- 1Department of Emergency, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2Department of Gastrointestinal and Anorectal Surgery, Nanning First People's Hospital, Nanning, China
- 3Department of Colorectal Surgery, The Eighth Hospital of Wuhan, Wuhan, China
- 4Department of Hepatobiliary Surgery, Guangxi Medical University Cancer Hospital, Nanning, China
- 5Second Department of General Surgery, Zhuzhou Central Hospital, Zhuzhou, China
- 6Guangxi Clinical Research Center for Colorectal Cancer, Nanning, China
Background: Tumor status can affect patient prognosis. Prognostic nutritional index (PNI), as a nutritional indicator, is closely related to the prognosis of cancer. However, few studies have examined the combined prognostic value of CEA and PNI in patients. This study investigated the relationship between CEA/PNI and prognosis of colon cancer patients.
Methods: A total of 513 patients with stage II–III colon cancer who underwent curative resection at two medical centers from 2009 to 2019 were included. Clinicopathological factors were assessed and overall survival (OS) was assessed in a cohort of 413 patients. Multivariate analysis was used to identify independent prognostic variables to construct histograms predicting 1-year and 3-year OS. Data from 100 independent patients in the validation group was used to validate the prognostic model.
Results: The median OS time was 33.6 months, and mortality was observed in 54 patients. Multivariate analysis revealed that preoperative CEA/PNI, lymph node metastasis, peripheral nerve invasion, operation mode, and postoperative chemotherapy were independent factors for prognosis evaluation and thus were utilized to develop the nomogram. The C-index was 0.788 in the learning set and 0.836 in the validation set. The calibration curves reached favorable consensus among the 1-, 3-year OS prediction and actual observation.
Conclusion: The combined use of CEA and PNI is an independent prognostic factor and thus can serve as a basis for a model to predict the prognosis of patients with stage II–III colon cancer.
Introduction
The latest global cancer data showed that colorectal cancer is among the top three with the highest incidence and mortality (1–3). Colon cancer accounts for approximately 65–70% of colorectal cancer cases (4, 5). Complete surgical resection remains the best treatment for patients with non-metastatic colon cancer. Despite receiving curative-intent treatment, 11.6–33% of patients with stage II–III colon cancer would still develop distant metastases or local recurrence 5 years after surgery (6–8). High preoperative CEA level increases the risk of death by 62% compared with preoperative CEA level in non-metastatic colon cancer (9). Therefore, identifying prognostic factors and individualizing postoperative therapy according to patient classification are necessary. Accumulating evidence indicates that nutritional status is associated with survival outcomes in patients with different cancers. Approximately 50–80% of admitted patients with malignant cancers are malnourished or at high risk for malnutrition (10–12). Therefore, an accurate understanding of the nutritional status of cancer patients is helpful to analyze and improve the prognosis of patients.
Prognostic nutritional index (PNI) was first designed by Buzby et al. in gastrointestinal cancer (13), and its relationship with the prognosis of malignant tumors has been subsequently widely studied (14–17). PNI has attracted extensive attention from clinicians due to its convenience in prognostic assessment (18–20). This index is calculated by the serum albumin and the total number of peripheral blood lymphocytes. Serum albumin, which is synthesized in and secreted from the liver, reflects the host's nutritional status and has a decreased level in malignant cancers (14–17, 21). Lymphocytes can recognize and eliminate tumor cells; the reduced numbers of different types of lymphocytes are thus likely to be associated with impaired tumor immunity, resulting in tumor progression (22, 23). CEA is elevated in peripheral blood in malignant tumors, especially in digestive tract tumors.
Serum CEA mainly reflects the tumor status, and PNI reflects the patient's overall condition, including nutritional and immune status. Their combination might be superior to either CEA or PNI alone for predicting the prognosis of patients with colon cancer. However, previous studies only focused on the prognostic association of a single variable with colorectal cancer alone. Research comparing different combinations of CEA and PNI in the prognosis of colon cancer remains lacking.
Methods
Patient Population
Databases from the Affiliated Cancer Hospital (learning set) and First Affiliated Hospital of Guangxi Medical University (validation set) were retrospectively reviewed. Approvals for the study were obtained from the two institutional review boards. The following patient clinicopathologic characteristics were obtained: gender, age, tumor location and size, operation mode (open vs. laparoscopic), preoperative blood test (CEA, CA199, albumin, and peripheral lymphocyte count), pathologic stage (T or N stage), differentiation degree of tumor cell, vascular or peri-neural infiltration, postoperative chemotherapy and information on deaths. Patients with stage II–III colon adenocarcinoma who received curative surgical excision from 2009 to 2019 at two medical centers were eligible. Exclusion criteria were as follows: (1) Younger than 18 years; (2) Preoperative neoadjuvant chemotherapy; (3) Palliative resection; (4) Multiple primary cancers; (5) Surgical history; (6) Incomplete preoperative laboratory data; (7) emergency surgery; and (8). incomplete follow-up information. The final learning and validation sets comprised 413 patients and 100 patients, retrospectively. The patients were started on semi-liquid to full liquid diet on admission. At 10 h before the operation, all patients were required to have oral sulfate-free polyethylene glycol electrolyte powder with 3,000 ml of water to remove all fecal contents.
