- First Department of Surgery, National and Kapodistrian University of Athens, Athens, Greece
The role of extended lymphadenectomy in the surgical treatment of gastric cancer has been debated for many years. So far six prospective randomized trials and a number of meta-analyses comparing D1- to D2-lymphadenectomy in open surgery have been published with contradicting results. The possible oncologic benefit of radical lymphadenectomy has been blurred by a number of reasons. In most of the trials the strategies under comparison were made similar after protocol violations. Imperfect design of the trials could not exclude the influence of cofounding factors. Inappropriate endpoints could not detect evidently the difference between the two surgical strategies. On the other hand radical lymphadenectomy was characterized by increased morbidity and mortality. This was mostly caused by the addition of pancreatico-splenectomy in all D2-dissections, even when not indicated. A careful analysis of the available evidence indicates that D2-lymphadenectomy performed by adequately trained surgeons without resection of the pancreas and/or spleen, unless otherwise indicated, decreases Gastric Cancer Related Deaths and increases Disease Specific Survival. This evidence is not compelling but cannot be ignored. D2-lymphadendctomy is nowadays considered to be the standard of care for resectable gastric cancer.
The Rationale for Extended Surgery
The benefit of resection of regional lymph nodes in gastric cancer been debated for many years and still remains under question. Τhe failure of limited surgery to control disease loco-regionally was reported as early as the 1950s by Gordon McNeer. In his autopsy series he found more than 20% cancer recurrence in the non-resected perigastric nodes or the gastric bed (1). As a remedy he proposed “a more thorough” operation with lymphadenectomy of the celiac axis and pancreatico-splenectomy (2). Although this operation improved loco-regional control, it didn’t affect survival (3) and didn’t gain popularity in the West.
In the East at that time the Japanese Research Society for Gastric Cancer established “General Rules” for the surgery of Gastric Cancer (4). The society classified the nodes participating in the lymphatic drainage of the stomach into tiers similarly to today’s classification. The perigastric nodes are the first tier, those lying along the big vessels are the second one, and the more remote, para-aortic lymph nodes nowadays constitute the third tier. Removal of the first tier constitutes D1 lymphadenectomy (formerly known as R1-resection), removal of the second tier D2 lymphadenectomy (formerly known as R2 resection), removal of the third a D3 lymphadenectomy. In the 1960’s the Japanese Society suggested that removal of the appropriate number of tiers would increase the chance of negative “lymphadenectomy margins”. Furthermore, under the influence of the work of Kanai, Kajitani and Aikou (5–7), pancreatosplenectomy became a standard part of D2-lymphadenectomy overlooking the fact that it required specific indications (8). Thus the typical Japanese operative strategy ended up pretty much similar to the one proposed by McNeer. Setting this operation as a standard the Japanese reported remarkably high survival rates. These were soon noticed by their Western colleagues and again the possible benefit of extended lymphadenectomy became a matter of investigation (9). Since then, a number of prospective randomized trials and meta-analyses have been conducted to compare D2- to D1-lymphadenectomy.
As discussed above, the concept of D2-lymphadenectomy was initially based on anatomical grounds. In agreement with the current 8th edition of TNM the first and second lymph node tiers are considered local and regional respectively. An ideal gastrectomy with D2-lymphadenectomy corresponds to extirpation of all locoregional disease. However this “ideal” resection can be verified only by thorough examination of the operative field. Examination of the resected specimen cannot rule out the possibility of lymphatic tissue left behind. Alternatively, we can compare the number of resected lymph nodes to the number of lymph nodes that normally exist on each tier. Wagner et al demonstrated in a cadaveric study that D2-lymphadenectomy would lead to an average retrieval of 27 lymph nodes, but he also noted that significant differences exist among the individuals in the total number of lymph nodes (10). On the other hand, Sharma et al retrieved an average of 35 nodes with a D2-dissection again in a cadaveric study in India (11) . The variation between the two studies may reflect a racial difference between the two populations, or a more extensive dissection, or even the cofounding effect of “less hygienic food consumption leading to lymphatic hyperplasia”. Nonetheless, it is understandable that the number of resected lymph nodes corresponds to the extent of lymphadenectomy but, on the other hand, it cannot stand for the completeness of surgery as regional treatment.
Existing Evidence
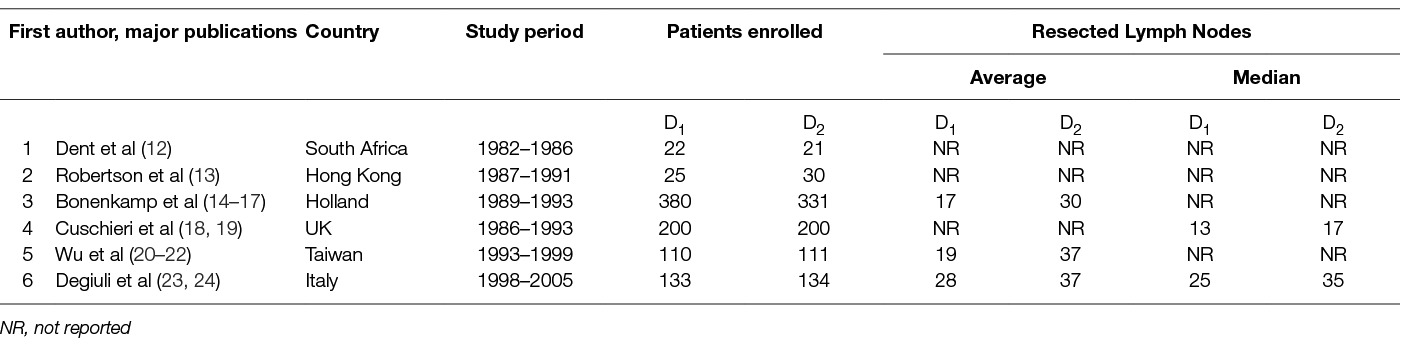
So far six randomized trials compared “limited” to “extended” lymphadenectomy as appreciated by their authors. The retrieved lymph nodes and other features of the trials are depicted in Table 1. Despite their major or minor limitations and the issues left unresolved, these trials contributed much not only to our knowledge regarding gastric cancer but also in general.
