
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 26 January 2021
Sec. Reconstructive and Plastic Surgery
Volume 7 - 2020 | https://doi.org/10.3389/fsurg.2020.559450
Chronic soft tissue defects are notoriously difficult to heal. Surgical reconstruction of chronic defects using tissue flaps is a routine approach for closure of challenging chronic defects. Due to the poor tissue quality of chronic defects and associated inflammation, infection and impaired blood supply the success of flap closure is marred by reported complication rates of 25–58%. Extracellular matrix (ECM)-based graft materials are commonly used for resolving chronic wounds and in plastic and reconstructive procedures to create a scaffold for tissue regeneration. We hypothesized combination use of ECM grafts with tissue flaps in a single-stage surgical procedure would reduce complications and improve outcomes in the closure of chronic soft tissue defects. We report a case series (n = 9) of chronic soft tissue defect reconstruction using this modified procedure of ECM graft augmented flap closure. Defects included pressure injuries and surgical dehiscence and ranged in wound age from 5 months to 7 years. Successful uncomplicated healing was achieved in six defects. Post-operative complications (dehiscence) occurred in two defects, however, these healed via secondary intention without additional surgical intervention. All healed defects exhibited acceptable cosmesis and “normal” function, with 100% patient satisfaction. Augmentation of tissue flaps with ECM graft materials in this modified single-stage procedure may improve outcomes and minimize typical complications encountered in flap closure of chronic defects attributed to inflammation, infection, hypoperfusion, and dead space.
Flap reconstruction is a well-established approach to the closure of chronic soft tissue defects however, post-operative complications such as infection, dehiscence, and re-occurrence are relatively common. The long-term success of flap closure is further complicated by patient co-morbidities such as obesity, diabetes and venous insufficiency. Retrospective analysis of 755 pressure injuries managed via flap closure demonstrated an overall complication rate of 25% at 30-day follow up (1). A prospective study of 276 pressure injuries closed by flap advancement demonstrated a complication rate of 58%, where wound dehiscence (31.2%) and re-occurrence (28.6%) were the most frequent complications (2). These complications associated with flap closure of chronic soft tissue defects are likely attributable to the poor quality of the underlying tissues which may be fibrotic and/or inflamed, the dead space potential between the advancing flap and underlying tissue, and poor vascularity of the tissues in general.
Extracellular matrix (ECM) grafts are absorbable bioscaffolds commonly used across a range of plastic and reconstructive procedures to scaffold soft tissue repair. These technologies provide a temporary scaffold for cellular infiltration and capillary formation while providing protective coverage and reinforcement of the defect until the bioscaffold is absorbed into the regenerating soft tissues (3). Many different ECM grafts are clinically available and differ in the origins of the source tissue (e.g., human, porcine, bovine, equine) and the processes used to decellularize the tissue to remove nuclear and cellular material while preserving the structure and composition of the tissue ECM.
Ovine forestomach matrix (OFM), is a decellularized ECM bioscaffold isolated from ovine forestomach tissue and has an established use in a range of clinical applications such as the management of acute and chronic wounds (4–9), skin grafting (10) and abdominal wall repair (11, 12). Previous studies have demonstrated OFM exerts a variety of biological functions. For example, OFM exhibits anti-inflammatory effects with broad-spectrum tissue protease modulation (13, 14), as well as stimulation of cell migration, differentiation and infiltration (15, 16). The matrix promotes neovascularization and is populated via cellular infiltration and completely remodeled into the regenerating tissues (16). Insights into the mechanisms behind these biological effects are provided by analysis of the structure and composition of OFM. To date, 151 different matrisomal proteins have been identified in the material that include a wide variety of collagens, adhesion proteins, and signaling molecules such as 12 growth factors including but not limited to fibroblast growth factor 2 (FGF2), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and connective tissue growth factor (CTGF) (15, 17). Structural studies have demonstrated that collagen fibril integrity and functional responses are preserved in OFM thus reflecting the retention of native ECM architecture (18). The material is highly porous and conducive to fluid imbibement and cellular infiltration, in addition to having robust mechanical properties suitable for incorporation into devices for load bearing indications (19).