Calculation of Laboratory Data
Peripheral venous blood samples (10 ml) were collected from patients under empty stomach conditions in the morning. Serum albumin, CEA, and peripheral lymphocyte count were tested within 3 days before surgery. PNI was calculated as follows: PNI = serum albumin level (g/L) + 5 × total lymphocyte count (/L) (24).
Model Construction and Internal Validation
Base on the results of multivariate analyses (P < 0.05), a nomogram was constructed by the combination of CEA/PNI with several other variables. External validation was applied to the validation set by the discriminatory power estimated by C-index and calibration curve.
Statistical Analysis
Statistical analysis was performed using SPSS 26.0 software (IBM SPSS 26.0, SPSS Inc.) and R software 3.2.5. Pearson correlation analysis was conducted to evaluate correlations between absolute lymphocyte count and basal serum albumin. After descriptive analysis, the association of PNI with all clinicopathological variables was tested. Chi-square test was used to analyze categorical data. T-test was utilized to analyze continuous data. Any probability of 0.05 or less was considered statistically significant. The Cox proportional hazard model was employed to determine the independent factors that influence OS based on the variables from the univariate analyses. Variable association with OS was analyzed using the Kaplan–Meier method, and differences were tested with the log-rank test. A P-value of < 0.05 was defined as significant in the univariate and multivariate analysis of prognostic factors for OS. The optimal diagnostic cut-off value of the total number of lymphocytes, ALB, PNI, and tumor size was determined by calculating the Youden index of the ROC curve.