The trial of Dent et al (12) in South Africa randomized 43patients. Major findings were that blood transfusion requirements, operating time and hospital stay were longer with extended lymphadenectomy. At a median follow-up of 3.1 years no benefit regarding survival was seen.
Robetson et al (13) randomized 55 patients in Hong Kong into D1 or D2 lymphadenectomy with compulsory pancreaticosplenectomy to find out again that operating time, transfusion requirements and hospital stay, all increased with extended lymphadenectomy. Morbidity was also higher with extended lymphadenectomy and was mostly due to abdominal sepsis. Contrary to the expectations overall survival was significantly worse and this was attributed to the impact of increased blood transfusion.
Cuschieri et al (18, 19) in the UK randomized 400 patients into two equal arms. 8 patients of the D1 arm and 113 of the D2 arm had a pancreaticosplenectomy while 54 patients of the D1 arm and 18 patients of the D2 arm had a splenectomy. Pancreas and/or spleen resection were blamed for the significantly higher morbidity seen after extended lymphadenectomy and the significantly longer hospitalization. Hospital mortality was also significantly higher. The 5-year Overall Survival, Disease Specific Survival and Disease Free Survival were similar in both treatment arms. The authors speculated that the possible benefit of extended lymphadenectomy was lost by the detrimental effects of pancreatico-splenectomy. The longest survival of the trial was seen with pancreas and spleen preserving D2-lymphadenectomy. But then again this concerned patients with more distal tumors, which may have acted as a cofounding factor.
In the “Dutch trial” (14–17) 380 patients underwent D1- and 331 D2- lymphadenectomy. 11% of the D1-patients and 38% of the D2- patients had a splenectomy. Among them 3% of the D1-patients and 30% of the D2-patients had a pancreatico-splenectomy. Again extended lymphadenectomy increased mortality, morbidity, reoperation rates and hospitalization. After 15 years the D2- group had a non-significant better Overall Survival, Disease Free Survival and Recurrence Risk. In contrast, Overall Recurrence Pattern and Gastric-Cancer-Related Death Rate were significantly lower. Patients with D2-lymphadenectomy without pancreatico-splenectomy showed again a significantly higher Overall Survival.
In the Taiwanese trial (20–22) conducted by Wu et al , 211 patients were randomly allocated into D1- or D2-lymphadenectomy. Extended lymphadenectomy increased operating times, blood loss, transfusion and hospital stay. Again morbidity was increased mostly due to abdominal sepsis but mortality did not differ. Extended lymphadenectomy led to significantly higher 5-year Overall Survival but no difference in the Recurrence Rates was seen in the cases with R0 resection.
Due to poor accrual, the Italian study (23, 24) closed after 8 years with a statistical power of approximately 80% as only 267 patients were randomized. Morbidity, mortality, 5-year Overall Survival and Disease Specific Survival were similar in both arms. However limited lymphadenectomy led to significantly better Disease Specific Survival in patients with pT1 tumors and patients older than 70-years old.
Is D2 Lymphadenectomy More Effective Than D1 ?
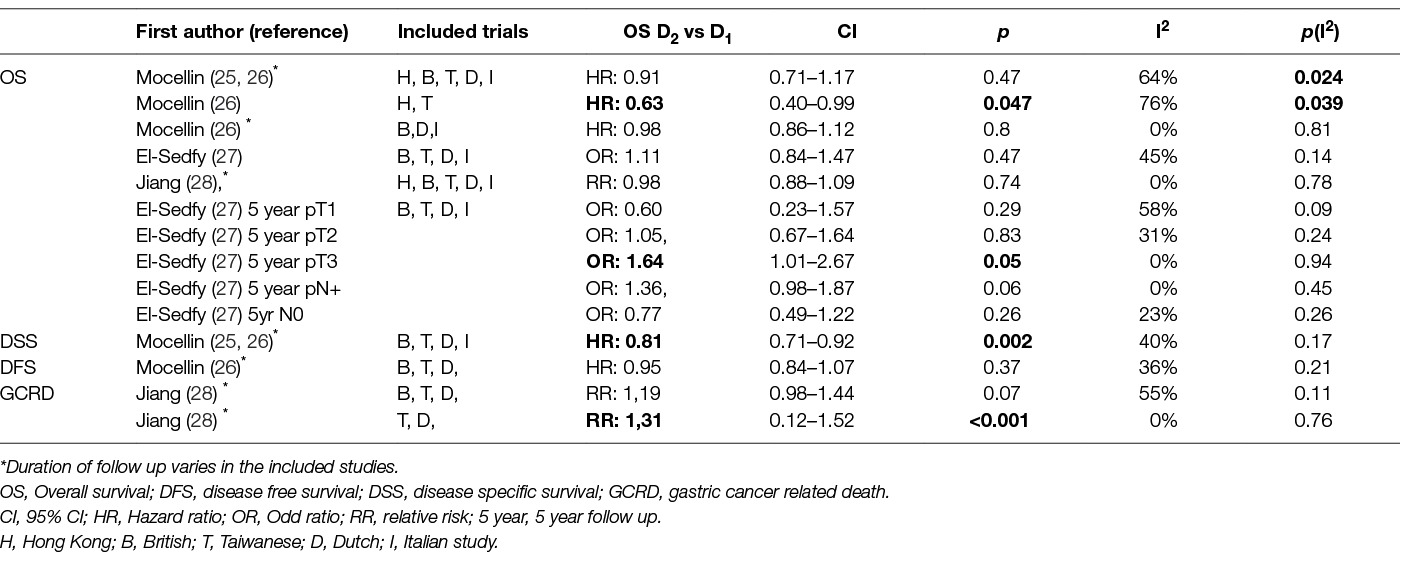
As it can be seen in Table 2 the Taiwanese trial (which excluded patients with early gastric cancer) demonstrated a benefit of D2-lymphadenectomy on 5-year Overall Survival and 5-year Disease Specific Survival (22). Similarly, the Dutch trial showed that D2 lymphadenectomy reduces significantly Reccurence Rates and Gastric Cancer Related Death after 15 years (17) In all other trials overall Survival Rates were similar after D1 and D2 dissection (12, 13, 17, 19, 22, 24). Regarding the oncological outcome of extended lymphadenectomy “stratified” by cancer stage, the univariate analysis of the Dutch trial data revealed a significant benefit for 15-year overall survival for Stage II and for N2 patients (by TNM 1997 definition) however the outcome of multivariate analysis was not reported (17). In contrast, the Italian trial showed that D1 lymphadenectomy may be better in aged patients and in early gastric cancer (pT1 disease D1: 98% vs D2: 83%, p = 0·015) (24). Nevertheless, none of the trials had enough power to reveal a survival difference for a certain cancer clinical TNM- or T-, or N- stage after extended lymphadenectomy. Also, the same outcome seemed to concern patients with early or with locally advanced cancer. None of the trials offered solid data for the election of the extend of lymphadenectomy in proportion with the disease burden. Of note, that none of the trials including Stage IA patients discriminated between T1a and T1b patients. This distinction seems of great importance as invasion of submucosa changes the malignant potential of the disease.
