- 1Lung Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 2Operating Room, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Outpatient, West China Hospital, Sichuan University, Chengdu, China
- 4Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China
Introduction: Malignant pleural effusion was encountered in about 8–15% of lung cancer patients at initial cancer diagnosis. The optimal therapeutic strategies for lung cancer with malignant pleural effusion (MPE) remain unclear.
Case Description: In this study, we reported a case of lung cancer with MPE, which was successfully managed with a multidisciplinary therapeutic strategy. The patient initially received gefitinib for 4 months with excellent response and he underwent salvage thoracoscopic lobectomy and systematic lymphadenectomy. Pathological complete response was confirmed for the patient and he discontinued gefitinib but received 4 cycles of adjuvant chemotherapy instead. The patient is still alive without disease progression for 62 months after surgery.
Conclusions: Combining targeted therapy, salvage surgery, and adjuvant therapy may be a promising treatment strategy for lung cancer with MPE harboring oncogene-targeted mutations.
Introduction
Malignant pleural effusion is commonly encountered in about 8–15% of lung cancer patients at the time of initial cancer diagnosis, which has been categorized as stage IVa disease in the eighth edition of the tumor-node-metastasis staging system (1). The prognosis of lung cancer patients with malignant pleural effusion (MPE) remains dismal with a median overall survival time of 5 months and a 5-year survival rate of 3% (2). Current guidelines recommended non-surgical therapy including local therapy (for example ambulatory small catheter drainage, pleurodesis, and pericardial window) with similar treatment strategies to other stage IV diseases consisting of systemic therapy and palliative therapy (3). In this study, we reported a case of lung cancer with MPE, which was successfully managed with the combined therapeutic strategy of targeted therapy followed by salvage surgery and adjuvant therapy.
Case Report
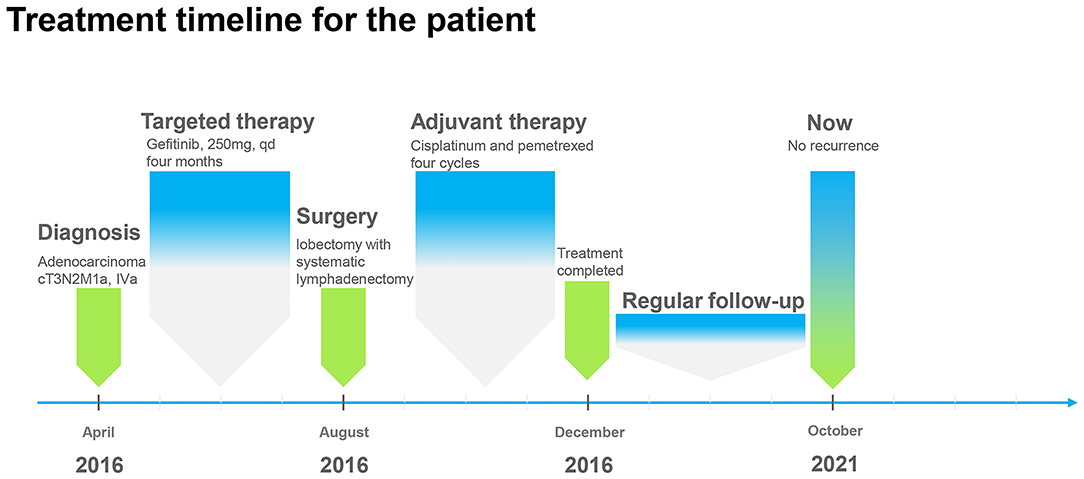
In April 2016, a 51-year old male patient complained of consistent cough for 2 months and was admitted to our center for a diagnosis of poorly differentiated lung adenocarcinoma [PCK(+), CK7 (focally +), TTF-1(+), CK18(+), CK5/6(-), P63(scattered +), CK14(-), CDX-2(-), CD56(+), CgA(-), Sgn(-), Ki-67(~50%)] in the right upper lobe with enlarged ipsilateral mediastinal lymph node and MPE confirmed by cytological examination of the fluid via both cell block and smear from the collected sample by thoracentesis, which was not further confirmed by pleural biopsy (cT3N2M1a, IVa) (Figure 1). The patient was