Results
Descriptive Statistics
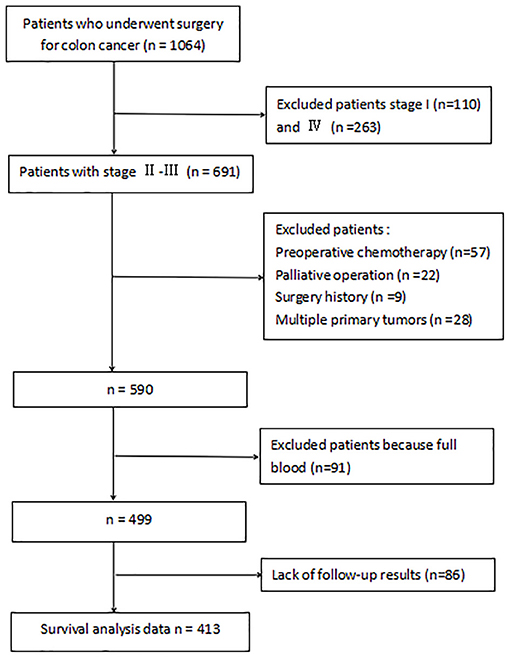
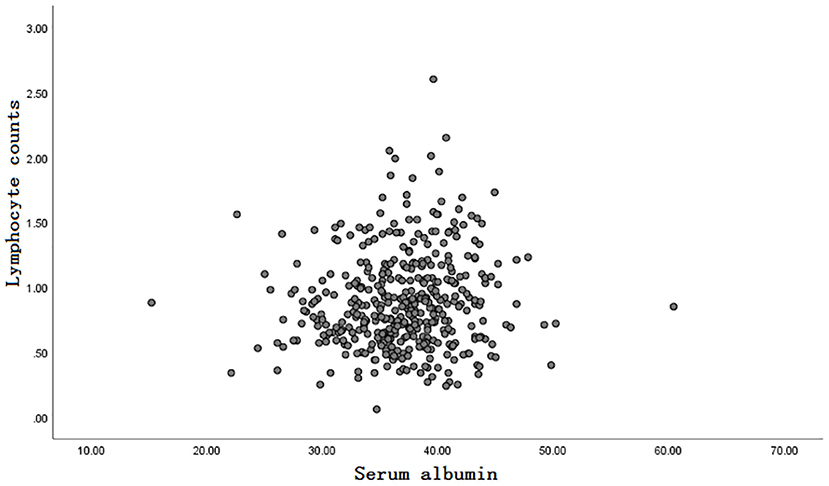
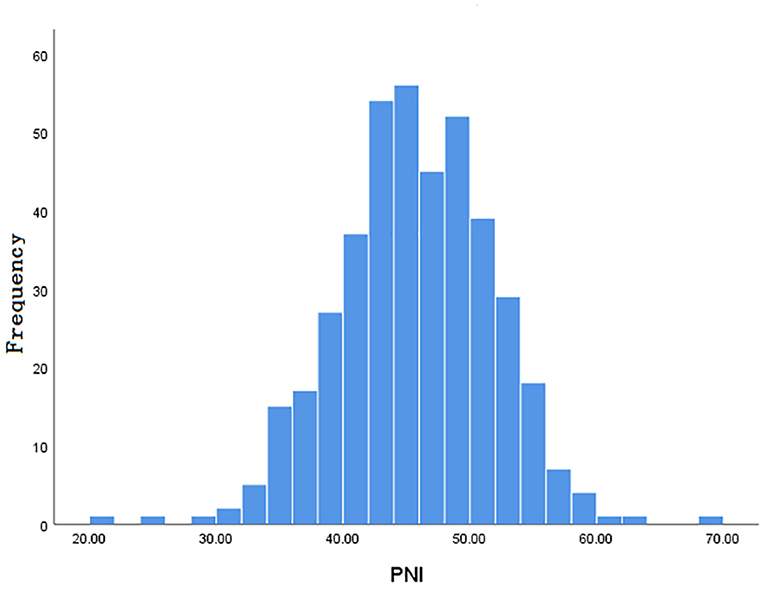
Inclusion and exclusion criteria are listed in Figure 1. A total of 413 patients were eligible for the learning set. The absolute lymphocyte count and basal serum albumin presented a poor correlation (r = 0.09; P = 0.068) (Figure 2). Mean PNI was 45 (SD 6.09; range 21.65–68.65), and its frequency distribution was normal (skewness −0.153, standard error [SE] 0.120; kurtosis 0.648, SE 0.240) (Figure 3).
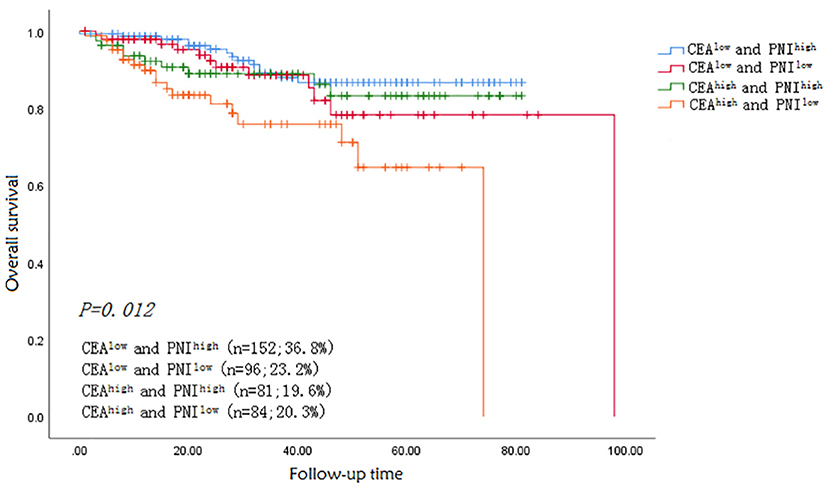
An optimal cut-off of PNI (45) was identified by calculating the Youden index and was used to categorize patients into two groups: PNI high (n = 180) and PNI low (n = 233). Table 1 shows the relation between PNI and the clinicopathological parameters of patients. PNIlow was highly common in patients with large tumors (≧5 cm), right colon and lymph node metastasis (P < 0.05). The mean serum CEA level was 15.4 ng/ml (range: 0.2–1500 ng/ml). The patients were also classified into two groups according to serum CEA concentration as follows: CEAlow(<5 ng/ml; n = 248) and CEA high (≧5 ng/ml; n = 165). The prognostic significance of the combination of CEA and PNI (CEA/PNI) was determined by grouping the patients into four as follows: CEAlow/PNI high (n = 152), CEA low/NI low (n = 96), CEA high/PNI high (n = 81) and CEA high/PNI low (n = 84).
