- Department of Interventional Radiology and Vascular Surgery, Peking University First Hospital, Beijing, China
Background: To date, there have been few studies examining the efficacy and safety of drug-coated balloon (DCB) angioplasty in the treatment of Tosaka class III in-stent restenosis (ISR) lesions in the clinical setting. Therefore, this study aimed to investigate the clinical efficacy and safety of DCBs in patients with Tosaka class III ISR femoropopliteal lesions.
Methods: This single-center study enrolled 28 femoropopliteal ISR Tosaka III patients who were treated by DCB angioplasty from September 2016 to September 2018. The patency, the freedom from target lesion revascularization (TLR) rate, clinical improvement, and safety endpoints were analyzed during a 14-month follow-up period.
Results: Out of the 28 patients, 32.1% presented with critical limb ischemia. The mean lesion length was 250.4 ± 93.9 mm. Technical success was achieved in all lesions (100%). Debulking devices were used in 50% of lesions, and bailout stents were performed in 3.6% of patients. Kaplan Meier estimates that the 14-month primary patency was 79.2% (95% CI 60.6–97.8%), whereas the freedom from TLR rate was 91.5% (95% CI 80.1–100%). Clinical symptoms improved by at least 1 Rutherford category in 82.1% of limbs. The ankle-brachial index (ABI) values improved from 0.51 ± 0.30 to 1.05 ± 0.22 at the final follow-up (P < 0.001). The rate of freedom from 30-day major adverse limb events (MALEs) was 100%. The mortality rate was 7.1%.
Conclusion: These results suggested that the use of DCBs is safe and effective in treating femoropopliteal Tosaka class III ISR lesions.
Introduction
Peripheral artery disease is the third leading cause of atherosclerotic cardiovascular morbidity and affects more than 200 million people globally (1). Recent guidelines recommend endovascular treatment of symptomatic aortoiliac and femoropopliteal patients as the first-line therapy (2, 3). Stents have been used to treat femoropopliteal lesions for decades and have shown better patency than plain old balloon angioplasty (POBA) (4). However, the 12-month patency in lesions longer than 10 cm remains poor with 50–65% rates, thereby leading to in-stent restenosis (ISR) (5–7). Treatment of femoropopliteal ISR lesions, especially Tosaka class III lesions, is intractable (8, 9).
Drug-coated balloons (DCBs) is an emerging therapeutic method, and multiple randomized controlled trials have demonstrated its superiority compared to POBA in reducing restenosis, minimizing target lesion revascularization, and decreasing late lumen loss (10–15). However, the majority of lesions included in these trials were de novo. Other studies have evaluated the use of DCBs in treating ISR lesions; however, these latter reports did not focus on Tosaka class III lesions and demonstrated conflicting outcomes (16–23). Therefore, the efficacy and safety of DCBs in Tosaka III ISR femoropopliteal lesions remains to be elucidated.
This study aimed to investigate the effectiveness and safety of DCBs in Tosaka class III femoropopliteal ISR lesions in real-world scenerio.
Materials and Methods
Patient Population
The local institutional Human Investigations Committee approved this retrospective study. Patient informed consent was waived for this study. Patients with femoropopliteal Tosaka class III ISR lesions treated with DCB from September 2016 to September 2018 were enrolled in this single-center study. The inclusion criteria were as follows: (1) patients age 18 years or older; (2) patients diagnosed with Rutherford Classification category 1 or greater; (3) the presence of femoropopliteal Tosaka III ISR lesions; and (4) the lesions were treatable with the available Acotec Orchid DCB. Pregnant patients or patients who were planning on becoming pregnant were excluded.
DCB treated a total of 120 femoropopliteal artery disease patients in the study period. Among them, 28 patients were diagnosed with femoropopliteal Tosaka III ISR lesions. According to institutional standard protocols, the patient's baseline characteristics including age, gender, cardiovascular risk factors, and clinical status stratified by the Rutherford classification were collated in a local database. Pre-procedural physical examination, ankle-brachial index (ABI) measurements, and vascular duplex sonography were performed.