generally in normal condition but was found to have type two diabetes mellitus with an Eastern Cooperative Oncology Group score of 1. With the primary tumor extracted via percutaneous needle biopsy for next-generation sequencing, it was confirmed to have epidermal growth factor receptor (EGFR) gene mutation (exon 21 L858R) and the patient was advised to receive gefitinib (250 mg, QD) for treatment. After taking gefitinib for 4 months, the patient was re-evaluated comprehensively and an excellent radiographic response to gefitinib was found on his chest computed tomography scan (Figure 2). Therefore, the patient was discussed in a multidisciplinary meeting in our center, and salvage surgery was recommended for him. In August 2016, after providing signed informed consent, the patient received lobectomy and systematic lymph node dissection under video-assisted thoracoscopic surgery (VATS) successfully and intraoperative findings did not reveal any pleural involvement, which was further confirmed by pleural biopsy. The patient was discharged on postoperative day 5 uneventfully and his postoperative pathological finding revealed no residual tumor neither in the right upper lobe nor in the mediastinal lymph node and pathological complete response to gefitinib was confirmed in the patient (ypT0N0M0) as shown in his pathological report that numerous chronic inflammatory cells, foamy histiocytes, and dense fibrosis were observed and no viable tumor was seen [PCK(-), EMA(-), CK7(-), TTF-1(-), NapsinA(-), CK5/6(-), P63(-), PGM-1 (inflammatory cells+), complete pathologic response]. As usual, after surgery, the patient discontinued gefitinib and underwent four cycles of adjuvant chemotherapy (cisplatinum 40 mg on day 1 to 3 and pemetrexed 800 mg on day 1) instead because chemotherapy was the regular regimen for postoperative adjuvant therapy then and finished his last course of adjuvant chemotherapy in December 2016 with only grade 2 of leukopenia without any drug-related grade 3 to 4 adverse events. Since then, the patient received no additional treatment (such as targeted therapy, chemotherapy, or radiotherapy) and was followed up regularly every 3–4 months with chest and abdominal CT scans and tumor biomarkers and annual positron emission tomography (PET)/CT scan. The patient's recent PET/CT scan (October 2021) still revealed no sign of recurrence (Figure 3), and he is still alive without disease for 62 months after surgery (disease-free survival: 62 months). The whole treatment timeline of the patient in our case was summarized in Figure 4.
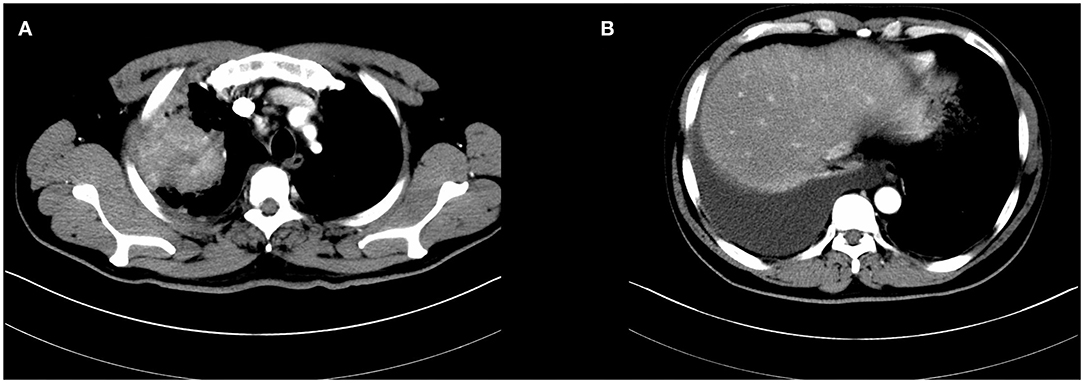
Figure 1. Initial chest computed tomography of the patient revealed a large mass in the right upper lobe with enlarged mediastinal lymph node and malignant pleural effusion (A,B).