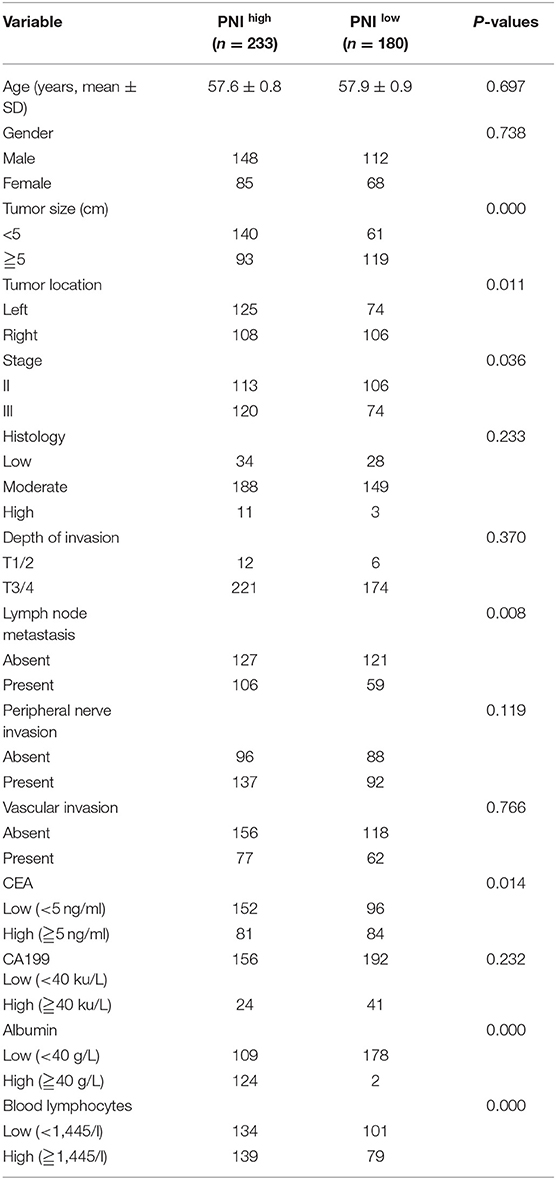
Table 1. Relationships between preoperative prognostic nutritional index (PNI) and clinicopathological variables in leaning set with stage II–III colon cancer.
Survival Analysis
The median follow-up time was 33.6 months. Fifty-four patients died during the follow-up period from all causes. The 1-, 3- and 5-year OS rates were significantly higher in patients in the CEAlow (98, 88, and 75%) than in the CEA high (91, 82, and 66%) group (p = 0.012). The OS rates of patients with CEA low/PNI high, CEA low/PNI low, CEA high/PNI high and CEA high/PNI low were 98.5, 97.5, 92.6, and 89.5% for 1-year, respectively, and 88.6, 87.4, 86, and 74% for 3-year, respectively (P < 0.005). Kaplan–Meier survival curve for OS in patients with colon cancer was stratified by CEA/PNI (Figure 4) (P = 0.005). Survival status was determined by ROC curves and compared using the AUCs. The AUCs of CEA, PNI, and CEA/PNI for OS were 0.568, 0.427, and 0.797, respectively, indicating that CEA/PNI was more useful than either indicator alone