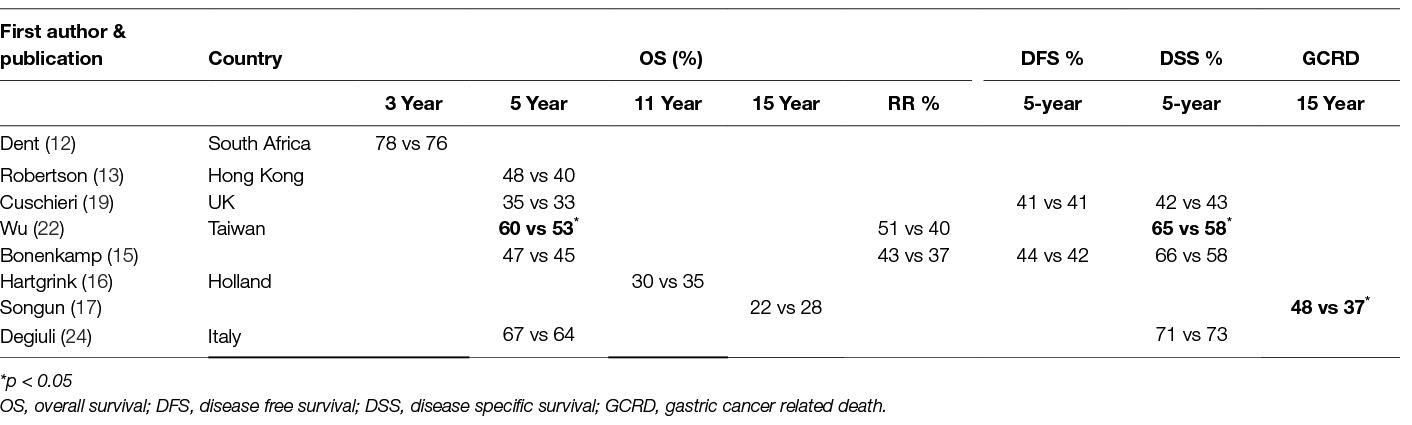
Table 2. Comparison of D1 to D2 Lymphadenectomy (D1:D2) in prospective randomized trials regarding oncologic outcome.
A number of meta-analyses of the final results from these randomized trials have been published also. Their conclusions regarding survival are summarized in Table 3 but as seen in the I2 column most of them suffer from heterogeneity. Also the duration of follow-up differs among the included studies. Mocellin et al (25, 26) included the 15 years results of the Dutch trial and Jiang et al (28) included the 10-year Dutch results. The rest of the included studies in all meta-analyses were based on the 5-year results. Despite these shortcomings, these meta-analyses still worth consideration. Most of them concluded that no difference seems to exist between D2 and D1 lymphadenectomy regarding Overall Survival. Only a meta-analysis of the subgroup of Hong Kong and Taiwan trials combined led to a significant improvement of overall survival, but this was at the cost of extremely high and significant heterogenity (26). In contrast, the subgroup of the European studies was highly homogenous but showed no difference between the two arms (26). Similarly Disease Free Survival was unaffected, but D2-dissection improved significantly Disease Specific Survival (25, 26). Along the same vein Jiang et al found that D2 dissection decreased significantly the risk of Gastric Cancer Related Death when the British trial was excluded as a cause of heterogeneity (28). Finally, El Sedfry et al showed that D2-dissection improves 5-year Overall Survival of patients with pT3 disease, but not of those with early gastric cancer or pT2 disease, a finding opposing the Italian study (27). This evidence altogether is not compelling but cannot be ignored and favor extended lymphadenectomy
Is D2 Lymphadenectomy Safe? Morbidity and Mortality
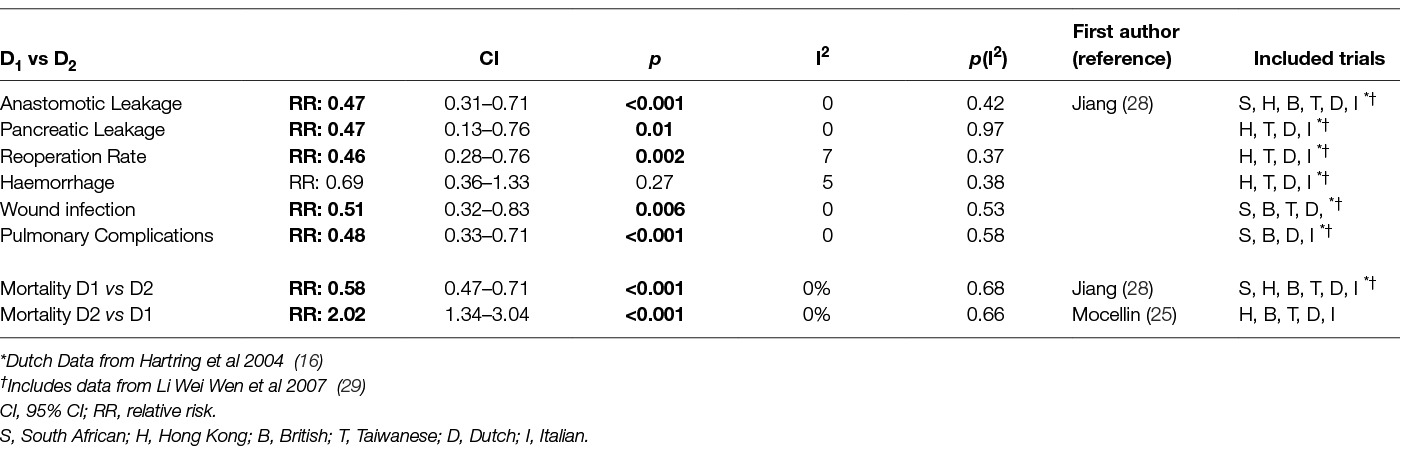
The technical complexity and difficulty of D2 – lymphadenectomy rose concerns on its morbidity and mortality. Tables 4; 5 depict the outcomes of the comparison between D1 and D2 lymphadenectomy in randomized trials and subsequent meta-analyses. Both the British and the Dutch trial and two recent meta-analyses of their data (14, 18, 25, 28) revealed increased mortality after D2 dissection. In the rest trials there was no difference in mortality. All trials except the Italian found increased morbidity and hospitalization with D2-gastrectomy (12–14, 18, 20, 21). Significantly more time was needed for the D2- operation as shown by the South African, Hong Kong and Taiwan trials (12, 13, 20, 21). Also in the same trials and the Dutch one D2- lymphadenectomy led to significantly greater blood loss and transfusion requirements (12–14, 20, 21). This was refuted by a subsequent meta-analysis which on the other hand showed that D2- dissection increased significantly all other complications: anastomotic leaks, pancreatitis, pulmonary complications, wound infection rates and re-operation rates (28).