Endovascular Procedures
Patients were treated by experienced vascular specialists in a catheter lab under local anesthesia and supplemented with intravenous sedation when required. All treatment procedures, including the use of DCB or additional devices, were determined at the vascular specialists' discretion. All patients were treated with Orchid DCB (Acotec, Beijing, China). The balloon was coated with 3.0 μg paclitaxel per mm2 and magnesium stearate as the excipient. During the procedure, 6–7 French sheaths were chosen for artery access. Intraluminal or subintimal crossings were performed on occlusions using a 0.035-/0.018-inch hydrophilic guidewire. Debulking devices, including Silverhawk/Turbohawk (Medtronic), Rotarex catheter (Straub Medical AG), or Angiojet (Boston Scientific), were employed at the discretion of the vascular specialists. At least one uncoated balloon was used for pre-dilation before each DCB. The diameter of the DCB was 0.5 or 1 mm larger than the pre-dilation uncoated balloon. Vessel diameter and lesion characteristics were visually estimated. Lesion length was measured by a radiopaque ruler placed under the patient's body on the examination bed. The degree of calcification was not evaluated due to the interference of the metal stents. The length of the DCB was sufficient to cover at least 1 cm distal and proximal to the lesion. If more than one DCB was used, the overlap was at least 1 cm. Implantation of self-expanding stents was allowed in flow-limiting dissection cases or where residual stenosis was >30%. Inflow and outflow lesions were often treated during the same intervention as determined by the operators.
Antiplatelet and Anticoagulation Protocol
Before the procedure, all the patients were administered a dual antiplatelet therapy of aspirin 100 mg/day and clopidogrel 75 mg/day for at least 7 days or a loading dose consisting of 300 mg aspirin and 300 mg/day clopidogrel 6 h before the procedure. During the procedure, heparin (5,000 IU) was administered for anti-coagulation following the long sheath insertion. All patients were prescribed dual antiplatelet therapy (aspirin 100 mg/day and clopidogrel 75 mg/day) for at least 6 months after the procedure and changed over to one agent after that. For patients with cardiovascular comorbidities, the administration of antiplatelet therapy was at the discretion of the doctors. Statin was prescribed for patients with dyslipidemia.
Follow-Up
All patients underwent a physical examination and ABI measurements to assess the results of treatment before discharge. Follow-up visits at 1, 3, 6, and 12 months and yearly after that were routinely scheduled at our hospital's outpatient department. During the follow-up consultations, symptoms inquiry, physical examination, duplex ultrasound scanning, and ABI measurements were conducted. Computed tomography angiography (CTA) was performed every year or when the ABI decreased more than 20%, or if the duplex ultrasound scanning showed significant restenosis (>50%), or if the patient complained of severe symptoms. Patients who did not return for follow-up were contacted by phone consultation every 6 months.
Endpoints and Definitions
The main endpoint of the study was the 14-month primary patency rate. The secondary endpoints were as follows: the 14-month freedom from target lesion revascularization (TLR) rate; the technical success rate; the 14-month mortality rate; and the 30-day major adverse limb events (MALEs) rate including death, major amputation, and TLR. Tosaka class III lesions were defined as totally occluded ISR lesions (8). In-stent chronic total occlusion (ISCTO) was defined as Tosaka class III lesions with a history of more than 3 months. Procedural technical success was defined as <30% residual stenosis in the final angiogram. Patency was defined as <50% restenosis based on CTA/angiography or duplex ultrasound scanning, with a 2.4 cutoff value for the peak systolic velocity ratio (PSVR). Primary patency was defined as uninterrupted patency without procedures, performed on or at the margin of the treated segment. TLR was defined as any intervention for treating the restenosis or other complication of the culprit vessels. Complications included local infection, dissection, thromboembolism, fistulas, hematoma, acute occlusion, renal failure, stroke, and myocardial infarction.
Statistical Analysis
Statistical analyses were performed using SPSS version 20 (SPSS, Chicago, IL, USA) software. Continuous data are shown as means ± standard deviation, and categorical data are presented as count and percentage. The normally-distributed continuous variables were compared using 2-sided Student's t-tests, and the non-normally-distributed continuous variables were compared using the Wilcoxon rank test. Mean values of the baseline and immediate postoperative or follow-up measurements were compared using a paired t-test. Categorical variables were compared using the 2-sided likelihood ratio chi-square test or Fisher exact test. Kaplan-Meier survival analysis with a log-rank test was used to estimate primary patency and freedom from TLR. A Cox regression analysis was used to identify independent predictors of restenosis and TLR. Variables associated with restenosis in the univariate analysis were entered into a multivariable model (P < 0.05). Outcomes were depicted as hazard ratio (HR) and 95% confidence interval (CI). Results were considered statistically significant when P < 0.05.