for predicting OS in patients with colon cancer. Multivariate analysis identified CEA/PNI as an independent prognostic indicator in patients with colon cancer. Lymph node metastasis, peripheral nerve invasion, operation mode, and postoperative chemotherapy were also noted as indicators (Table 2).

Table 2. Univariate and Multivariate analyses of prognostic factors for overall survival in the learning set with stage II–III colon cancer.
Model Construction and External Validation
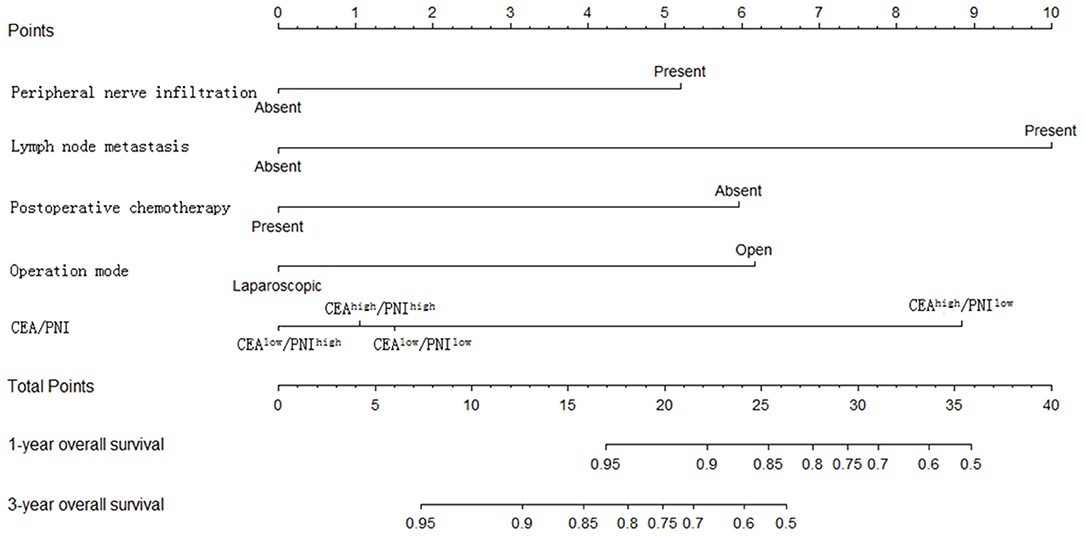
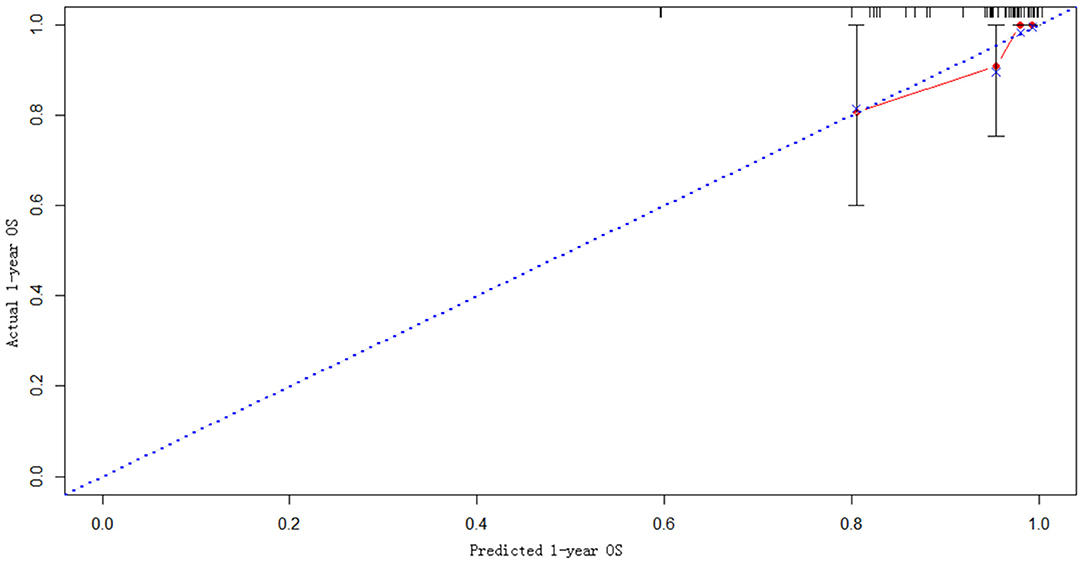
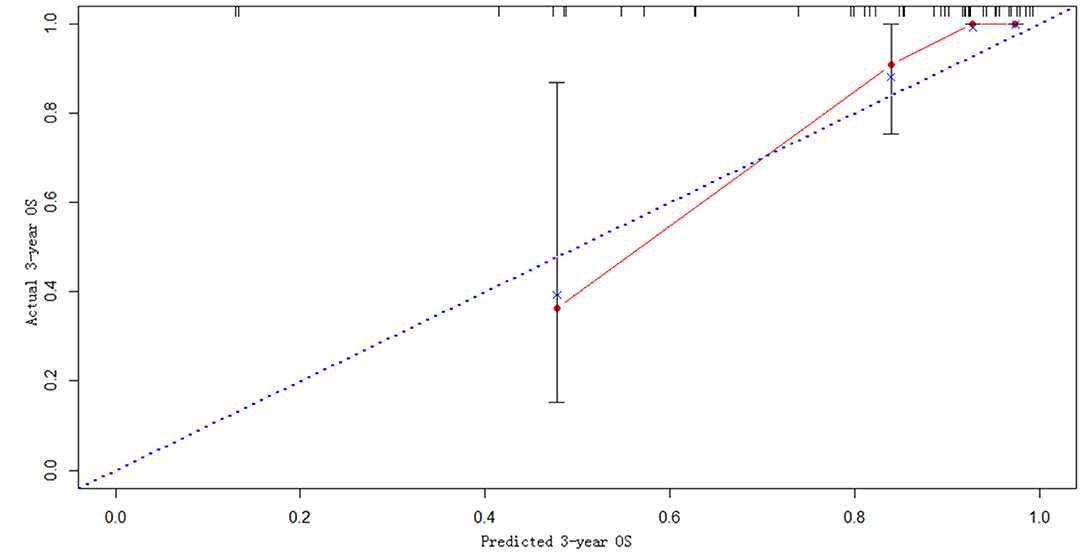
The statistically significant clinicopathological variables found in the cohort of 413 patients in the learning set were used to develop a nomogram that predicts OS after curative resection (Figure 5). The validation dataset comprised 100 patients. The C-index for OS after curative resection was 0.788 in the learning set and 0.836 in the validation set. Calibration curves showed that the prediction curves coincided with the diagonal lines (Figures 6, 7).