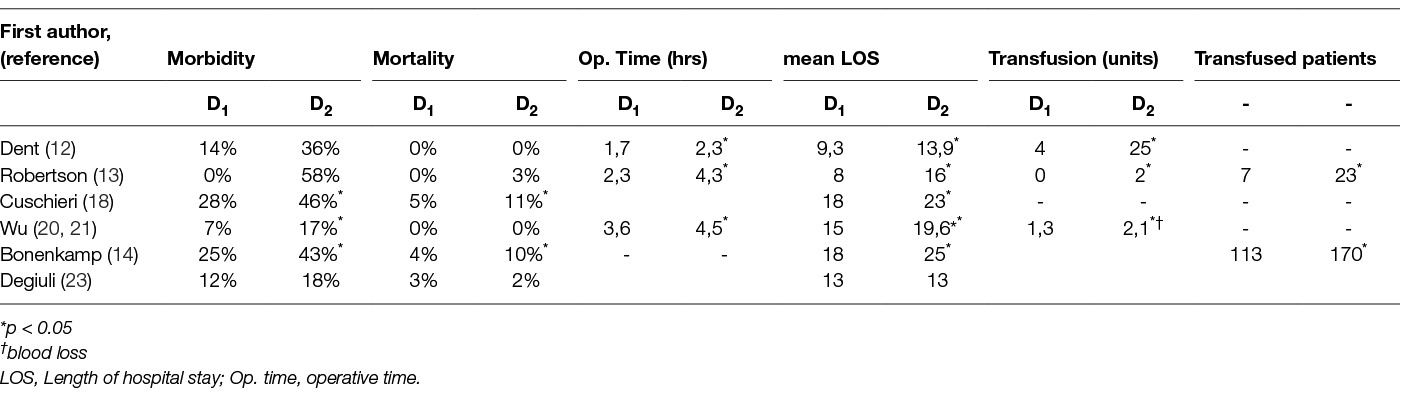
Table 4. Morbidity, mortality and perioperative characteristics in prospective randomized trials comparing D1 to D2 Lymphadenectomy.
The Role of Pancreas And/or Spleen Resection in Morbidity and Mortality
A major part for the increase of morbidity and mortality has been attributed to the resection of the spleen and/or the pancreas, once though a compulsory step for D2-lymphadenectomy (Tables 6, 7). Although the Italian trial showed that post-operative hospital stay was not affected by splenectomy implying unaltered morbidity (23), the Taiwanese and the UK trial reported an increase in morbidity after both splenectomy and/or pancreaticosplenectomy (18, 20, 21). Furthermore, the difference in morbidity between D1- and D2- lymphadenectomy became non-significant in the British trial after allowance for splenectomy or pancreatico-splenectomy (18). In other words the increased morbidity seen after D2 lymphadenectomy was chiefly due to the removal of pancreas and/or spleen. In contrast, the Dutch trial reported no association of the increased morbidity of D2- lymphadenectomy to pancreaticosplenectomy (14). With regards to mortality both the Dutch and the Taiwanese trial reported no effect of pancreatico-splenectomy (14, 20, 21). In contrast, the British trial reported a significant increase of mortality after pancreatectomy and/or splenectomy (18). This was confirmed by a meta-analysis of the British and Dutch data which also showed that postoperative mortalities of D1- and D2- dissection were even after resection of the spleen and/or the pancreas (Table 8) (28) .
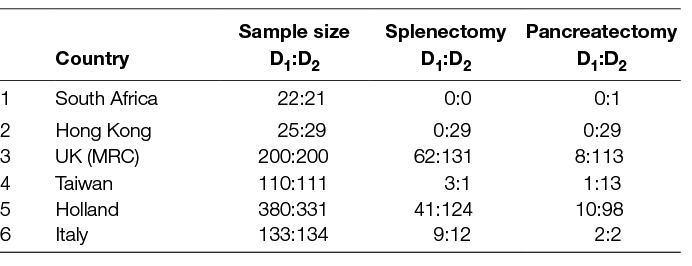
Table 6. Patients with Splenectomy or Pancreaticosplenectomy in the prospective randomized trials comparing D1- to D2-Lymphadenectomy.