Results
Patient Characteristics
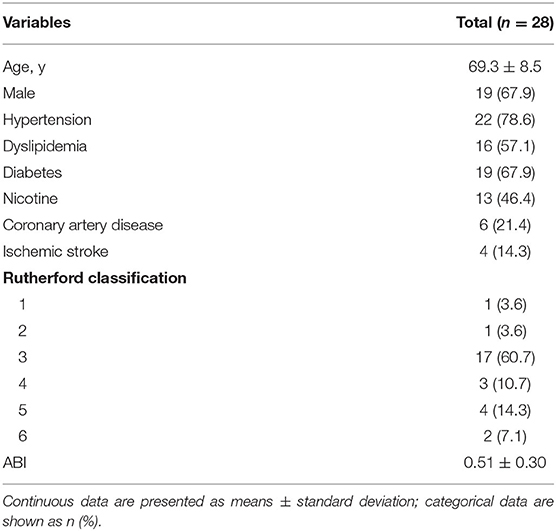
Demographic information for the patients is shown in Table 1. The patients' mean age was 69.3 ± 8.5 years, and 67.9% of patients were male. Cardiovascular risk factors were highly prevalent, including hypertension, dyslipidemia, and diabetes. Thirteen (46.4%) patients had a history of smoking. Most patients (92.9%) presented with severe claudication (60.8%) or critical limb ischemia (32.1%) at baseline. The mean pre-procedure ABI was 0.51 ± 0.30.
Lesion and Procedural Characteristics
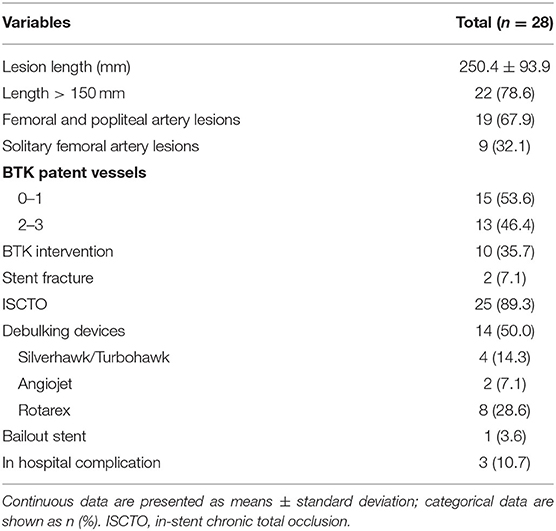
The average total target lesion length was 250.4 ± 93.9 mm (Table 2). The majority of lesions (78.6%) were longer than 150 mm. Approximately two-thirds (67.9%) of the lesions were in both the femoral and the popliteal arteries, while 32.1% of lesions were restricted to the femoral arteries alone. Stent fractures were observed in two patients (7.1%). Nearly 90% of the lesions were ISCTO. Technical success was obtained in all cases. Debulking devices were used in 14 of the 28 lesions (50.0%). Specifically, Silverhawk/Turbohawk was used in four patients, Angiojet was used in two patients, and Rotarex was used in eight patients. An average number of 1.71 ± 0.60 DCBs were used in each patient. A bailout stent was performed in only one patient (3.6%). Three complications (10.7%) occurred in the process of endovascular treatment, including 2 cases of thrombosis and 1 case of distal embolization. All conditions were managed via endovascular procedures, and open surgery was not required. The complications did not elongate the length of hospitalization. Within 30 days after the procedure, no MALEs had occurred. The rate of freedom from 30-day MALEs was 100%.