Discussion
An important association was found between CEA/PNI and OS in a single-center cohort of patients with stage II–III colon cancer. The results revealed that preoperative CEA/PNI is a prognosis-associated marker for patients with colon cancer. To the best of author's knowledge, this study takes the lead in evaluating the prognostic significance of preoperative CEA collaborated with PNI in patients with stage II–III colon cancer. Their combination was considered superior to either CEA or PNI alone to predict the prognosis of patients with colon cancer. Univariate analysis showed that factors affecting the prognosis of colon cancer included CEA, lymph node metastasis, peripheral nerve invasion, vascular invasion, albumin, peripheral blood lymphocytes, CEA/PNI, operation mode and postoperative chemotherapy. The final multivariate model strongly suggests that CEA/PNI, lymph node metastasis, CEA, peri-nerve invasion, operation mode and postoperative chemotherapy are independent prognostic variables.
Consistent with previous reports (6, 16), PNI was positively associated with the prognosis of colon cancer, indicating the values of immune and nutritional status as prognostic indicators. However, the mechanism by which PNI affects prognosis is unknown. PNI includes the measures of serum albumin and peripheral blood lymphocytes. Albumin, which constitutes up to 60% of plasma, is produced in the liver, reflects the nutritional status, is regulated by inflammatory cytokines and may play crucial roles in tumorigenesis and cancer progression (25). Serum albumin is positively correlated with the prognosis of colorectal cancer (26). Lymphocytes including NK cells, NKT cells, CD4+ T, and CD8+ T cells and B-cells are closely related to tumor immunity. Accumulating evidence implies that the decreased numbers of lymphocytes are associated with unfavorable prognosis in various cancers (27, 28). Several studies emphased that a cut-off value of PNI of 44 or higher is associated with a long OS on colorectal cancer (14, 29), and this result is highly consistent with our findings. Given that PNI is a continuous variable, the ultimate result may be uncertain in the process of conversion to the classification variable (14).
Preoperative CEA lacks sensitivity and specificity in disease diagnosis (30, 31) but is commonly used to assess the prognosis. The prognostic role of preoperative CEA concentration in early-stage CRC is controversial. This study revealed that preoperative CEA level is inversely correlated with the prognosis of patients with non-metastatic colon cancer. Multivariate regression analysis revealed that this factor is not an independent prognostic factor. For patients with early colon cancer, postoperative CEA levels can be used for predicting outcomes (32, 33). Studies from Asia suggested that postoperative CEA concentration ≥5 ng/ml is an important factor for poor prognosis in patients with stage II colorectal cancer. Preoperative high CEA concentration is a useful marker in follow-up, especially for stage II–III colon cancer patients. Further research suggested that elevated preoperative CEA level (≥5 ng/ml) loses its informative value when postoperative CEA level is normal (33). Although preoperative CEA may not be an independent prognosis factor for patients with stage II–III diseases (33, 34), it has predictive value in advanced/metastatic colon cancer (35) and can be combined with other markers or examination methods to evaluate the prognosis of early colon cancer (36).
Predictive models are particularly important for prognostic judgment and patient-physician clinical decision-making. This study used multivariate factors to develop a novel nomogram that is well-calibrated and externally validated. Subsequent verification revealed that the C-index reached 0.836, and the predicted value was highly consistent with the observed value in calibration curves.
However, this study has several limitations. The main pitfall is the retrospective design, second is the lack of validation data sets with sufficient samples, third is the unclear optimal variables cut-off value and the last is the small number of included patients. Therefore, multi-center and randomized control trials are needed to confirm the results. The main strength of the study is the prolonged follow-up periods for most survivors. Additionally, CEA, serum albumin and lymphocyte counts are inexpensive and easily obtained in any hospital.
In conclusion, multivariate regression analysis revealed that compared with CEA or PNI alone, the combination of CEA/PNI might provide relatively satisfactory prognostic information for patients with colon cancer. The presented prognostic model is inexpensive and can be easily constructed in clinical work.
Conclusion
The combination of CEA and PNI is an independent prognostic factor, and a model based on this factor may be helpful in predicting the prognosis of patients with stage II–III colon cancer.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by First Affiliated Hospital of Guangxi Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Y-sX, GL, and CZ contributed equally to this study, performed the experiments, analyzed the data, and wrote the manuscript. Y-sX designed the experiments. H-gZ and S-lL collected the data. YC and L-xH checked and revised the manuscript. ZL confirmed all the data in the manuscript. C-yL and YC firstly modified the language of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by grant from Health Commission of Guangxi Zhuang Autonomous Region (Z20210188).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Gopaul Roodrajeetsing who works at Victoria Hospital in Mauritius for his contribution to the language polishing of this manuscript.
