
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 14 July 2021
Sec. Visceral Surgery
Volume 8 - 2021 | https://doi.org/10.3389/fsurg.2021.678853
This article is part of the Research Topic Case Reports in Visceral Surgery: Tumors of the Liver View all 7 articles
Background: Neuroendocrine tumors are heterogeneous malignancies that originate from the neuroendocrine system. Previous studies show that this cancer type mainly localizes in the gastrointestinal tract and often metastasizes to the liver. Primary liver neuroendocrine tumors are very rare and primary hepatic neuroendocrine tumors (PHNET) with concurrent hepatocellular carcinoma (HCC) are extremely rare. To the best of our knowledge, only few PHNET cases have been identified, making their diagnosis difficult. Here, we report the biggest ever reported and “deceiving” lesion of a mixed neuroendocrine-non-neuroendocrine neoplasm in the liver, aiming to raise awareness and improve treatment of the disease.
Case Presentation: Here, we report a preoperative misdiagnosed case that presented with hepatocellular carcinoma clinical features and no extrahepatic tumors. Postoperative pathology confirmed that it was a mixed neuroendocrine-non-neuroendocrine neoplasm. The patient was then referred for etoposide and cisplatin-based chemotherapy. No disease recurrence was observed at the 6-month follow-up.
Conclusion: We report a very rare and easily misdiagnosed case and we speculate that there were “undifferentiated cells” undergoing neuroendocrine and hepatocellular carcinoma differentiation, during which some hepatocellular carcinoma cells express neuroendocrine features. We recommend proper surgery and postoperative platinum-based chemotherapy in the management of this disease.
Neuroendocrine tumors are heterogeneous malignancies that originate from the neuroendocrine system. Previous studies show that this cancer type mainly localizes in the gastrointestinal tract, including the small intestine (30.8%), rectum (26.3%), colon (17.6%), pancreas (12.1%), and appendix (5.7%), and often metastasizes to the liver (1). Primary liver neuroendocrine tumors are very rare and primary hepatic neuroendocrine tumors (PHNET) with concurrent hepatocellular carcinoma (HCC) are extremely rare. To the best of our knowledge, only few PHNET cases have been identified. It is rarer the two components occur simultaneously, making their diagnosis difficult. Here, we report the biggest ever reported lesion of a mixed neuroendocrine-non-neuroendocrine neoplasm confirmed by postoperative pathology in the liver, aiming to raise awareness and improve treatment of the disease.
A 39-year-old man without known, significant medical history, was admitted to our department with >2 months of anorexia. The patient mainly complained of the discovery of focal liver lesions for 7 days. He denied any tobacco or alcohol use and any treatments. His family history did not reveal liver disease. At admission, the patient was afebrile and had normal vital signs. Physical examination yielded normal findings. Laboratory tests revealed: total bilirubin = 23.7 μmol/L↑ (reference range: 5–21 μmol/L), direct bilirubin = 5.6 μmol/L (reference range: 0–7 μmol/L), indirect bilirubin = 18.1 μmol/L↑ (reference range: 1.5–18 μmol/L), γ-glutamyl transpeptidase = 125U/L↑ (reference range: 8–57 U/L), AFP serum levels = 22468.30 ng/mL↑ (reference range: 0–6.6 ng/mL), and normal CA19-9 (carbohydrate antigen 19–9) and CA125 (carbohydrate antigen 125) serum levels. The patient was HBsAg (hepatitis B surface antigen) and HBc-Ab (hepatitis B core antibody) positive and had a hepatitis B DNA copy number of 1.03E3IU/mL↑. Abdominal computed tomography (CT) (Figure 1) revealed marked liver enlargement and a large soft tissue mass on the right hepatic lobe with an estimated volume of 17.4 × 16.1 × 20.1 cm. The mass exhibited uneven internal density with multiple dotted high-density and flaky low-density shadows. The lesion was enhanced in the early phase and washed out in the delayed phase. Display of the right branch of portal vein was unclear. Given these results, hepatic cancer with portal vein cancerous thrombus was suspected.
