:503–7.
38. Puerto Huerta MdCI L, Cañete Villafranca CR. Arnica montana en el tratamiento del dolor después de la odontectomía de terceros molares retenidos. Medisan. (2015) 19:619. Available online at: http://scielo.sld.cu/pdf/san/v19n5/san07195.pdf
39. Ramelet A BG, Lorenz P, Imfeld M. Homoeopathic Arnica in postoperative haematomas: a double-blind study. Dermatology. (2000) 201:347–8. doi: 10.1159/000051551
40. Robertson A, Suryanarayanan R, Banerjee A. Homeopathic Arnica montana for post-tonsillectomy analgesia: a randomised placebo control trial. Homeopathy. (2007) 96:17–21. doi: 10.1016/j.homp.2006.10.005
41. Seeley B DA, Ahn M, Maas C. Effect of homeopathic arnica montana on bruising in face-lifts: results of a randomized, double-blind, placebo-controlled clinical trial. Arch Facial Plast Surg. (2006) 8:54–9. doi: 10.1001/archfaci.8.1.54
42. Sorrentino L, Piraneo S, Riggio E, Basilico S, Sartani A, Bossi D, et al. Is there a role for homeopathy in breast cancer surgery? A first randomized clinical trial on treatment with Arnica montana to reduce post-operative seroma and bleeding in patients undergoing total mastectomy. J Intercult Ethnopharmacol. (2017) 6:1–8. doi: 10.5455/jice.20161229055245
43. Souza LM. Ação Anti-Edematosa: Arnica montana 6ch X Diclofenaco de Sódio 50 mg. Pesquisa Brasileira em Odontopediatria e Clínica Integrada. (2011) 11:491–6. doi: 10.4034/PBOCI.2011.114.06
44. Stevinson C DV, Fountain-Barber A, Hawkins S, Ernst E. Homeopathic arnica for prevention of pain and bruising: randomized placebo-controlled trial in hand surgery. J R Soc Med. (2003) 96:60–5. doi: 10.1258/jrsm.96.2.60
45. Totonchi A, Guyuron B. A randomized, controlled comparison between arnica and steroids in the management of postrhinoplasty ecchymosis and edema. Plast Reconstr Surg. (2007) 120:271–4. doi: 10.1097/01.prs.0000264397.80585.bd
46. Wolf M LR, Rose O. Adjuvante Arnica-Medikation zur schwellungs- und schmerzreduzierenden Wirkung bei operativ versorgten hüftgelenksnahen Frakturen: Kontrollierte, nicht randomisierte Therapiestudie. AHZ. (2002) 247:141–4. doi: 10.1055/s-2006-936804
47. Wolf M TC, Mayer W, Heger M. Wirksamkeit von Arnica bei Varizenoperation: Ergebnisse einer randomisierten, doppelblinden, Placebo-kontrollierten Pilot-Studie. Forsch Komplementarmed Klass Naturheilkd. (2003) 10:242–7. doi: 10.1159/000074778
48. McNicol ED, Ferguson MC, Schumann R. Single-dose intravenous diclofenac for acute postoperative pain in adults. Cochrane Database Syst Rev. (2018) 8:CD012498. doi: 10.1002/14651858.CD012498.pub2
49. Derry S, Wiffen PJ, Moore RA. Single dose oral diclofenac for acute postoperative pain in adults. Cochrane Database Syst Rev. (2015) 2015:CD004768. doi: 10.1002/14651858.CD004768.pub3
50. Baillargeon L, Drouin J, Desjardin L, Leroux D, Audet D. Les effets de l'Arnica montana sur la coagulation sanguine: essai clinique randomisé. Le Médecin de famille canadien. (1993) 39:2362–7.
51. Lotan AM, Gronovich Y, Lysy I, Binenboym R, Eizenman N, Stuchiner B, et al. Arnica montana and Bellis perennis for seroma reduction following mastectomy and immediate breast reconstruction: randomized, double-blind, placebo- controlled trial. Eur J Plastic Surg. (2020) 43:285–94. doi: 10.1007/s00238-019-01618-7
52. Albertini H, Goldberg W, Sanguy B, Toulza C. Bilan de 60 observations randomisées - Hypericum-Arnica contre placébo dans les névralgies dentaires. Homéopathie. (1984) 1:47–9.
Keywords: Arnica montana, wound healing, pain, homeopathy, meta-analysis
Citation: Gaertner K, Baumgartner S and Walach H (2021) Is Homeopathic Arnica Effective for Postoperative Recovery? A Meta-analysis of Placebo-Controlled and Active Comparator Trials. Front. Surg. 8:680930. doi: 10.3389/fsurg.2021.680930
Received: 15 March 2021; Accepted: 05 November 2021;
Published: 17 December 2021.
Edited by:
Sergio Olate, University of La Frontera, ChileReviewed by:
Fatih Zor, Wake Forest School of Medicine, United StatesCopyright © 2021 Gaertner, Baumgartner and Walach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katharina Gaertner, a2F0aGFyaW5hLmdhZXJ0bmVyQHVuaS13aC5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.