Follow-Up Outcomes
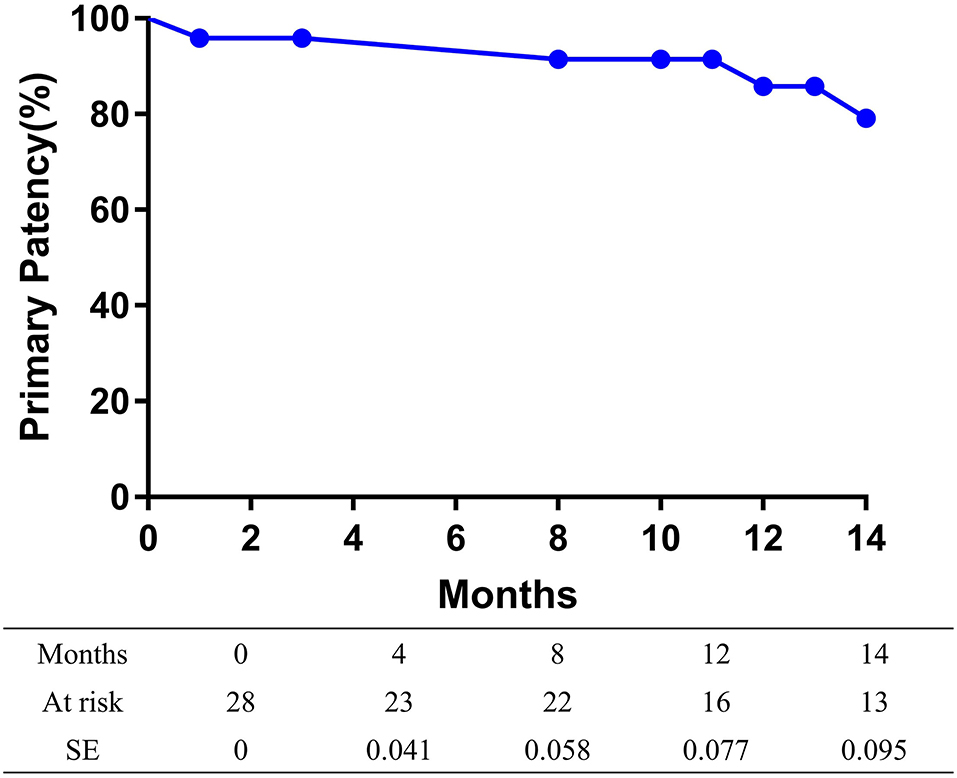
The overall follow-up rate was 85.7% (24/28) and the mean follow-up time was 21.5 ± 10.3 months. Symptoms improved by at least 1 Rutherford category in 82.1% (23/28) of limbs at follow-up. The mean ABI value at follow-up was 1.05 ± 0.22, increasing significantly from baseline (p < 0.001). A total of two patients died at 8 and 29 months after the procedures, with heart attacks being the cause of death in both cases. The 14-month mortality rate was 7.1%. At 14 months after treatment, the primary patency rate and the freedom from TLR rate, as determined by the Kaplan-Meier estimates, were 79.2% (95% CI 60.6–97.8%; Figure 1) and 91.5% (95% CI 80.1–100%; Figure 2), respectively.