On the 3rd day after admission, TACE (transcatheter arterial chemoembolization) was used to embolize the tumor feeding arteries to slow tumor development. An emulsion of oxaliplatin (50 mg) and lipiodol emulsion (20 mL) was administered via the feeding arteries and embolization performed using a gelatin sponge. The patient was then put on home-based recuperation. After 2 weeks, serum bilirubin was observed to have returned to normal levels, while AFP levels fell to 458.20 ng/mL relative to 22468.30 ng/mL at admission (reference range: 0–6.6 ng/mL). Figure 2 was a reexamination of CT 2 weeks after TACE, in which tumor growth was not observed and a large portion of the tumor was necrotic, and good results were achieved. Then an extended right hemihepatectomy laparotomy was performed by laparotomy under general anesthesia. During surgical exploration, laparotomy revealed the mass's bumpy surface. The remaining liver tissue was normal without obvious pathological changes. The operation did not reveal asities or liver cirrhosis. Extensive abdominal exploration did not find additional primary tumor sites.
The resected liver tissue had a volume of 26 × 23 × 12 cm (Figure 3). Multi-section incision revealed a 21 × 20 × 12 cm mass that was gray-yellow/gray-brown, and soft to touch, with geographic necrosis. The rest of the liver tissue section was grayish red. Microscopically, the neoplastic cells were disposed in nets and sheets. The tumor is mainly comprised of neoplastic cells with enlarged nuclei, inconspicuous nucleoli, and granular chromatin, arranged in nests or rosette structures. Nuclear molding change and mitoses were frequently observed (Figure 4A). The resected liver tissue had a volume of 26 × 23 × 12 cm (Figure 3). Multi-section incision revealed a 21 × 20 × 12 cm mass that was gray-yellow/gray-brown, and soft to touch, with geographic necrosis. The rest of the liver tissue section was grayish red. Microscopically, the neoplastic cells were disposed in nets and sheets. The tumor is mainly comprised of neoplastic cells with enlarged nuclei, inconspicuous nucleoli, and granular chromatin, arranged in nests or rosette structures. Nuclear molding change and mitoses were frequently observed (Figure 4A). A small nest strongly expressed SYN on the cell membrane (Figure 4F). The small round cells on the right side of the dotted line lost this staining pattern, where the cells on the left side of the dotted line still remained this pattern. The cells outside the dotted line expressed GPC and AFP diffusely (Figures 4C,D). And the small nest showed weak AFP expression. All small round cells showed CgA positivity (Figure 4E). The Ki-67 ratio was about 70%. Postoperative pathological findings (Figure 4) confirmed a mixed neuroendocrine-non-neuroendocrine neoplasm. Postoperative pathological findings (Figure 4) confirmed a mixed neuroendocrine-non-neuroendocrine neoplasm. The patient was referred to an internist for etoposide and cisplatin-based chemotherapy. At 6-month follow-up, no recurrence was seen and the patient had remained disease-free.
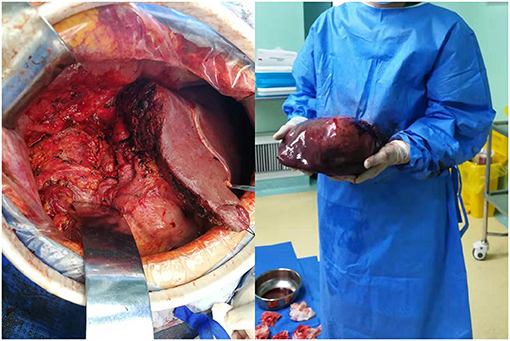
Figure 3. Tumor appearance. (Left) Operating field after excision of right, caudate and quadrate lobe of liver; (Right) gross specimen.
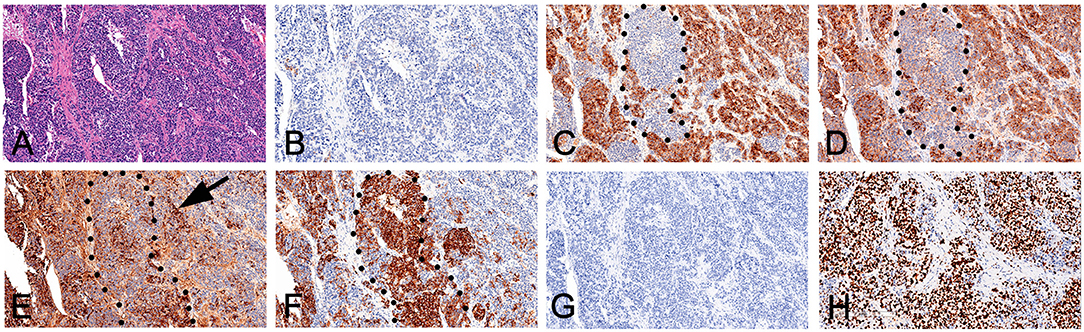
Figure 4. Immunohistochemical expression. (A) HE staining; (B) Hepatocyte(+); (C) Glypican-3 (+); (D) AFP (+); (E) CgA (+); (F) SYN (+); (G) Arginase-1 (–); and (H) KI-67.