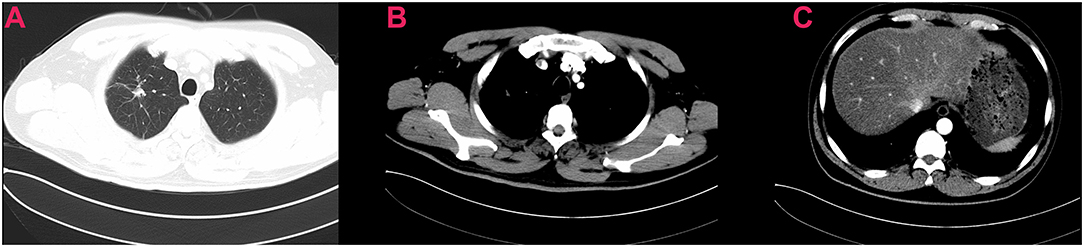
Figure 2. Preoperative chest computed tomography of the patient showing excellent response to neoadjuvant targeted therapy (A–C).
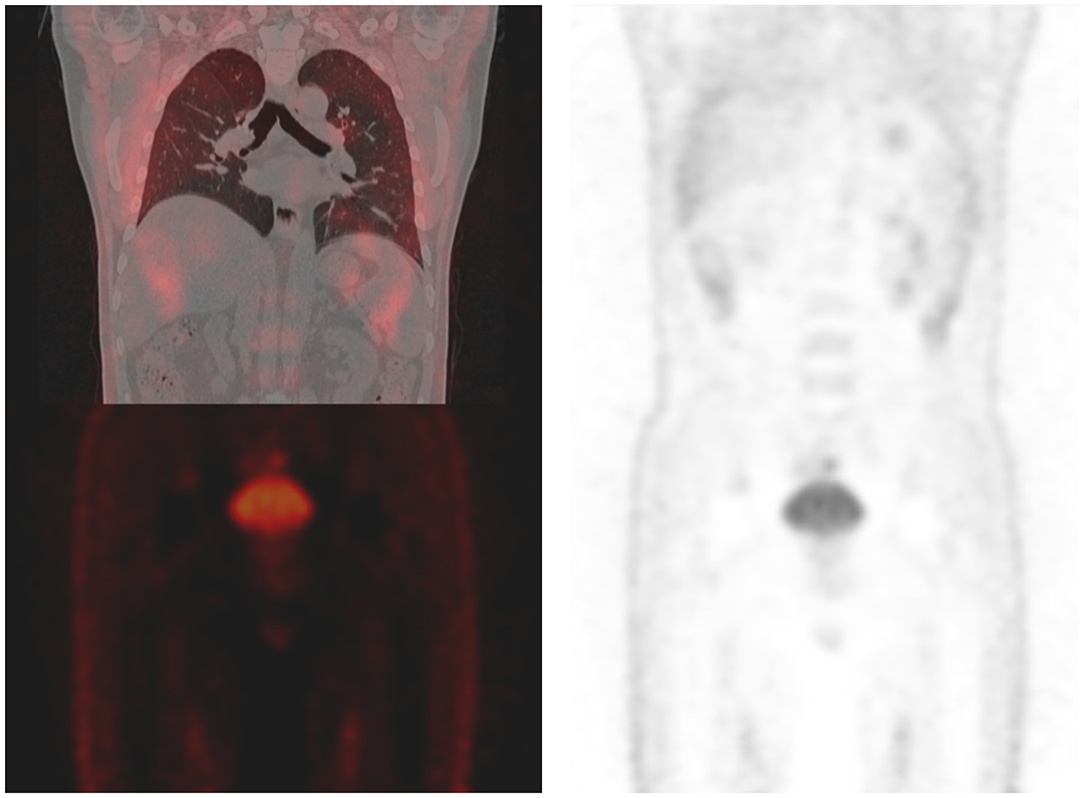
Figure 3. Recent positron emission tomography/computed tomography of the patient shows no sign of recurrence.
Discussion
Previous studies have shown that surgery could benefit certain carefully selected patients with pleural metastasis and the reported 5-year OS of these patients treated with surgical resection ranged from 16 to 31% (4). Our previous study also indicated that surgical resection of the primary tumor could bring survival benefits for patients with unexpected pleural metastasis found during operation (5). However, it should be noted that patients with MPE yielded significantly worse survival than those without after surgery for pleural metastasis (6), suggesting that MPE may represent diffused pleural dissemination and surgery may be precluded for MPE (7). However, previous studies applied surgery with systemic therapy for treating advanced lung cancer with MPE and found that the long-term outcomes of combined therapy for these patients remain conflicting as some found that surgery with systemic therapy could benefit patients while others found a worse outcome after surgery (8). Therefore, for MPE, induction therapy followed by salvage surgery and subsequent systemic therapy was investigated. However, most of the previous studies applied chemoradiotherapy for induction therapy in patients with MPE and the rate of complete response was extremely low (9, 10). Moreover, the majority of these patients after surgery relapsed during follow-up (9). Therefore, the role of salvage surgery in treating advanced lung cancer with MPE remains further to be elucidated.