Considering the regenerative properties of ECM grafts, it was hypothesized that complications following flap reconstruction of chronic wounds may be reduced by inclusion of an ECM graft to stabilize and augment the surgical flap and underlying tissues. This pilot case series presents our initial findings implementing this strategy.
Retrospective data was collected from operation notes, clinical photography, and clinical records. A total of n = 9 defects from 9 patients were included in the case series (Table 1). All patients had various comorbidities known to complicate healing and were to undergo a planned flap reconstruction of a chronic defect. All defects were chronic and non-healing with an age range of 5 months to 7 years. All defects were prepared via sharp debridement or aggressive excision of chronic tissues. The OFM ECM graft (Myriad™ Soft Tissue Matrix, Aroa Biosurgery Limited, Auckland, New Zealand), was rehydrated in sterile saline (~ 5 min), trimmed to size, then placed into the base of the defect. Defects were then closed by local flap advancement and 3-0 Monocryl suture. Jackson-Pratt (JP) drains and incisional negative-pressure wound therapy (NPWT) were used as required (Table 1). Defects were monitored for up to 3–6 months (Table 1) for dehiscence, infection or recurrence.
We hypothesized that the concurrent placement of an underlaid ECM graft during flap reconstruction of chronic wounds may reduce surgical complications by reducing inflammation of the proximal tissues and stabilizing the flap. Using this strategy, uncomplicated healing was achieved in n = 7 of the n = 9 study participants with defects (Table 1). Post-operative complication, namely dehiscence, occurred in n = 2 of the defects. However, both of these defects progressed to heal secondarily with no additional surgical intervention required. All healed defects demonstrated good cosmesis comparable to adjacent tissues. Assessment of healed defects demonstrated excellent functionality and motion and all patients were satisfied with their respective outcomes.
Fifty-three-year-old obese male who had a single-port laparoscopic gastric band placement performed 10 years prior. The band eroded through the skin and was surgically removed but closure was complicated by secondary dehiscence. At the time of intervention, the patient had a non-healing abdominal wound for 11 months, a more recent revision surgery (5 months prior) had also failed with a dehisced surgical site. Previous management of the defect was carried out via alginate dressings, cadexmer iodine, and saline gauze. The defect was causing moderate pain, but was clean with no evidence of infection and hypertrophic granulation tissue was present (Figure 1A). Surgical incision was made at the defect margins, through subcutaneous adipose tissue and down to the fascia (Figure 1B). An ECM graft (Myriad™, “Thick”) was trimmed to fit the defect and placed onto the fascia (Figure 1C). Due to the depth of the defect and potential for dead space, second and third layers of ECM graft were prepared and placed in the defect in a layered arrangement. The defect was then closed by flap advancement with subcutaneous running suture with JP drain placed and incisional NPWT initiated. After seven days, cutaneous tissues demonstrated good apposition and tissues exhibited no inflammation (Figure 1D), the NPWT and drain were removed and patient was discharged with instruction to wear abdominal binder and 6 weeks heavy lifting/strenuous activity restriction. At 6 months, the site remained closed with no dehiscence or other complications.