References
1. Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO–ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. (2018) 29:44–70. doi: 10.1093/annonc/mdx738
2. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. (2017) 66:683–91. doi: 10.1136/gutjnl-2015-310912
3. Freddie Bray JFIS. Global cancer statistics 2018 GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
4. Yin J, Song L, Lu H, Zheng X. Prediction of different stages of rectal cancer: Texture analysis based on diffusion-weighted images and apparent diffusion coefficient maps. World J Gastroentero. (2020) 26:2082–96. doi: 10.3748/wjg.v26.i17.2082
5. Rebecca L, Siegel M, Kimberly D, Miller M, Ahmedin Jemal DP. Cancer Statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
6. Peng J, Zhang R, Zhao Y, Wu X, Chen G, Wan D, et al. Prognostic value of preoperative prognostic nutritional index and its associations with systemic inflammatory response markers in patients with stage III colon cancer. Chin J Cancer. (2017) 36:96. doi: 10.1186/s40880-017-0260-1
7. Abdallah EA, Souza E, Silva V, Braun AC, Gasparini VA, Kupper BEC, Tariki MS, et al. A higher platelet-to-lymphocyte ratio is prevalent in the presence of circulating tumor microemboli and is a potential prognostic factor for non-metastatic colon cancer. Transl Oncol. (2021) 14:100932. doi: 10.1016/j.tranon.2020.100932
8. Alonso S, Saltz L. The landmark series: chemotherapy for non-metastatic colon cancer. Ann Surg Oncol. (2021) 28:995–1001. doi: 10.1245/s10434-020-09375-9
9. Becerra AZ, Probst CP, Tejani MA, Aquina CT, González MG, Hensley BJ, et al. Evaluating the prognostic role of elevated preoperative carcinoembryonic antigen levels in colon cancer patients: results from the national cancer database. Ann Surg Oncol. (2016) 23:1554–61. doi: 10.1245/s10434-015-5014-1
10. Emilie Reber RSLB. Efficacy and efficiency of nutritional support teams. J Clin Med. (2019) 9:1281–99. doi: 10.3390/jcm8091281
11. Bouleuc C, Anota A, Cornet C, Grodard G, Thiery-Vuillemin A, Dubroeucq O, et al. Impact on health-related quality of life of parenteral nutrition for patients with advanced cancer cachexia: results from a randomized controlled trial. Oncologist. (2020) 25:e843–51. doi: 10.1634/theoncologist.2019-0856
12. Mantzoroua M, Antonios K, Stamatios TCG. Clinical value of nutritional status in cancer: what is its impact and how it affects disease progression and prognosis. Nutr Cancer. (2017) 29:1151–76. doi: 10.1080/01635581.2017.1367947
13. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. (1980) 139:160–7. doi: 10.1016/0002-9610(80)90246-9
14. Luvián Morales J, González Trejo S, Carrillo JF, Herrera Goepfert R, Aiello Crocifoglio V, Gallardo Rincón D, et al. Association of the prognostic nutritional index and overall survival in patients with colorectal cancer: A STROBE compliant retrospective cohort study. Cancer Med-US. (2019) 8:3379–88. doi: 10.1002/cam4.2212
15. Li B, Lu Z, Wang S, Hou J, Xia G, Li H, et al. Pretreatment elevated prognostic nutritional index predicts a favorable prognosis in patients with prostate cancer. BMC Cancer. (2020) 20:361. doi: 10.1186/s12885-020-06879-1
16. Mirili C, Yilmaz A, Demirkan S, Bilici M, Basol Tekin S. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int J Clin Oncol. (2019) 24:1301–10. doi: 10.1007/s10147-019-01461-7
17. Jian Li RXDH. Prognostic nutritional index predicts outcomes of patients after gastrectomy for cancer: a systematic review and meta-analysis of nonrandomized studies. Nutr Cancer. (2019) 71:557–68. doi: 10.1080/01635581.2019.1577986
18. Sasaki M, Miyoshi N, Fujino S, Ishikawa S, Saso K, Takahashi H, et al. Development of novel prognostic prediction models including the prognostic nutritional index for patients with colorectal cancer after curative resection. J Anus Rectum Colon. (2019) 3:106–15. doi: 10.23922/jarc.2018-041
19. Noh GT, Han J, Cho MS, Hur H, Min BS, Lee KY, et al. Impact of the prognostic nutritional index on the recovery and long-term oncologic outcome of patients with colorectal cancer. J Cancer Res Clin. (2017) 143:1235–42. doi: 10.1007/s00432-017-2366-x
20. Zhao Y, Deng Y, Peng J, Sui Q, Lin J, Qiu M, et al. Does the preoperative prognostic nutritional index predict survival in patients with liver metastases from colorectal cancer who underwent curative resection? J Cancer. (2018) 9:2167–74. doi: 10.7150/jca.25346
21. Kang HW, Seo SP, Kim WT, Yun SJ, Lee S, Kim W, et al. Low preoperative serum cholesterol level is associated with aggressive pathologic features and poor cancer-specific survival in patients with surgically treated renal cell carcinoma. Int J Clin Oncol. (2018) 23:142–50. doi: 10.1007/s10147-017-1172-4
22. Ichikawa N, Homma S, Yoshida T, Mitsuhashi T, Iijima H, Ogasawara K, et al. An increase in the peripheral lymphocyte-to-monocyte ratio after primary site resection is associated with a prolonged survival in unresectable colorectal carcinoma. Surg Today. (2020) 50:604–14. doi: 10.1007/s00595-019-01927-1
23. Maffei F, Zolezzi MJ, Angelini S, Zenesini C, Musti M, Festi D, et al. Micronucleus frequency in human peripheral blood lymphocytes as a biomarker for the early detection of colorectal cancer risk. Mutagenesis. (2014) 29:221–5. doi: 10.1093/mutage/geu007
24. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5.