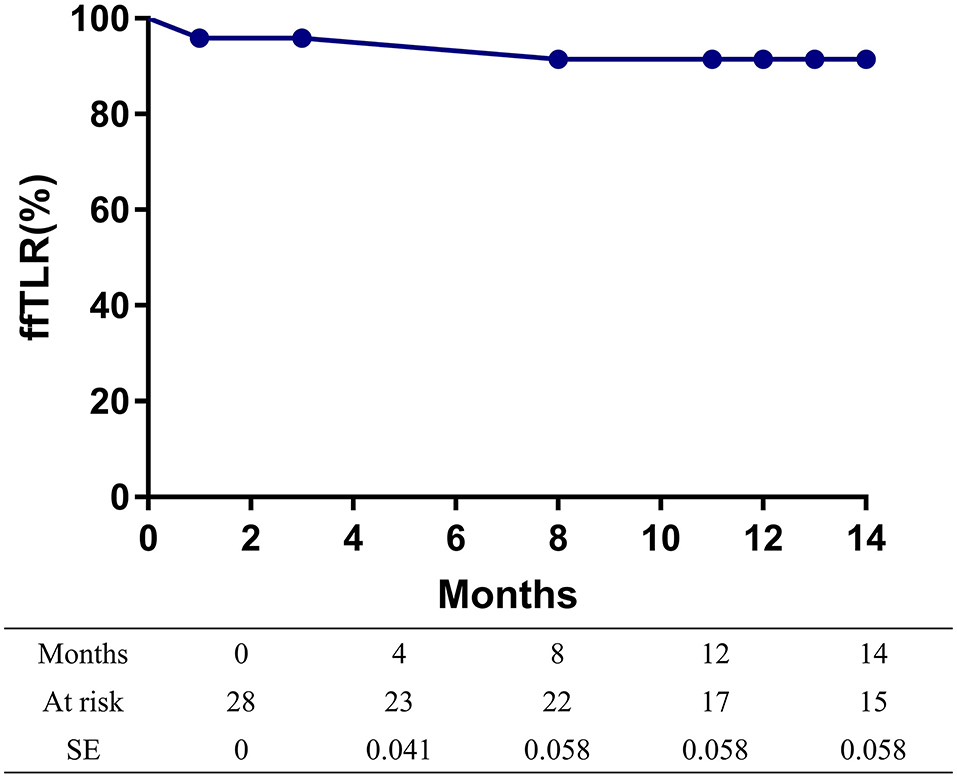
Figure 2. Kaplan-Meier curves of the freedom from target lesion revascularization (TLR) of patients.
As shown in Table 3, the univariate analysis determined that the risk factor for restenosis was bailout stenting (P < 0.05). However, the Cox multivariate analysis showed that bailout stenting was not a risk factor for restenosis (P = 1.000, HR = 1.000, 95% CI 0–18,184.735). The univariate analysis also showed that diabetes and bailout stenting were possible risk factors for TLR (P < 0.05). However, the Cox multivariate analysis showed that neither was an independent risk factor for TLR.
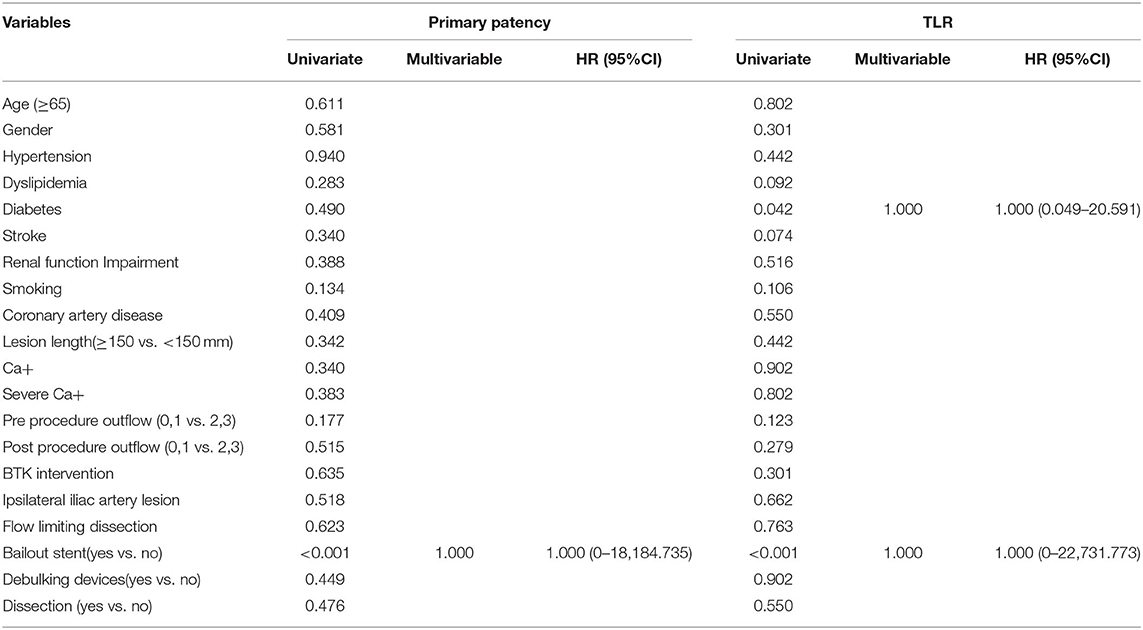
Table 3. Univariate and multivariate analyses of the risk factors for 12-month primary patency and target lesion revascularization (TLR).