Here, we report a very rare and easily misdiagnosed case. To the best of our knowledge, this is so far, the largest recorded mixed neuroendocrine-non-neuroendocrine neoplasm. Multiple points deserve close attention. First, clinical examination was highly suggestive of hepatocellular carcinoma (HCC) (1). The lesion was enhanced in the early phase and washed out in the delayed phase with possible simultaneous portal vein invasion (2). The patient was infected with HBV and had abnormal AFP levels. However, postoperative pathology revealed a mixed neuroendocrine-non-neuroendocrine neoplasm, highlighting the importance of pathological diagnosis. Second, some cells were positive for neuroendocrine carcinoma (NEC) and HCC markers, indicating that some “undifferentiated cells” were plastic during the differentiation process, irrespective of whether they were hepatic malignant tumor cells or hepatic progenitor cells.
Primary neuroendocrine tumors with HCC in the liver are extremely rare (2). The first case of HCC with carcinoid tumors was reported in 1984 (3). The lesions fall into 2 classes, collision and combined (4). Collision type tumors are distinguished by fibrillar component, while in combined type tumors the 2 features are mixed and cannot be recognized. Microscopically, they fall into 3 types, transitional, intermediate, and separate. The former 2 types represent colocalization of neuroendocrine components and non-neuroendocrine components. In the transitional type, NEC and HCC components intermingle in transitional areas. In the intermediate type, intermediate components simultaneously express hepatocyte markers and neuroendocrine markers, intermingling with the NEC and HCC components. In the separate type, the 2 features occur independently (5). Neuroendocrine tumors are known to mainly localize in the gastrointestinal tract, including the small intestine (30.8%), rectum (26.3%), colon (17.6%), pancreas (12.1%), and appendix (5.7%), and to most frequently metastasize to the liver (1). Primary neuroendocrine tumors in the liver are very rare. Information about primary mixed NEC and HCC is summarized in Table 1 (2–4, 6–18). Most primary neuroendocrine tumor patients have underlying liver disease. The presence of hepatitis B and C virus suggests a chronic process. Most patients are middle-aged and elderly men and the majority have the combined type. Tumor markers are usually manifested with primary liver cancer's characteristics, evaluated AFP and normal CA125/199 levels.
For our patient, immunohistochemical analysis revealed the NEC component to be positive for both CgA and SYN, and negative for Glypain-3, which is usually positive in HCC. Interestingly, some HCC components were positive for neuroendocrine markers (Figure 4E, Arrow). Moreover, NEC cells were poorly positive for AFP.
Where do these NEC cells come from? Gould et al., have suggested that they may originate from neuroendocrine differentiation of a single malignant stem cell or a precursor of other hepatic malignant tumors (19). Pettinato et al. hypothesized that hepatic progenitor cells translocate to the intrahepatic bile duct epithelium during embryonic development and may have the capacity to progress into NECs (6). Both hepatic malignant tumors or hepatic progenitor cells are capable of differentiating toward a hepatocellular or biliary fate. Thus, we speculate that benign or malignant undifferentiated cells may have undergone hepatocellular and neuroendocrine differentiation during proliferation, with the 2 components intermingling. As Figure 4 showed, hepatocellular dominant areas were strongly positive for hepatocyte-related antibodies and poorly positive for neuroendocrine markers. In neuroendocrine dominant areas, the reverse was observed. While intermediate cells stained positive for both hepatocyte and neuroendocrine markers. Due to their merging, it was impossible to clearly define the cancer type. Based on 2019 WHO classification of digestive system tumors (20), we diagnosed the case as a mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN). Thus, some PHNET with AFP or other index changes may not be real PHNET, but hepatic progenitor cells undergoing hepatocellular neuroendocrine differentiation, and may fall under the MiNEN category.
Surgical resection is the first choice of treatment in cases of pure PHNET (21, 22). Studies by Givi et al. have shown that the median survival time of patients undergoing surgery is about 159 months, while that of patients without surgical treatment is only 47 months (23). However, the prognosis of HCC with PHNET remains unclear due to its rarity. A significantly higher Ki-67 proliferative index in NEC relative to HCC suggests a poorer prognosis (5). In PHNET, laboratory tests reveal some tumor markers, including AFP, CEA, and CA-199 to often be within the normal range. Indicators of hepatitis, cirrhosis, and other hepatic diseases are also negative. However, these indicators change accordingly when PHNET combines with HCC. Final diagnosis still requires histopathological analysis and careful exclusion of extrahepatic primary tumor.