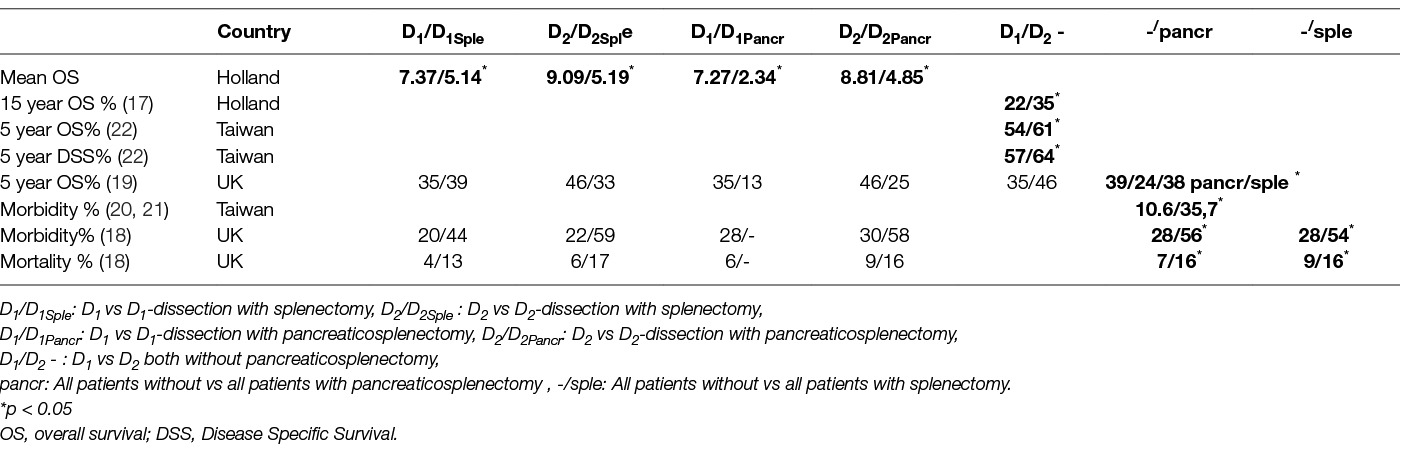
Table 7. Effect of Splenectomy and Pancreatico-splenectomy on morbidity, mortality and oncologic outcome in prospective randomized trials comparing D1- to D2-Lymphadenectomy.
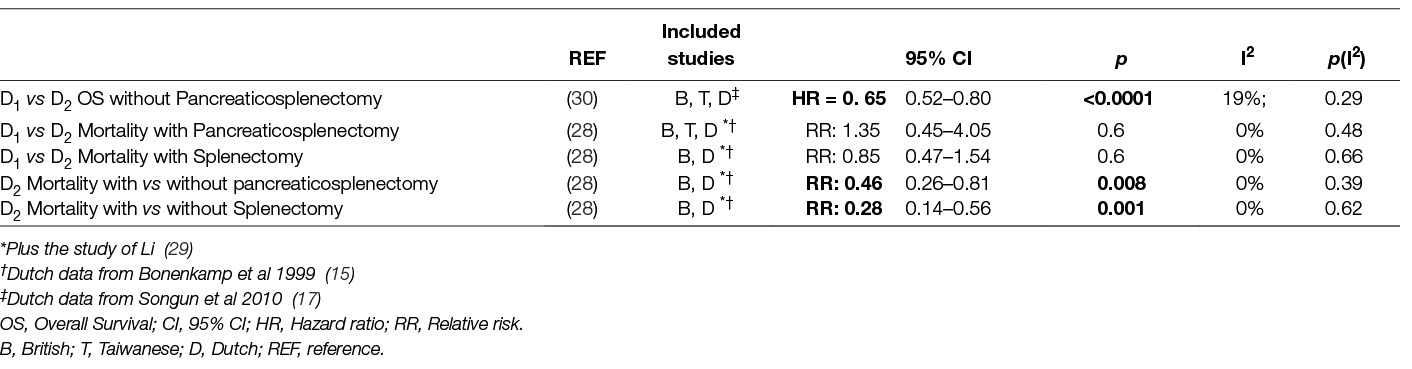
Table 8. Effect of Splenectomy and Panceatico-splenectomy in mortality and oncologic outcome in meta-analyses comparing D1- to D2-Lymphadenectomy.
Taken all these together we may conclude that D2-dissection leads to increased morbidity which on the other hand can be decreased significantly by avoiding resection of the spleen and the pancreas unless properly indicated.
The Role of Pancreas And/or Spleen Resection on Survival
Pancreatico-splenectomy, but not splenectomy alone, decreased Overall Survival significantly in the UK trial (19)(Tables 7 and 8). After 5 years the group with D2-dissection plus pancreaticosplenectomy presented the poorest survival. It was speculated that the possible benefits of extended lymphadenectomy were thus blurred. In the Dutch trial splenectomy or pancreaticosplenectomy decreased mean Overall Survival after 15 years in both the D1 and D2 dissection group (17). Interestingly, D2 lymphadenectomy without pancreaticosplenectomy led to a significant improvement of 15-year Overall Survival. This was supported by the Taiwanese trial where extended lymphadenectomy with preservation of the pancreas and spleen increased 5 year Overall Survival and Disease Free Survival (22). Of note, neither pancreatectomy nor splenectomy seemed to affect the Hazard Ratio for death (22). A meta-analysis of the data from the Dutch, the British and the Taiwanese trials regarding patients with and without splenectomy and/or pancreatectomy showed a benefit of D2- compared to D1-lymphadenectomy on Overall Survival. Remarkably the heterogeneity among the included trials was low (30). Given the known effect of pancreaticosplenectomy on morbidity, its detrimental effect on survival is not unexpected. Long term survival after oncologic gastrectomy is known to be influenced by morbidity and blood transfusion rate (31–35). As a consequence avoiding spleen and/or pancreas resection increases survival, at least indirectly.