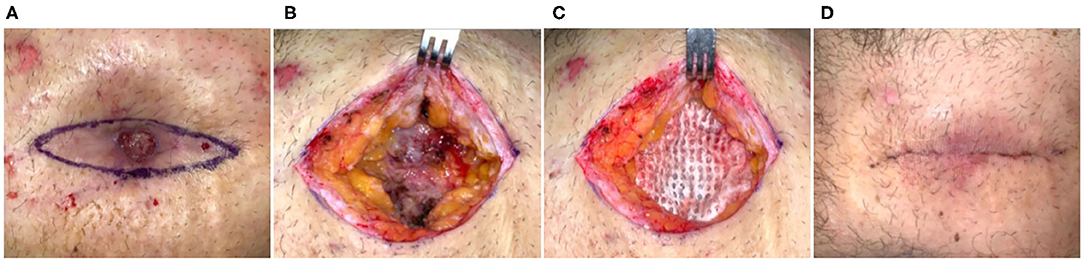
Figure 1. Flap advancement and ECM stabilization of an abdominal dehiscence. Case 1—(A) Erosion of the abdominal tissues resulting from a gastric band and failed previous surgical reconstruction of the ~11-month-old defect. (B) Wide excision of the defect down to the underlying fascia and adipose tissue. (C) Placement of the ECM graft into the base of the defect prior to flap advancement and closure. (D) Seven days post-op. Remained healed at 6 months.
Seventy-three-year-old female who was non-ambulatory secondary to Parkinson's disease, Lupus, and rheumatoid arthritis. Sacral pressure injury had been present for 7 years and had been previously managed with medical honey and enzymatic debridement. The patient had undergone a reconstructive procedure 1 month prior, using excision of the defect and flap coverage. The flap had subsequently dehisced and while there were no signs of infection, the defect was chronically inflamed (Figure 2A). Defect was excised with side margins down to the coccyx, with a partial ostectomy (Figure 2B). An ECM graft (Myriad™, “Thick”) was trimmed to fit the defect and into the base of the defect and covering the bone protrusion of the coccyx (Figure 2C). The excision was closed via a flap advancement with suture used to the close the primary incision line (Figure 2D). JP drains were placed and incisional NPWT initiated. A dehiscence of the site developed, which subsequently went onto heal via secondary intention at 3 months. At 6 months the defect remained healed.
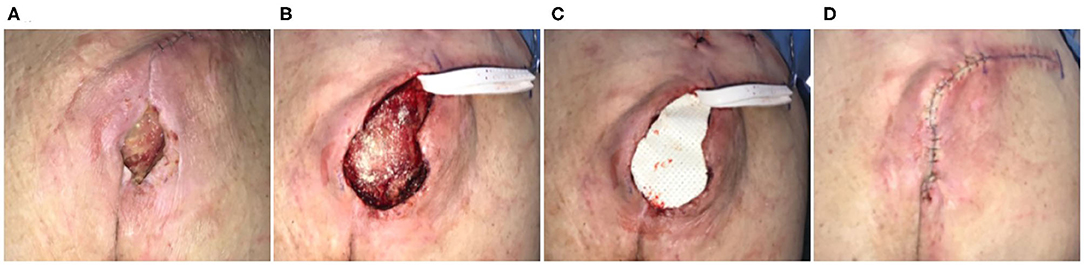
Figure 2. Surgical reconstruction of recalcitrant sacral pressure injury. Case 2—(A) Eleven-month-old pressure injury that had previously failed a flap reconstruction 1 month prior; secondary procedure performed using ECM graft to stabilize the flap. (B) Excision of ulcer with partial ostectomy. (C) Placement of the ECM graft and (D) flap closure.
Twenty-six-year-old female with a 13-month-old non-healing full thickness defect following open reduction and internal fixation surgery of the left ankle (Figure 3A). Orthopedic hardware remained in place, and the wound was culture negative. The defect had previously been managed through use of alginate dressings. Surgical excision of proximal chronic tissues was carried out (Figure 3B). Trimmed ECM graft (Myriad™ “Thin”) was placed contacting the tissues of the defect bed and allowed to rehydrate in situ via absorption of blood components (Figure 3B). The defect was closed by flap advancement (Figure 3C) and underwent incisional NPWT for seven days. The defect exhibited signs of infection and secondary dehiscence 1-week post-surgery (Figure 3D). Additional ECM graft material was packed in the dehiscence cavity (Figure 3D) and covered with a gentian violet/methylene blue foam secondary dressing. Over the following weeks, the defect demonstrated marked improvement with healthy granulation tissue development (Figure 3E). Therefore, application of additional ECM graft material, hydrolyzed collagen dressings covered with a non-adherent dressing layer and continued use of gentian violet/methylene blue secondary dressings were used to heal the defect via secondary intention. Successful closure of the defect by secondary intention was achieved 8 weeks after the initial flap surgery (Figure 3F).