In conclusion, we reported a rare preoperative misdiagnosis case showing deceiving clinical features. Postoperative pathology confirmed the final diagnosis. We speculate that there were “incomplete differentiation cells” undergoing neuroendocrine and hepatocellular carcinoma differentiation because of HBV infection or other chronic hepatic diseases, which could explain some hepatocellular carcinoma cells could express neuroendocrine features simultaneously. We recommend proper surgery and postoperative platinum-based chemotherapy in the management of this disease. We will also conduct a long-term follow-up of the patient in this article to better understand the disease.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
This case report was approved in full by the Ethics Committee of the Zhongnan Hospital of Wuhan University (Wuhan, China). Written informed consent was obtained from this patient. Data were collected from the daily medical nursing records by staff experienced in gathering clinical information.
JL and QL designed the idea. XQ and BC collected the data. JL and DG processed the data and wrote the manuscript. All authors have read and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank QL, from the Department of Hepato-Biliary Pancreatic Surgery, Zhongnan Hospital of Wuhan University, for performing the surgery.
PHNET, Primary hepatic neuroendocrine tumor; AFP, alpha-fetoprotein; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 199; CA125, carbohydrate antigen 125; NEC, neuroendocrine carcinoma; HCC, Hepatocellular carcinoma; SCNEC, small cell neuroendocrine carcinoma; LCNEC, large cell neuroendocrine carcinoma; MiNEN, mixed neuroendocrine–non-neuroendocrine neoplasm.
1. Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. (2018) 68:471–87. doi: 10.3322/caac.21493
2. Garcia MT, Bejarano PA, Yssa M, Buitrago E, Livingstone A. Tumor of the liver (hepatocellular and high grade neuroendocrine carcinoma): a case report and review of the literature. Virchows Arch. (2006) 449:376–81. doi: 10.1007/s00428-006-0251-0
3. Barsky SH, Linnoila I, Triche TJ, Costa J. Hepatocellular carcinoma with carcinoid features. Hum Pathol. (1984) 15:892–4. doi: 10.1016/S0046-8177(84)80152-5
4. Yang C-S, Wen M-C, Jan Y-J, Wang J, Wu C-C. Combined primary neuroendocrine carcinoma and hepatocellular carcinoma of the liver. J Chin Med Assoc. (2009) 72:430–3. doi: 10.1016/S1726-4901(09)70400-9
5. Nomura Y, Nakashima O, Akiba J, Ogasawara S, Fukutomi S, Yamaguchi R, et al. Clinicopathological features of neoplasms with neuroendocrine differentiation occurring in the liver. J Clin Pathol. (2017) 70:563–70. doi: 10.1136/jclinpath-2016-203941
6. Pettinato G, Manivel JC, Saldana MJ, Peyser J, Dehner LP. Primary bronchopulmonary fibrosarcoma of childhood and adolescence: reassessment of a low-grade malignancy. Clinicopathologic study of five cases and review of the literature. Hum Pathol. (1989) 20:463–71. doi: 10.1016/0046-8177(89)90012-9
7. Tajima Y, Nakajima T, Sugano I, Nagao K, Kondo Y, Saito J. Hepatocellular carcinoma containing endocrine cells. An autopsy report of triplecancer involving the liver, kidney and thyroid. Acta Pathol Jpn. (1992) 42:904–10. doi: 10.1111/j.1440-1827.1992.tb01897.x
8. Ishida M, Seki K, Tatsuzawa A, Katayama K, Hirose K, Azuma T, et al. Primary hepatic neuroendocrine carcinoma coexisting with hepatocellular carcinoma in hepatitis C liver cirrhosis: report of a case. Surg Today. (2003) 33:214–8. doi: 10.1007/s005950300048
9. Yamaguchi R, Nakashima O, Ogata T, Hanada K, Kumabe T, Kojiro M. Hepatocellular carcinoma with an unusual neuroendocrine component. Pathol Int. (2004) 54:861–5. doi: 10.1111/j.1440-1827.2004.01770.x