25. Hu Z, Li Y, Mao W, Chen B, Yang L, Meng X. Impact of nutritional indices on the survival outcomes of patients with colorectal cancer. Cancer Manag Res. (2020) 12:2279–89. doi: 10.2147/CMAR.S243172
26. Yamamoto M, Saito H, Uejima C, Tanio A, Tada Y, Matsunaga T, et al. Combination of serum albumin and cholinesterase levels as prognostic indicator in patients ith colorectal cancer. Anticancer Res. (2019) 39:1085–90. doi: 10.21873/anticanres.13217
27. Feng F, Zheng G, Wang Q, Liu S, Liu Z, Xu G, et al. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. (2018) 18:148. doi: 10.1186/s12876-018-0877-9
28. Guo Y, Liu J, Zhang W, Xiao S, Zheng G, Liu S, et al. Prognostic value of fibrinogen and lymphocyte count in intermediate and high risk gastrointestinal stromal tumors. Cancer Manage Res. (2020) 12:8149–57. doi: 10.2147/CMAR.S262570
29. Galizia G, Lieto E, Auricchio A, Cardella F, Mabilia A, Podzemny V, et al. Naples prognostic score, based on nutritional and inflammatory status, is an independent predictor of long-term outcome in patients undergoing surgery for colorectal cancer. Dis Colon Rectum. (2017) 60:1273–84. doi: 10.1097/DCR.0000000000000961
30. Benson RAB, Desch CE, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, et al. 2000 update of American Society of Clinical Oncology colorectal cancer surveillance guidelines. J Clin Oncol. (2000) 18:3586–8. doi: 10.1200/JCO.2000.18.20.3586
31. Chao MGP. Caution is required before recommending routine carcinoembryonic antigen and imaging follow-up for patients with early-stage colon cancer. J Clin Oncol. (2009) 27:e279–80. doi: 10.1200/JCO.2009.25.6156
32. Lin J, Lin C, Yang S, Wang H, Jiang J, Lan Y, et al. Early postoperative CEA level is a better prognostic indicator than is preoperative CEA level in predicting prognosis of patients with curable colorectal cancer. Int J Colorectal Dis. (2011) 26:1135–41. doi: 10.1007/s00384-011-1209-5
33. Konishi T, Shimada Y, Hsu M, Tufts L, Jimenez-Rodriguez R, Cercek A, et al. Association of Preoperative and Postoperative Serum Carcinoembryonic Antigen and Colon Cancer Outcome. JAMA Oncol. (2018) 4:309–15. doi: 10.1001/jamaoncol.2017.4420
34. Chen L, Jiang B, Wang Z, Liu M, Yang H, Xing J, et al. Combined preoperative CEA and CD44v6 improves prognostic value in patients with stage I and stage II colorectal cancer. Clin Transl Oncol. (2014) 16:285–92. doi: 10.1007/s12094-013-1069-2
35. Kirat HT, Ozturk E, Lavery IC, Kiran RP. The predictive value of preoperative carcinoembryonic antigen level in the prognosis of colon cancer. Am J Surg. (2012) 204:447–52. doi: 10.1016/j.amjsurg.2011.11.007
Keywords: colon cancer, tumor marker, nutritional status, prognosis, nomogram
Citation: Xu Y-s, Liu G, Zhao C, Lu S-l, Long C-y, Zhong H-g, Chen Y, Huang L-x and Liang Z (2021) Prognostic Value of Combined Preoperative Carcinoembryonic Antigen and Prognostic Nutritional Index in Patients With Stage II–III Colon Cancer. Front. Surg. 8:667154. doi: 10.3389/fsurg.2021.667154
Received: 08 March 2021; Accepted: 25 June 2021;
Published: 20 July 2021.
Edited by:
Massimiliano Veroux, University of Catania, ItalyReviewed by:
Giuseppe Portale, Azienda ULSS 6 Euganea, ItalyAntoniono Zanghi, Università degli Studi di Catania, Italy
Copyright © 2021 Xu, Liu, Zhao, Lu, Long, Zhong, Chen, Huang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-song Xu, aGJ4eXM4MUAxNjMuY29t
 Yan-song Xu
Yan-song Xu Gang Liu2
Gang Liu2 Yi Chen
Yi Chen