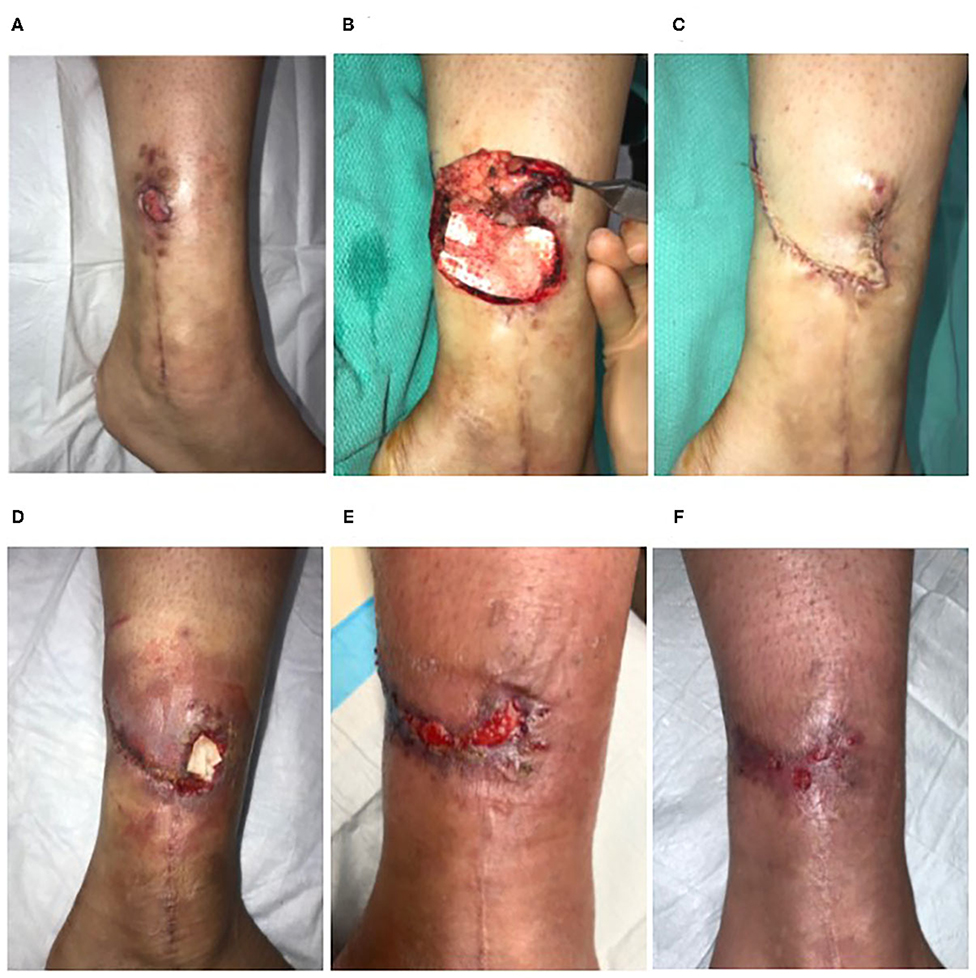
Figure 3. Reconstruction of a non-healing lower extremity surgical defect. Case 3—(A) Thirteen-month-old non-healing defect following open reduction and internal fixation surgery of the left ankle. (B) Surgical excision of proximal chronic tissues and placement of the ECM graft into the defect. (C) Flap advancement and closure. (D) The defect exhibited signs of infection and secondary dehiscence 1-week post-surgery and was treated with a second ECM graft. (E) At 6 weeks, the defect demonstrated marked improvement with healthy granulation tissue development. (F) Successful closure of the defect by secondary intention was achieved 8-weeks after the initial flap reconstruction without additional surgical intervention.