10. Nakanishi C, Sato K, Ito Y, Abe T, Akada T, Muto R, et al. Combined hepatocellular carcinoma and neuroendocrine carcinoma with sarcomatous change of the liver after transarterial chemoembolization. Hepatol Res. (2012) 42:1141–5. doi: 10.1111/j.1872-034X.2012.01017.x
11. Aboelenen A, El-Hawary AK, Megahed N, Zalata KR, El-Salk EM, Fattah MA, et al. Right hepatectomy for combined primary neuroendocrine and hepatocellular carcinoma. A case report Int J Surg Case Rep. (2014) 5:26–9. doi: 10.1016/j.ijscr.2013.10.018
12. Nishino H, Hatano E, Seo S, Shibuya S, Anazawa T, Iida T, et al. Histological features of mixed neuroendocrine carcinoma and hepatocellular carcinoma in the liver: a case report and literature review. Clin J Gastroenterol. (2016) 9:272–9. doi: 10.1007/s12328-016-0669-0
13. Yun EY, Kim TH, Lee SS, Kim HJ, Kim HJ, Jung WT, et al. [A case of composite hepatocellular carcinoma and neuroendocrine carcinoma in a patient with liver cirrhosis caused by chronic hepatitis B]. Korean J Gastroenterol. (2016) 68:109–13. doi: 10.4166/kjg.2016.68.2.109
14. Choi GH, Ann SY, Lee SI, Kim SB, Song IH. Collision tumor of hepatocellular carcinoma and neuroendocrine carcinoma involving the liver: Case report and review of the literature. World J Gastroenterol. (2016) 22:9229–34. doi: 10.3748/wjg.v22.i41.9229
15. Okumura Y, Kohashi K, Wang H, Kato M, Maehara Y, Ogawa Y, et al. Combined primary hepatic neuroendocrine carcinoma and hepatocellular carcinoma with aggressive biological behavior (adverse clinical course): a case report. Pathol Res Pract. (2017) 213:1322–6. doi: 10.1016/j.prp.2017.06.001
16. Yilmaz DB, Bayramoglu Z, Ünay G, Ayik E, Başsorgun CI, Elpek GÖ. Incidental collision tumor of hepatocellular carcinoma and neuroendocrine carcinoma. J Clin Transl Hepatol. (2018) 6:339–44. doi: 10.14218/JCTH.2017.00076
17. Ikeda A, Aoki K, Terashima T, Itokawa Y, Kokuryu H. A fat containing combined neuroendocrine carcinoma and hepatocellular carcinoma in the liver: a case report. Ann Hepatol. (2020) 22:100183. doi: 10.1016/j.aohep.2020.01.006
18. Mao J-X, Teng F, Sun K-Y, Liu C, Ding G-S, Guo W-Y. Two-in-one: a pooled analysis of primary hepatic neuroendocrine carcinoma combined/collided with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. (2020) 19:399–403. doi: 10.1016/j.hbpd.2020.03.012
19. Gould VE, Banner BF, Baerwaldt M. Neuroendocrine neoplasms in unusual primary sites. Diagn Histopathol. (1981) 4:263–77.
20. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. (2019) 76:182–8. doi: 10.1111/his.13975
21. Gravante G, De Liguori Carino N, Overton J, Manzia TM, Orlando G. Primary carcinoids of the liver: a review of symptoms, diagnosis and treatments. Dig Surg. (2008) 25:364–8. doi: 10.1159/000167021
22. Yang K, Cheng YS, Yang JJ, Jiang X, Guo JX. Primary hepatic neuroendocrine tumor with multiple liver metastases: A case report with review of the literature. World J Gastroenterol. (2015) 21:3132–8. doi: 10.3748/wjg.v21.i10.3132
Keywords: primary hepatic neuroendocrine tumor, neuroendocrine tumor, HCC, case reports, heterogeneous malignancies
Citation: Lan J, Guo D, Qin X, Chen B and Liu Q (2021) Mixed Neuroendocrine Carcinoma and Hepatocellular Carcinoma: A Case Report and Literature Review. Front. Surg. 8:678853. doi: 10.3389/fsurg.2021.678853
Received: 10 March 2021; Accepted: 14 June 2021;
Published: 14 July 2021.
Edited by:
Gabriel Sandblom, Karolinska Institutet (KI), SwedenReviewed by:
Grzegorz Wiktor Kaminski, Military Institute of Medicine, PolandCopyright © 2021 Lan, Guo, Qin, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quanyan Liu, c3Bzc2xxeUB2aXAuMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.