Similar to the dilemma encountered in neoadjuvant therapy for stage III lung cancer (11), the optimal induction regimens for lung cancer with MPE remain far from being established. As the promising effects of oncogene-targeted therapy for lung cancer, targeted therapy has already become the first-line therapy for advanced lung cancer harboring sensitizing mutations. Considering that EGFR tyrosine kinase inhibitors (TKIs) could yield a significantly higher response rate than chemoradiotherapy and confer survival benefit over chemoradiotherapy in advanced lung cancer harboring sensitizing mutations (12), in our case, the patient received gefitinib as the initial treatment because of the EGFR gene mutation. Surprisingly, the patient showed complete response to gefitinib after 4-month treatment. As we all know, the majority of patients receiving the first generation of EGFR-TKIs will progress within 1 year due to resistance mutation (12). Therefore, in our case, salvage surgery was recommended because of the radiographic finding of complete response. Moreover, we have successfully performed VATS lobectomy with systematic lymphadenectomy for the patient. Because of no residual tumor in the right upper lobe and mediastinal lymph node (ypT0N0), the patient only received 4 cycles of adjuvant chemotherapy without postoperative targeted therapy (as discussed by the multidisciplinary team considering that no residual tumor was revealed in the patient) for minimizing postoperative recurrence and was regularly followed up thereafter. And the patient is still alive without any sign of recurrence or metastasis for nearly 62 months. Therefore, this is an interesting case in a stage IVa lung cancer patient, who was managed successfully with a therapeutic combination of targeted therapy, salvage surgery, and adjuvant therapy.
Previously, Kubo et al. (13) reported a total of 7 cases of lung cancer with MPE treated with gefitinib, who responded effectively to gefitinib and chest drainage. However, the time to treatment failure for these patients was about 0.2–19.0 months. Tsai et al. (14) reported a case with stage IV oligometastatic adenocarcinoma of the lung successfully managed with neoadjuvant afatinib (pathological complete response) followed by surgery and adjuvant afatinib as well as radiotherapy for oligometastasis (the third lumbar vertebra) and the patient was alive for 32 months after initial diagnosis. A previous study also confirmed that salvage surgery after targeted therapy could serve as a promising therapeutic option for advanced lung cancer (15). Therefore, targeted therapy may serve as induction therapy for lung cancer with MPE. Arrieta et al. (10) reported two cases of lung cancer patients with malignant pleural effusion but without extra-thoracic disease, who were treated with neoadjuvant targeted therapy followed by salvage surgery and adjuvant targeted therapy. One case was treated with erlotinib and was alive without disease for 32 months while another was treated with afatinib and was alive without disease for 28.25 months. Song et al. (15) reported three cases of lung cancer with malignant pleural effusion treated with targeted therapy followed by salvage surgery and found that the postoperative survival was 6–44 months, proving that salvage surgery after targeted therapy was feasible and promising for treating advanced lung cancer with malignant pleural effusion. Li et al. (16) also reported a case of advanced lung cancer with malignant pleural effusion treated with salvage surgery followed by targeted therapy who finally developed progression of pubic bone metastasis after 13 months after surgery. Here we summarized these similar cases of lung cancer with malignant pleural effusion successfully managed with targeted therapy followed by salvage surgery in Table 1. Therefore, taking our case together, we believe that the combination of targeted therapy followed by salvage surgery and adjuvant therapy seems to be a promising therapeutic strategy for lung cancer with MPE harboring oncogene-targeted mutations.