We hypothesized that complications following flap reconstruction of chronic defects may be reduced by the complementary use of an ECM graft to stabilize and augment the surgical flap and underlying tissues. This pilot case series explored using a one-stage procedure of flap reconstruction augmented with ECM graft placement. Simultaneous use of ECM grafts in flap closure is scarcely documented in the literature. The use of an amnion-derived ECM graft material has been shown to improve random flap survival in a preclinical murine model (20), however, this work primarily focused on combination use of amnion graft with mesenchymal stem cell supplementation and has not been translated into human studies. Various clinical reports exist of utilizing ECM grafts in the salvage of compromised or failed flaps (21, 22), but these reports describe reactive use of an ECM graft in a second intervention rather than a deliberate initial strategy. In periodontal surgery, ECM grafts are commonly used in combination with coronally positioned flaps for the treatment of gingival recession, acting to provide root coverage and increase the thickness of the gingiva (23, 24).
To our knowledge this is the first published work regarding the use of ECM grafts to augment flap reconstruction. The ECM graft utilized in the current study is derived from ovine (sheep) forestomach tissue, specifically the propria submucosa, a layer of ECM that extends through the forestomach tissue (15). Once isolated the propria submucosa undergoes a decellularization process to remove the ovine cells and nucleic acids leaving a layer of intact ECM. Decellularization utilizes a combination of detergents, chelating agents and salts to firstly lyse the ovine cells, then solubilize the cell membranes and nucleic acids (15). Grafts are fabricated using individual layers of ECM and presented either as a three- (~1.0 mm) or five-layer graft (~1.5 mm), in sizes up to 200 cm2. While advanced ECM bioscaffolds have typically been difficult to access due to cost, pricing of the OFM ECM graft (~USD$250–USD$2,500) enables novel usage of this technology in reconstructive procedures.
We hypothesized that an ECM graft may help to reduce flap complications via a combination of biological and physically mediated mechanisms. For example, the protease modulating effects of OFM (13) may rectify the underlying unbalanced environment in chronic soft tissue defects with high levels of tissue inflammation and proteolytic activity. Promotion of neovascularization by ECM material (16) may assist in establishment of blood supply from both the flap and the underlying tissues of the defect. Increasing the local blood supply to the defect through vascularization also minimizes risk of infection via increased perfusion of protective immune system elements and/or systemically administered antibiotics (25). Indeed, proper site preparation is vital for successful closure of chronic defects with positive post-debridement cultures being a proven predictor of failure of flap closure (26), as such our procedure included aggressive debridement and removal of chronic defective tissues. Additionally, placement of ECM graft material in the defect bed may provide occlusion of dead space between the flap and underlying soft tissues.
Results from the current pilot case series were encouraging, but the limited sample size must be noted. Additionally, five participants received incisional NPWT, the contribution of which cannot be fully assessed with this limited sample size. While incisional NPWT is known to reduce surgical site infections, the risk of dehiscence of surgical primary closures has been shown to be equivalent to standard wound dressings (27).
Flap reconstruction is an effective and prevalent method for the repair of chronic soft tissue defects. The present case series piloted use of an ECM graft to augment flap reconstruction of chronic soft tissue defects. Outcomes of these initial cases may warrant future controlled studies for evaluation of this technique relative to unmodified flap closure.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
All patients provided written informed consent for their images and data to be used for research and publication purposes.
MD contributed to the design of the study, clinical management, data collection, data analysis, and preparation of the manuscript. KB and AW contributed to case management and data collection. KH, KD, and DG contributed to clinical management. All authors contributed to manuscript revision, read, and approved the submitted version.
MD received an educational travel grant from Aroa Biosurgery Limited.
MD consults for Aroa Biosurgery Limited (Auckland, New Zealand). Myriad™ Soft Tissue Matrix was provided by Aroa Biosurgery Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors acknowledge that assistance of Barnaby May and Christopher Miller (Aroa Biosurgery Limited) for assistance in the preparation of the manuscript.