Discussion
In this single-center study, 28 patients with Tosaka class III femoropopliteal lesions were treated with DCBs and followed up for 14 months. The data showed that DCB treatment is safe in Tosaka class III patients, and the results were promising in terms of patency rates, freedom from TLR, and improved clinical symptoms.
The use of DCBs in treating femoral ISR lesions is expected to be superior to POBA due to the anti-hyperplasia properties of paclitaxel (24). In a prospective multicenter randomized trial, the authors claimed that treating superficial femoral artery ISR lesions with DCBs was associated with less recurrent restenosis and a better clinical outcome than POBA after a 12-month follow-up (18). In the PACUBA trial, DCBs showed a superior 12-month primary patency compared to POBA in lesions with an average length >17 cm (40.7 vs. 13.4%) (21). Another report has demonstrated that at the 24-month follow-up, DCBs were associated with a significant reduction of TLR compared to POBA (23). However, long-term results showed that at 3 years, the freedom from TLR rates was similar between the DCB and POBA groups (20). Considering the late catch-up phenomenon of drug-coated devices (17, 25), the long-term efficacy of DCBs in treating ISR lesions needs to be further investigated.
Tosaka et al. demonstrated that restenotic patterns were important predictors of recurrent ISR (8). They reported that the rate of recurrent ISR at 2 years after POBA was 84.8% in class III patients compared with 49.9% in class I patients and 53.3% in class II patients. Similar results were found in ISR lesions treated by DCBs. Recent research reported that the treatment of complex ISR lesions (classes II and III) was associated with an increased rate of recurrent restenosis compared with class I lesions (33.3% and 36.3 vs. 12.5%) (17). In the DEBATE-ISR study, the presence of a Tosaka class III occlusion was associated with a worse 3-year outcome in both the DCB and the angioplasty groups (20). However, contrasting results were reported by other researchers. In the PLAISIR Trial, no significant differences between Tosaka class I (25 lesions) and class II lesions (29 lesions) were noted in terms of freedom from TLR at 1 year after DCB treatment (22). According to the 1-year results of the PACUBA Trial, there was no significant difference between stenosis (Tosaka class I or II) and occlusion (Tosaka class III) in terms of primary patency and freedom from TLR (21). In our study, the 14-month primary patency was close to 80%, and the freedom from TLR rate was 91.5%. These results are promising, and DCBs should be considered the first-line treatment for femoropopliteal lesions.
Removing the neointimal hyperplasia and extra-cellular matrix of ISR with debulking devices is theoretically an attractive option. In a single-center randomized study, the combination of laser atherectomy and DCB in treating Tosaka class III ISR lesions was correlated with improved 12-month primary patency and freedom from TLR rates in comparison with DCB alone (26). Similarly, the combination of directional atherectomy with DCB was associated with greater freedom from TLR rate than POBA after directional atherectomy (27). In our study, 50% of lesions were treated with a combination of debulking and DCB. The use of debulking devices may help to improve clinical outcomes.
There are potential limitations in the present study. First, this was a nonrandomized and noncomparative study, and it was therefore not possible to compare DCBs and other treatment options in managing Tosaka class III lesions. Second, it was a single-center study, and bias related to the sample size and loss to follow-up may limit the results' generalizability. Third, the multivariable analyses could not be performed due to the small sample size. Further studies with larger sample sizes are warranted, and the risk factors of restenosis should be evaluated.