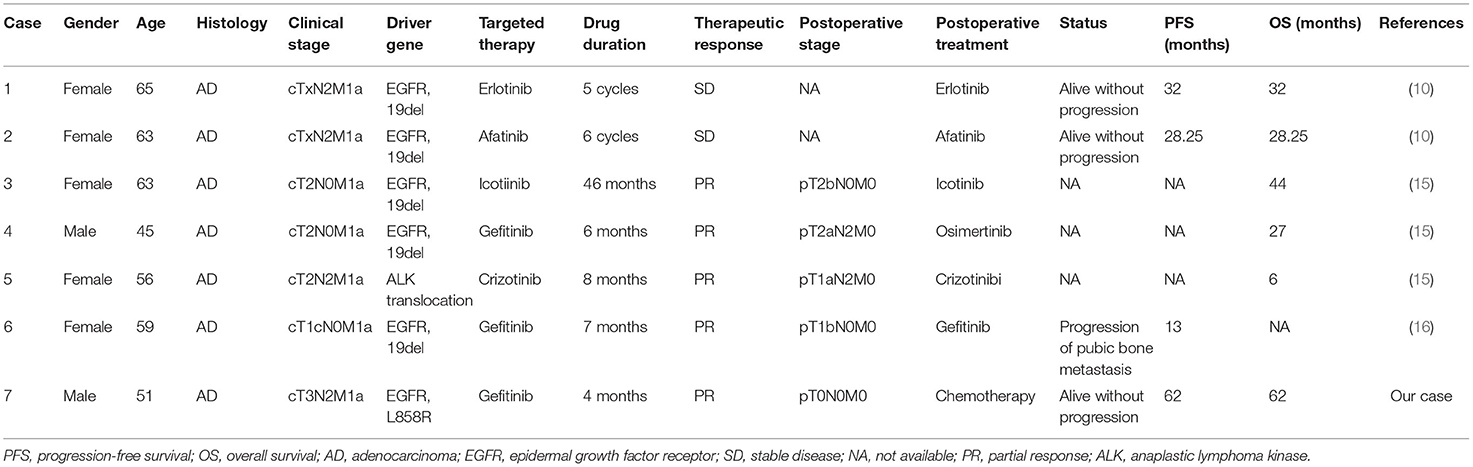
Table 1. Literature review for case reports regarding targeted therapy followed by salvage surgery for treating lung cancer with malignant pleural effusion.
However, several limitations existed in our case report. First, we drew our conclusions based on only one case, which could decrease the evidence level of our conclusions. Second, our case was diagnosed with MPE only confirmed by cytological examination of the fluid without thoracoscopic pleural biopsy, which may lead to false-positive results. Therefore, for such patients, a well-detailed algorithm should be designed to ensure proper patient selection in the future. Moreover, expanding surgical indications for patients with MPE may carry significant risks, such as perioperative morbidity and mortality as well as postoperative recurrence and metastasis, and should be done only after great deliberation and thoughtful consideration of all risks. In our opinion, the salvage surgery may be considered for patients with MPE, whose tumors showed a significant radiographic response (CT or PET/CT) to initial therapy with the disappearance of pleural effusion. However, the widely accepted criteria to decide salvage surgery for patients with MPE remains further to be established. Therefore, our conclusions should be taken with caution and further similar cases are encouraged to add evidence to our conclusions.