1. Tran BNN, Chen AD, Kamali P, Singhal D, Lee BT, Fukudome EY. National perioperative outcomes of flap coverage for pressure ulcers from 2005 to 2015 using American College of Surgeons National Surgical Quality Improvement Program. Arch Plast Surg. (2018) 45:418. doi: 10.5999/aps.2018.00262
2. Bamba R, Madden JJ, Hoffman AN, Kim JS, Thayer WP, Nanney LB, et al. Flap reconstruction for pressure ulcers: an outcomes analysis. Plastic Reconstr Surg Glob Open. (2017) 5:e1187. doi: 10.1097/GOX.0000000000001187
3. S. Badylak. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. (2002) 13:377–460. doi: 10.1016/S1084952102000940
4. Ferreras DT, Craig S, Malcomb R. Use of an ovine collagen dressing with intact extracellular matrix to improve wound closure times and reduce expenditures in a US Military Veteran Hospital Outpatient Wound Center. Surg Technol Int. (2017) 30:61–9.
5. Bohn GA, Gass K. Leg ulcer treatment outcomes with new ovine collagen extracellular matrix dressing: a retrospective case series. Adv Skin Wound Care. (2014) 27:448–454. doi: 10.1097/01.ASW.0000453728.12032.6f
6. Liden BA, May BC. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care. (2013) 26:164–7. doi: 10.1097/01.ASW.0000428862.34294.d4
7. Simcock J, Than M, Ward B, May B. Treatment of ulcerated necrobiosis lipoidica with ovine forestomach matrix. J Wound Care. (2013) 22:383–4. doi: 10.12968/jowc.2013.22.7.383
8. González: Use of collagen extracellular matrix dressing for the treatment of a recurrent venous ulcer in a 52-year-old patient. J Wound Ostomy Continence Nurs. (2016) 43:310–2. doi: 10.1097/WON.0000000000000231
9. E. J. Lullove. Use of ovine-based collagen extracellular matrix and gentian violet/methylene blue antibacterial foam dressings to help improve clinical outcomes in lower extremity wounds: a retrospective cohort study. Wounds. (2017) 29:107–114.
10. Simcock J, May BC. Ovine forestomach matrix as a substrate for single-stage split-thickness graft reconstruction. Eplasty. (2013) 13:e58.
11. Ferzoco SJ. Early experience outcome of a reinforced bioscaffold in inguinal hernia repair: a case series. Int J Surg Open. (2018) 12:9–11. doi: 10.1016/j.ijso.2018.06.001
12. Sawyer MA. New ovine polymer-reinforced bioscaffold in hiatal hernia repair. JSLS. (2018) 22:e2018.00057. doi: 10.4293/JSLS.2018.00057
13. Negron L, Lun S, May BC. Ovine forestomach matrix biomaterial is a broad spectrum inhibitor of matrix metalloproteinases and neutrophil elastase. Int Wound J. (2014) 11:392–7. doi: 10.1111/j.1742-481X.2012.01106.x
14. Street M, Thambyah A, Dray M, Amirapu S, Tuari D, Callon KE, et al. Augmentation with an ovine forestomach matrix scaffold improves histological outcomes of rotator cuff repair in a rat model. J Orthop Surg Res. (2015) 10:165. doi: 10.1186/s13018-015-0303-8
15. Lun S, Irvine SM, Johnson KD, Fisher NJ, Floden EW, Negron L, et al. A functional extracellular matrix biomaterial derived from ovine forestomach. Biomaterials. (2010) 31:4517–29. doi: 10.1016/j.biomaterials.2010.02.025
16. Irvine SM, Cayzer J, Todd EM, Lun S, Floden EW, Negron L, et al. Quantification of in vitro and in vivo angiogenesis stimulated by ovine forestomach matrix biomaterial. Biomaterials. (2011) 32:6351–61. doi: 10.1016/j.biomaterials.2011.05.040