In conclusion, DCB angioplasty was shown to be a safe and effective treatment option in patients for Tosaka class III lesions at 14 months follow-up.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Human Investigations Committee of Peking University First Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
The authors acknowledge support from Scientific Research Seed Fund of Peking University First Hospital under grant No. 2018SF023, Youth Clinical Research Project of Peking University First Hospital under grant No. 2018CR16, and Interdisciplinary Clinical Research Project of Peking University First Hospital under grant No. 2018CR33.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. (2013) 382:1329–40. doi: 10.1016/S0140-6736(13)61249-0
2. Committee TS, Jaff MR, White CJ, Hiatt WR, Fowkes GR, Dormandy J, et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Endovasc Ther. (2015) 22:663–77. doi: 10.1177/1526602815592206
3. Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. (2017) 135:e686–725. doi: 10.1161/CIR.0000000000000470
4. Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, et al. Editor's Choice - 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. (2018) 55:305–68. doi: 10.1016/j.ejvs.2017.07.018
5. Bosiers M, Deloose K, Callaert J, Moreels N, Keirse K, Verbist J, et al. Results of the Protege EverFlex 200-mm-long nitinol stent (ev3) in TASC C and D femoropopliteal lesions. J Vasc Surg. (2011) 54:1042–50. doi: 10.1016/j.jvs.2011.03.272
6. Lammer J, Zeller T, Hausegger KA, Schaefer PJ, Gschwendtner M, Mueller-Huelsbeck S, et al. Heparin-bonded covered stents versus bare-metal stents for complex femoropopliteal artery lesions: the randomized VIASTAR trial (Viabahn endoprosthesis with PROPATEN bioactive surface [VIA] versus bare nitinol stent in the treatment of long lesions in superficial femoral artery occlusive disease). J Am Coll Cardiol. (2013) 62:1320–7. doi: 10.1016/j.jacc.2013.05.079
7. Liistro F, Grotti S, Porto I, Angioli P, Ricci L, Ducci K, et al. Drug-eluting balloon in peripheral intervention for the superficial femoral artery: the DEBATE-SFA randomized trial (drug eluting balloon in peripheral intervention for the superficial femoral artery). JACC Cardiovasc Interv. (2013) 6:1295–302. doi: 10.1016/j.jcin.2013.07.010
8. Tosaka A, Soga Y, Iida O, Ishihara T, Hirano K, Suzuki K, et al. Classification and clinical impact of restenosis after femoropopliteal stenting. J Am Coll Cardiol. (2012) 59:16–23. doi: 10.1016/j.jacc.2011.09.036
9. van den Berg JC. In-stent restenosis management: the best is yet to come. J Cardiovasc Surg. (2017) 58:508–17. doi: 10.23736/S0021-9509.17.09953-0
10. Scheinert D, Duda S, Zeller T, Krankenberg H, Ricke J, Bosiers M, et al. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv. (2014) 7:10–9. doi: 10.1016/j.jcin.2013.05.022
11. Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, et al. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. (2015) 373:145–53. doi: 10.1056/NEJMoa1406235
12. Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. (2015) 131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004
13. Tepe G, Schnorr B, Albrecht T, Brechtel K, Claussen CD, Scheller B, et al. Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial. JACC Cardiovasc Interv. (2015) 8 :102–8. doi: 10.1016/j.jcin.2014.07.023
14. Jia X, Zhang J, Zhuang B, Fu W, Wu D, Wang F, et al. Acotec drug-coated balloon catheter: randomized, multicenter, controlled clinical study in femoropopliteal arteries: evidence from the AcoArt I trial. JACC Cardiovasc Interv. (2016) 9:1941–9. doi: 10.1016/j.jcin.2016.06.055
15. Bausback Y, Willfort-Ehringer A, Sievert H, Geist V, Lichtenberg M, Del Giudice C, et al. Six-month results from the initial randomized study of the ranger paclitaxel-coated balloon in the femoropopliteal segment. J Endovasc Ther. (2017) 24:459–67. doi: 10.1177/1526602817710770