Protocol Violations and Other Issues
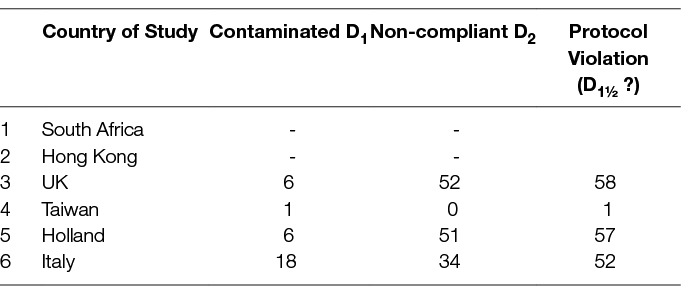
A strict adherence to the study protocol is of paramount importance in any clinical trial. Quality control in the comparison of two lymphadenectomy strategies should ensure the complete removal of the lymph node stations as predetermined by the protocol, and only these. Removing more or less stations constitutes protocol violation. If fewer stations are removed then compliance to the protocol is defective, if more stations are resected then contamination ensues. In particular for lymphadenectomy in gastric cancer, contamination has been defined as the resection of more than two lymph node stations that should not have been removed i.e., over-resection (36). On the other hand, non-compliance has been defined as the non-resection of more than two lymph node stations that should have been excised i.e., under-resection (36). Contamination of D1-operations and non-compliance of D2-operations would obviously mitigate any expected difference. As it can be seen in Table 9, in all European Trials more than 50% of the enrolled patients received something between D1- and D2- dissection regardless of the allocated treatment. Proving a difference between the two lymphadenectomies is difficult under these circumstances. This can be understood by the re-analysis of 15-year data from the Dutch trial. The overall survival initially reported had a difference of 8% (21% vs 29% p = 0.351 for D1 and D2-dissection respectively). After excluding both non-compliant and contaminated operations the difference became 10% (23% vs 33% p = 0, 26 for the D1 and D2 groups respectively) but still remained not significant (37). However the 15-year survival of the group of 139 patients with a compliant and contaminated D2 dissection (i.e., those who had a proper D2-resection and beyond) was higher than the survival of the non-contaminated D1-group (those who had a proper D1-dissection); and more importantly it was higher than the survival of the remaining 192 patients who underwent what the authors perceived as “D2 dissection” (37).
The role of training and of the learning curve also cannot be overemphasized. Conducting a proper D1- or D2- lymph node dissection and being able to discriminate between these two operations demands appropriate training. This improves operative time, lymph node yield, morbidity and mortality. Parkih et al in UK reported that the curve reaches a plateau after 18—24 months or 15–25 procedures (38), Lee et al reported that 8 months or 23–35 cases are needed (39) while Wu et al defined the number of needed cases to 25 (21). Furthermore training influences oncologic outcome because Overall Survival is significantly improved by a history of 100 gastrectomies (40). Such an in-depth training was reported to exist for the surgeons of the Taiwanese trial. Also in the Italian trial all surgeons had a history of at least 21 D2 dissections due to their participation in the Italian multicenter phase II study (41). Consequently, they were adequately trained regarding morbidity and mortality. As expected, morbidity and mortality in the Taiwanese and Italian studies were more or less the same. In contrast in the Dutch trial the training actually happened during the study. As the investigators state, during the first 4 (42) or 6 months (14) one Japanese surgeon trained the 8 (42) or 11 (14) regional supervising surgeons (the training period and number of regional supervisors-trainees are unclear in literature). They in turn, supervised and advised the regional surgeons. Additional training material (booklet and videotape) were given to the regional surgeons. Also during the trial the Japanese and/or a supervising surgeon proctored the regional surgeons (14, 42). 67 patients were allocated to the D2-arm in the first 10 months of the study i.e., the training period and some more months. The Japanese instructor operated 34 patients and the supervising surgeons 33 (42). In other words, by the time each supervisor started supervising and proctoring the regional surgeons he himself had performed the maximum of 4 D2 dissections (42). The trial went on and, surprisingly, no learning curve was detected for any surgeon. Similarly, in the British trial the operating surgeons were trained with a booklet and an instructional video. Standardization was also pursued through an “operative form” filled by the surgeon. No proctorship or mentorship was available throughout the trial (18). Given the comparable training of surgeons in these two European trials the similarity in the morbidity and mortality outcomes is not unpredictable. Regarding the South African and the Hong Kong trial, no data exist on the training of the participating surgeons and no comments can be made. Finally, as far as the oncologic outcome is concerned the criteria defined by Kim et al (40) are fulfilled only by the Taiwanese trial, which of note is the only one who demonstrated a survival difference.
Beyond any doubt the comparison of two different surgical strategies against a known lethal disease such as gastric cancer should have survival as a chief endpoint. However Overall Survival includes also all those patients who died from causes unrelated to gastric cancer. Although theoretically these patients will be equally distributed in the arms of a well conducted randomized comparison, this is not always the case. For example, the patients of the D2 arm were accidentally older in the South African trial (12), and there is always a possibility of unknown cofounding factors not taken into account. Disease Specific Survival and Disease Related Death Rate are more specific endpoints because they reflect directly those who survived and those who died from gastric cancer. The validity of these endpoints can be seen in the Dutch trial where no difference was seen in Overall Survival but the difference in Gastric Cancer Related Deaths i.e., the principal aim of surgery was statistically significant (17).
Any trial should have the power to prove the expected difference in the treatment outcome. One can speculate that this was not probably the case in the trials of Dent et al (12) and Robertson et al (13) where the size of each arm was small. Furthermore, the population where the trial is conducted should be of enough size to support the accomplishment of the trial. This was not the case with the Italian study which closed due to pure accrual (24). Moreover, the expected outcome study should be clearly and pragmatically defined. This can be seen in the British trial where the initial estimate was a 20% survival for the D1 arm and a 12% improvement with D2 dissection (19). These estimates were incorrect and survival after D1-dissection was found to be around 30% (19, 43). To prove a 10% improvement in the outcome more than 1,000 patients should have been enrolled and the recruitment period would have exceeded 15 years (43), which brings us to the next issue: the timeframe during which a trial is conducted must be narrow. During the years medical care improves, patients are diagnosed earlier, staging becomes more accurate, perioperative care becomes more effective, postoperative treatment evolves; even surgery itself changes with the implementation of endoscopic, minimally invasive and other techniques.