17. Dempsey SG, Miller CH, Hill RC, Hansen KC, May BC. functional insights from the proteomic inventory of ovine forestomach matrix. J Proteome Res. (2019) 18:1657–68. doi: 10.1021/acs.jproteome.8b00908
18. Sizeland KH, Wells HC, Kelly SJ, Nesdale KE, May BC, Dempsey SG, et al. Collagen fibril response to strain in scaffolds from ovine forestomach for tissue engineering. ACS Biomater Sci Eng. (2017) 3:2550–8. doi: 10.1021/acsbiomaterials.7b00588
19. Floden EW, Malak SF, Basil-Jones MM, Negron L, Fisher JN, Lun S, et al. Biophysical characterization of ovine forestomach extracellular matrix biomaterials. Journal of Biomedical Materials Research Part B: Applied Biomaterials. (2011) 96:67–75. doi: 10.1002/jbm.b.31740
20. Chehelcheraghi F, Eimani H, Homayoonsadraie S, Torkaman G, Amini A, Majd HA, et al. Effects of acellular amniotic membrane matrix and bone marrow-derived mesenchymal stem cells in improving random skin flap survival in rats. Iran Red Crescent Med J. (2016) 18:e25588. doi: 10.5812/ircmj.25588
21. Shanti RM, Smart RJ, Meram A, Kim D. Porcine urinary bladder extracellular matrix for the salvage of fibula free flap skin paddle: technical note and description of a case. Craniomaxillofac Trauma Reconstr. (2017) 10:318–22. doi: 10.1055/s-0036-1593473
22. Kruper GJ, VandeGriend ZP, Lin H-S, Zuliani GF. Salvage of failed local and regional flaps with porcine urinary bladder extracellular matrix aided tissue regeneration. Case Rep Otolaryngol. (2013) 2013:917183. doi: 10.1155/2013/917183
23. Guan W, Liao H, Guo L, Wang C, Cao Z. Root coverage using a coronally advanced flap with or without acellular dermal matrix: a meta-analysis. J Periodontal Implant Sci. (2016) 46:22–34. doi: 10.5051/jpis.2016.46.1.22
24. de Queiroz Côrtes A, Sallum AW, Casati MZ, Nociti FH Jr, Sallum EA. A two-year prospective study of coronally positioned flap with or without acellular dermal matrix graft. J Clin Periodontol. (2006) 33:683–9. doi: 10.1111/j.1600-051X.2006.00969.x
25. Suh HP, Hong JP. The role of reconstructive microsurgery in treating lower-extremity chronic wounds. Int Wound J. (2019) 16:951–9. doi: 10.1111/iwj.13127
26. Shuck J, Nolan J, Kanuri A, Evans KK, Attinger CE. The effect of positive post-debridement cultures on local muscle flap reconstruction of the lower extremity. Plastic Reconstr Surg. (2015) 136:9–10. doi: 10.1097/01.prs.0000472283.01460.a1
Keywords: flap reconstruction, chronic wounds, soft tissue defects, extracellular matrix, ovine forestomach matrix
Citation: Desvigne MN, Bauer K, Holifield K, Day K, Gilmore D and Wardman AL (2021) Case Report: Surgical Closure of Chronic Soft Tissue Defects Using Extracellular Matrix Graft Augmented Tissue Flaps. Front. Surg. 7:559450. doi: 10.3389/fsurg.2020.559450
Received: 06 May 2020; Accepted: 15 December 2020;
Published: 26 January 2021.
Edited by:
Charles M. Malata, Anglia Ruskin University, United KingdomReviewed by:
Oren Lapid, Amsterdam University Medical Center (UMC), NetherlandsCopyright © 2021 Desvigne, Bauer, Holifield, Day, Gilmore and Wardman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Micheal N. Desvigne, bWRlc3ZpZ25lQGRlc3ZpZ25lbWQuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.