Conclusion
Lung cancer with MPE has an extremely poor prognosis and targeted therapy followed by salvage surgery and adjuvant therapy seems to be a promising therapeutic strategy for lung cancer with MPE harboring oncogene-targeted mutations.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by West China Hospital, Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
H-YD and YR collected data and drafted the manuscript. XT, DL, and KW designed the study and revised the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Skok K, Hladnik G, Grm A, Crnjac A. Malignant pleural effusion and its current management: a review. Medicina. (2019) 55:80490. doi: 10.3390/medicina55080490
2. Carter J, Miller JA, Feller-Kopman D, Ettinger D, Sidransky D, Maleki Z. Molecular profiling of malignant pleural effusion in metastatic non-small-cell lung carcinoma. The effect of preanalytical factors. Annal Am Thoracic Soc. (2017) 14:1169–76. doi: 10.1513/AnnalsATS.201609-709OC
3. Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR, et al. NCCN guidelines insights: non-small cell lung cancer, version 1.2020. J Natl Comprehens Cancer Netw. (2019) 17:1464–72. doi: 10.6004/jnccn.2019.0059
4. David EA, Clark JM, Cooke DT, Melnikow J, Kelly K, Canter RJ. The role of thoracic surgery in the therapeutic management of metastatic non-small cell lung cancer. J Thoracic Oncol. (2017) 12:1636–45. doi: 10.1016/j.jtho.2017.08.008
5. Deng HY, Zheng X, Zhu DX, Zhou Q. Is surgical resection of primary tumour superior to exploratory thoracotomy without resection in treating lung cancer patients with unexpected pleural metastasis detected during operation? Interact Cardiovasc Thorac Surg. (2020) 30:582–87. doi: 10.1093/icvts/ivz315
6. Ren YJ, She YL, Dai CY, Jiang GN, Fei K, Chen C. Primary tumour resection showed survival benefits for non-small-cell lung cancers with unexpected malignant pleural dissemination. Interact Cardiovasc Thorac Surg. (2016) 22:321–6. doi: 10.1093/icvts/ivv353
7. Porcel JM. Malignant pleural effusions because of lung cancer. Curr Opin Pulm Med. (2016) 22:356–61. doi: 10.1097/MCP.0000000000000264
8. Fukui T, Yokoi K. The role of surgical intervention in lung cancer with carcinomatous pleuritis. J Thorac Dis. (2016) 8:S901–s07. doi: 10.21037/jtd.2016.06.36
9. Yamaguchi M, Ichinose Y, Shimamatsu S, Yoshida T, Toyokawa G, Nosaki K, et al. Preoperative concurrent chemoradiotherapy followed by extrapleural pneumonectomy for patients with non-small cell lung cancer with malignant pleural effusion and/or pleural nodules: ten-year results of a prematurely terminated single institute phase II trial. Surg Oncol. (2015) 24:78–83. doi: 10.1016/j.suronc.2015.02.004
10. Arrieta O, Escamilla-Lopez I, Lyra-Gonzalez I, Barron F, Ramirez-Tirado LA, Vergara E, et al. Radical aggressive treatment among non-small cell lung cancer patients with malignant pleural effusion without extra-thoracic disease. J Thorac Dis. (2019) 11:595–601. doi: 10.21037/jtd.2019.01.36
11. Xiong L, Lou Y, Bai H, Li R, Xia J, Fang W, et al. Efficacy of erlotinib as neoadjuvant regimen in EGFR-mutant locally advanced non-small cell lung cancer patients. J Int Med Res. (2019) 2019:300060519887275. doi: 10.1177/0300060519887275
12. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. (2009) 361:947–57. doi: 10.1056/NEJMoa0810699
13. Kubo A, Koh Y, Kawaguchi T, Isa S, Okamoto I, Fukuoka J, et al. Malignant pleural effusion from lung adenocarcinoma treated by gefitinib. Internal Med. (2011) 50:745–8. doi: 10.2169/internalmedicine.50.4652
14. Tsai PC, Yeh YC, Huang CS. Pathological complete response after afatinib treatment of stage IV oligometastatic adenocarcinoma of the lung: the role of pulmonary surgery. J Int Med Res. (2019) 5:178. doi: 10.1186/s40792-019-0741-3
15. Song W, Di S, Liu J, Fan B, Zhao J, Zhou S, et al. Salvage surgery for advanced non-small cell lung cancer after targeted therapy: a case series. Thoracic Cancer. (2020) 11:1061–67. doi: 10.1111/1759-7714.13366
Keywords: lung cancer, malignant pleural effusion, targeted therapy, salvage surgery, adjuvant therapy
Citation: Deng H-Y, Li D, Ren Y, Wang K and Tang X (2021) Targeted Therapy Followed by Salvage Surgery and Adjuvant Therapy: A Promising Therapy for Lung Cancer With Malignant Pleural Effusion From a Case Report. Front. Surg. 8:659983. doi: 10.3389/fsurg.2021.659983
Received: 28 January 2021; Accepted: 10 November 2021;
Published: 10 December 2021.
Edited by:
Yongbing Chen, Soochow University, ChinaReviewed by:
Mark William Hennon, University at Buffalo, United StatesOlivia Lauk, University Hospital Zuerich, Switzerland
Copyright © 2021 Deng, Li, Ren, Wang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojun Tang, dGFuZ3hpYW9qdW43M0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Han-Yu Deng
Han-Yu Deng Deyan Li2†
Deyan Li2†