Conclusion
From the studies conducted so far there is plenty of evidence on the equivalence, and there is limited evidence on the superiority of D2- lymphadenectomy. If morbidity and mortality are kept to a minimum, then probably D2 lymphadenectomy is advantageous, in particular for patients with locally advanced tumors. However it seems that this advantage is easily wiped out by unrequired resection of the pancreas and/or spleen and by inadequately trained surgeons. It has to be noted however that the existing studies are neither recent nor flawless and the conclusions drawn may not pertain to today’s modern medicine. Since the closure of the last trial on the row, some 12 years ago, much progress has been made in the diagnosis, staging, peri-, and post-operative care of patients with gastric cancer. Perhaps in the near future as medicine advances, the necessity and extend of lymphadenectomy will have to be redefined. The new framework of surgical oncology should be individualized and take into consideration “novel” parameters like neoadjuvant treatment, biological therapies and, more importantly, the molecular characteristics of each tumor. Until new solid evidence appears, with new, contemporary, well designed and properly conducted randomized trials, implementation of D2-lymphadenectomy into the daily practice of well-trained surgeons seems justified. For this reason, the vast majority of the scientific societies worldwide issued guidelines with D2- lymphadenectomy as the standard treatment for resectable gastric cancer (44–49) provided that surgical expertise and postoperative care are of sufficient standard and that treatment will be delivered in high volume specialized centers.
Author Contributions
IK and AM both participated on the conception and design of the manuscript; data acquisition, interpretation and analysis; drafting, revising and final approval of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
References
1. Mcneer G, Vandenberg H, Donn FY, Bowden L. A critical evaluation of subtotal gastrectomy for the cure of cancer of the stomach. Ann Surg (1951) 134(1):2–7. doi: 10.1097/00000658-195107000-00002
2. Mcneer G, Sunderland DA, Mcinnes G, Vandenberg HJ, Lawrence W. A more thorough operation for gastric cancer; anatomical basis and description of technique. Cancer (1951) 4(5):957–67. doi: 10.1002/1097-0142(195109)4:5<957::AID-CNCR2820040509>3.0.CO;2-M
3. Papachristou DN, Fortner JG. Local recurrence of gastric adenocarcinomas after gastrectomy. J Surg Oncol (1981) 18(1):47–53. doi: 10.1002/jso.2930180108
5. Kanai H. Significance of combined pancreatosplenectomy in gastric resection for gastric carcinoma. J Jpn Soc Cancer Ther (1967) 2:328–38.
6. Kajitani T, Hoshino T. Combined resection of pancreas in gastric cancer. Gekachiryo (1964) 10:80–6.
7. Aikou T. Clinicopathological study on the significance of combined pancreatosplenectomy, especially, gross indication of the basis on thebase of oncological features. Igaku Kenkyu (1980) 50:533–50.
8. Okajima K, Isozaki H. Splenectomy for treatment of gastric cancer: Japanese experience. World J Surg (1995) 19(4):537–40. doi: 10.1007/BF00294715
9. Wanebo HJ, Kennedy BJ, Chmiel J, Steele G, Winchester D, Osteen R. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg (1993) 218(5):583–92.
10. Wagner PK, Ramaswamy A, Rüschoff J, Schmitz-Moormann P, Rothmund M. Lymph node counts in the upper abdomen: anatomical basis for lymphadenectomy in gastric cancer. Br J Surg (1991) 78(7):825–7. doi: 10.1002/bjs.1800780719
11. Sharma D, Thakur A, Toppo S, Chandrakar SK. Lymph node counts in indians in relation to lymphadenectomy for carcinoma of the oesophagus and stomach. Asian J Surg (2005) 28(2):116–20. doi: 10.1016/S1015-9584(09)60274-8
12. Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg (1988) 75(2):110–2. doi: 10.1002/bjs.1800750206
13. Robertson CS, Chung SC, Woods SD, Griffin SM, Raimes SA, Lau JT, et al. A prospective randomized trial comparing R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg (1994) 220(2):176–82. doi: 10.1097/00000658-199408000-00009
14. Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet (1995) 345(8952):745–8. doi: 10.1016/S0140-6736(95)90637-1
15. Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med (1999) 340(12):908–14. doi: 10.1056/NEJM199903253401202
16. Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol (2004) 22(11):2069–77. doi: 10.1200/JCO.2004.08.026
17. Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol (2010) 11(5):439–49. doi: 10.1016/S1470-2045(10)70070-X
18. Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet (1996) 347(9007):995–9. doi: 10.1016/S0140-6736(96)90144-0
19. Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer (1999) 79(9-10):1522–30. doi: 10.1038/sj.bjc.6690243
20. Wu CW, Hsiung CA, Lo SS, Hsieh MC, Shia LT, Whang-Peng J. Randomized clinical trial of morbidity after D1 and D3 surgery for gastric cancer. Br J Surg (2004) 91(3):283–7. doi: 10.1002/bjs.4433
21. Wu CW, Chang IS, Lo SS, Hsieh MC, Chen JH, Lui WY, et al. Complications following D3 gastrectomy: post hoc analysis of a randomized trial. World J Surg (2006) 30(1):12–16. doi: 10.1007/s00268-005-7951-5
22. Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol (2006) 7(4):309–15. doi: 10.1016/S1470-2045(06)70623-4
23. Degiuli M, Sasako M,Ponti A, Italian Gastric Cancer Study Group. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg (2010) 97(5):643–9. doi: 10.1002/bjs.6936
24. Degiuli M, Sasako M, Ponti A, Vendrame A, Tomatis M, Mazza C, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg (2014) 101(2):23–31. doi: 10.1002/bjs.9345
25. Mocellin S, Mcculloch P, Kazi H, Gama-Rodrigues JJ, Yuan Y, Nitti D. Extent of lymph node dissection for adenocarcinoma of the stomach. Cochrane Database Syst Rev (2015) 12(8):CD001964. doi: 10.1002/14651858.CD001964.pub4
26. Mocellin S, Nitti D. Lymphadenectomy extent and survival of patients with gastric carcinoma: a systematic review and meta-analysis of time-to-event data from randomized trials. Cancer Treat Rev (2015) 41(5):448–54. doi: 10.1016/j.ctrv.2015.03.003
27. El-Sedfy A, Dixon M, Seevaratnam R, Bocicariu A, Cardoso R, Mahar A, et al. Personalized Surgery for Gastric Adenocarcinoma: A Meta-analysis of D1 versus D2 Lymphadenectomy. Ann Surg Oncol (2015) 22(6):1820–7. doi: 10.1245/s10434-014-4168-6
28. Jiang L, Yang KH, Chen Y, Guan QL, Zhao P, Tian JH, et al. Systematic review and meta-analysis of the effectiveness and safety of extended lymphadenectomy in patients with resectable gastric cancer. Br J Surg (2014) 101(6):595–604. doi: 10.1002/bjs.9497
29. Li W-W, Chen L-K, Huang H, Tan J-M, Li M-Y. Clinical study of D2 lymphadenectomy for gastric cancer. Chin J Cancer Prev Treat (2007) 14:1891–3.