16. Stabile E, Virga V, Salemme L, Cioppa A, Ambrosini V, Sorropago G, et al. Drug-eluting balloon for treatment of superficial femoral artery in-stent restenosis. J Am Coll Cardiol. (2012) 60:1739–42. doi: 10.1016/j.jacc.2012.07.033
17. Virga V, Stabile E, Biamino G, Salemme L, Cioppa A, Giugliano G, et al. Drug-eluting balloons for the treatment of the superficial femoral artery in-stent restenosis: 2-year follow-up. JACC Cardiovasc Interv. (2014) 7:411–5. doi: 10.1016/j.jcin.2013.11.020
18. Krankenberg H, Tubler T, Ingwersen M, Schluter M, Scheinert D, Blessing E, et al. Drug-coated balloon versus standard balloon for superficial femoral artery in-stent restenosis: the randomized Femoral Artery In-Stent Restenosis (FAIR) trial. Circulation. (2015) 132:2230–6. doi: 10.1161/CIRCULATIONAHA.115.017364
19. Gray BH, Buchan JA. The treatment of superficial femoral artery in-stent restenosis: the jury is still out. JACC Cardiovasc Interv. (2016) 9:1393–6. doi: 10.1016/j.jcin.2016.04.029
20. Grotti S, Liistro F, Angioli P, Ducci K, Falsini G, Porto I, et al. Paclitaxel-eluting balloon vs standard angioplasty to reduce restenosis in diabetic patients with in-stent restenosis of the superficial femoral and proximal popliteal arteries: three-year results of the DEBATE-ISR Study. J Endovasc Ther. (2016) 23:52–7. doi: 10.1177/1526602815614555
21. Kinstner CM, Lammer J, Willfort-Ehringer A, Matzek W, Gschwandtner M, Javor D, et al. Paclitaxel-eluting balloon versus standard balloon angioplasty in in-stent restenosis of the superficial femoral and proximal popliteal artery: 1-year results of the PACUBA Trial. JACC Cardiovasc Interv. (2016) 9:1386–92. doi: 10.1016/j.jcin.2016.04.012
22. Bague N, Julia P, Sauguet A, Pernes JM, Chatelard P, Garbe JF, et al. Femoropopliteal in-stent restenosis repair: midterm outcomes after paclitaxel eluting balloon use (PLAISIR Trial). Eur J Vasc Endovasc Surg. (2017) 53:106–13. doi: 10.1016/j.ejvs.2016.10.002
23. Ott I, Cassese S, Groha P, Steppich B, Voll F, Hadamitzky M, et al. ISAR-PEBIS (Paclitaxel-eluting balloon versus conventional balloon angioplasty for in-stent restenosis of superficial femoral artery): a randomized trial. J Am Heart Assoc. (2017) 6:e006321. doi: 10.1161/JAHA.117.006321
24. Schorn I, Malinoff H, Anderson S, Lecy C, Wang J, Giorgianni J, et al. The Lutonix(R) drug-coated balloon: a novel drug delivery technology for the treatment of vascular disease. Adv Drug Deliv Rev. (2017) 112:78–87. doi: 10.1016/j.addr.2017.05.015
25. Schmidt A, Piorkowski M, Gorner H, Steiner S, Bausback Y, Scheinert S, et al. Drug-coated balloons for complex femoropopliteal lesions: 2-year results of a real-world registry. JACC Cardiovasc Interv. (2016) 9:715–24. doi: 10.1016/j.jcin.2015.12.267
26. Gandini R, Del Giudice C, Merolla S, Morosetti D, Pampana E, Simonetti G. Treatment of chronic SFA in-stent occlusion with combined laser atherectomy and drug-eluting balloon angioplasty in patients with critical limb ischemia: a single-center, prospective, randomized study. J Endovasc Ther. (2013) 20:805–14. doi: 10.1583/13-4308MR.1
Keywords: drug-coated balloon, femoropopliteal lesions, in-stent restenosis, Tosaka class, patency
Citation: Zhang B, Niu G, Yan Z, Zou Y, Tong X and Yang M (2021) Drug-Coated Balloon for the Treatment of Femoropopliteal Tosaka Class III In-stent Restenosis Lesions. Front. Surg. 7:616414. doi: 10.3389/fsurg.2020.616414
Received: 15 October 2020; Accepted: 14 December 2020;
Published: 13 January 2021.
Edited by:
Shahab Toursavadkohi, University of Maryland, United StatesReviewed by:
Thodur Madabushi Vasudevan, The Alfred Hospital, AustraliaGeorge Galyfos, National and Kapodistrian University of Athens, Greece
Copyright © 2021 Zhang, Niu, Yan, Zou, Tong and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Yang, ZHJ5YW5nbWluQGdtYWlsLmNvbQ==
 Bihui Zhang
Bihui Zhang Guochen Niu
Guochen Niu Ziguang Yan
Ziguang Yan Yinghua Zou
Yinghua Zou Min Yang
Min Yang