30. Jiang L, Yang KH, Guan QL, Zhao P, Chen Y, Tian JH. Survival and recurrence free benefits with different lymphadenectomy for resectable gastric cancer: a meta-analysis. J Surg Oncol (2013) 107(8):807–14. doi: 10.1002/jso.23325
31. Tsujimoto H, Ichikura T, Ono S, Sugasawa H, Hiraki S, Sakamoto N, et al. Impact of postoperative infection on long-term survival after potentially curative resection for gastric cancer. Ann Surg Oncol (2009) 16(2):311–8. doi: 10.1245/s10434-008-0249-8
32. Liang YX, Guo HH, Deng JY, Wang BG, Ding XW, Wang XN, et al. Impact of intraoperative blood loss on survival after curative resection for gastric cancer. World J Gastroenterol (2013) 19(33):5542–50. doi: 10.3748/wjg.v19.i33.5542
33. Li QG, Li P, Tang D, Chen J, Wang DR. Impact of postoperative complications on long-term survival after radical resection for gastric cancer. World J Gastroenterol (2013) 19(25):4060–5. doi: 10.3748/wjg.v19.i25.4060
34. Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol (2013) 20(5):1575–83. doi: 10.1245/s10434-012-2720-9
35. Jiang N, Deng JY, Ding XW, Zhang L, Liu HG, Liang YX, et al. Effect of complication grade on survival following curative gastrectomy for carcinoma. World J Gastroenterol (2014) 20(25):8244–52. doi: 10.3748/wjg.v20.i25.8244
36. Bunt TM, Bonenkamp HJ, Hermans J, van de Velde CJ, Arends JW, Fleuren G, et al. Factors influencing noncompliance and contamination in a randomized trial of "Western" (r1) versus "Japanese" (r2) type surgery in gastric cancer. Cancer (1994) 73(6):1544–51. doi: 10.1002/1097-0142(19940315)73:6<1544::AID-CNCR2820730604>3.0.CO;2-4
37. de Steur WO, Hartgrink HH, Dikken JL, Putter H, van de Velde CJ. Quality control of lymph node dissection in the Dutch Gastric Cancer Trial. Br J Surg (2015) 102(11):1388–93. doi: 10.1002/bjs.9891
38. Parikh D, Johnson M, Chagla L, Lowe D, Mcculloch P. D2 gastrectomy: lessons from a prospective audit of the learning curve. Br J Surg (1996) 83(11):1595–9. doi: 10.1002/bjs.1800831134
39. Lee JH, Ryu KW, Lee JH, Park SR, Kim CG, Kook MC, et al. Learning curve for total gastrectomy with D2 lymph node dissection: cumulative sum analysis for qualified surgery. Ann Surg Oncol (2006) 13(9):1175–81. doi: 10.1245/s10434-006-9050-8
40. Kim CY, Nam BH, Cho GS, Hyung WJ, Kim MC, Lee HJ, et al. Learning curve for gastric cancer surgery based on actual survival. Gastric Cancer (2016) 19(2):631–8. doi: 10.1007/s10120-015-0477-0
41. Degiuli M, Sasako M, Ponti A, Calvo F. Survival results of a multicentre phase II study to evaluate D2 gastrectomy for gastric cancer. Br J Cancer (2004) 90(9):1727–32. doi: 10.1038/sj.bjc.6601761
42. Bonenkamp JJ, van de Velde CJ, Sasako M, Hermans J. R2 compared with R1 resection for gastric cancer: morbidity and mortality in a prospective, randomised trial. Eur J Surg (1992) 158(8):413–8.
43. Fayers PM, Cuschieri A, Fielding J, Craven J, Uscinska B, Freedman LS. Sample size calculation for clinical trials: the impact of clinician beliefs. Br J Cancer (2000) 82(1):213–9. doi: 10.1054/bjoc.1999.0902
44. Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer (2014) 14(2):87–104. doi: 10.5230/jgc.2014.14.2.87
45. Martin-Richard M, Custodio A, García-Girón C, Grávalos C, Gomez C, Jimenez-Fonseca P, et al. Seom guidelines for the treatment of gastric cancer 2015. Clin Transl Oncol (2015) 17(12):996–1004. doi: 10.1007/s12094-015-1456-y
46. Moehler M, Baltin CT, Ebert M, Fischbach W, Gockel I, Grenacher L, et al. International comparison of the German evidence-based S3-guidelines on the diagnosis and multimodal treatment of early and locally advanced gastric cancer, including adenocarcinoma of the lower esophagus. Gastric Cancer (2015) 18(3):550–63. doi: 10.1007/s10120-014-0403-x
47. Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A,Arnold D ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2016) 27(suppl 5):v38–v49. doi: 10.1093/annonc/mdw350
48. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer (2017) 20:1–19.
Keywords: gastric cancer, lymphadenectomy, gastrectomy, D2-lymphadenectomy, D2-dissection, extended lymphadenectomy, radical lymphadenectomy
Citation: Karavokyros I and Michalinos A (2018). Favoring D2-Lymphadenectomy in Gastric Cancer. Front. Surg. 5:42. doi: 10.3389/fsurg.2018.00042
Received: 11 December 2017; Accepted: 03 May 2018;
Published: 07 June 2018
Reviewed by:
Wanda Petz, Istituto Europeo di Oncologia s.r.l., ItalyGianni Mura, San Donato Hospital, Italy
Copyright © 2018 Karavokyros and Michalinos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ioannis Karavokyros, aW9rYXJhdm9reXJvc0Btc24uY29t
 Ioannis Karavokyros
Ioannis Karavokyros Adamantios Michalinos
Adamantios Michalinos