
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Surg. , 15 May 2017
Sec. Otorhinolaryngology - Head and Neck Surgery
Volume 4 - 2017 | https://doi.org/10.3389/fsurg.2017.00026
Background: The recurrence of pleomorphic adenoma (PA) has been extensively debated, mostly in relation to the extent of parotidectomy.
Methods: A systematic review was undertaken to clarify the surgical and pathological variables related to PA recurrence. Inclusion criteria were as follows: English literature, and prospective or retrospective studies. Exclusion criteria were as follows: single case reports, reviews, and lack of PA recurrence data.
Results: Pathology-related variables associated with recurrence include the histological subtype, the thickness and incompleteness of the tumor capsule, pseudopodia, and satellite nodules. Surgery-related variables associated with recurrence are the presence of intact margins and tumor puncture or spillage. Other factors are the size of the tumor and the age of patient. Myxoid subtypes of PA tend to have incomplete and thinner capsules and to recur more frequently. Surgical variables related to recurrence include positive margins and tumor spillage.
Conclusion: Myxoid and/or large PA, especially in young patients, should be approached more cautiously to avoid recurrences.
Pleomorphic adenoma (PA) is the most common parotid gland neoplasm accounting for 50–60% of all parotid tumors (1, 2). The standard surgical treatment is an open parotidectomy I-V (3), which is associated with recurrence rates below 2–3% (4). By contrast, in earlier studies, recurrence rates following enucleation were as high as 40% (5, 6).
Despite this progress, the exact causes of PA recurrence have remained elusive. The widely held hypothesis is subtotal tumor removal due to inadequate surgery, while the characteristics of the tumor capsule or other histological aspects are rarely examined. We hypothesize here that the various reasons advanced for the recurrence of PA can be grouped into pathology-related (capsule thickness or lack of capsule, pseudopodia, satellite nodules, multi-centricity) and surgery-related (rupture of the tumor, spillage of tumor contents, insufficient margins of resection because of nerve branches, inadequate excision related to the type of surgery) factors. These clinicopathological and surgical features of PA are the basis for this review.
The PubMed database was searched in December 2016 with the terms “pleomorphic adenoma” AND “recurrence” AND “parotid gland” AND “pathology” (search pathology) as well as with the terms “pleomorphic adenoma” AND “recurrence” AND “parotid gland” AND “surgery” (search surgery). Neither language nor time of publication restriction was placed.
The search “pathology” revealed 266 results, while the search surgery yielded 345. Of these, 210 publications were present in both searches, leaving 401 articles. The abstracts of these two searches were examined independently by two authors (Naif H. Alotaibi and Pavel Dulguerov) and any disagreement resolved through discussion.
We included randomized and non-randomized trials, and prospective and retrospective case series. Criteria for exclusion and the number of publications excluded are detailed in Table 1. After abstract-based exclusions, the remaining 79 articles were thoroughly evaluated. This led to the further exclusion of 49 additional publications for various reasons detailed in Table 1. References from the relevant articles were also searched, which yielded an additional 22 publications (1–3, 5–23).
The outcome criteria were not predefined but were built as the literature review progressed. Any criterion that was found significant in one study was further searched in all relevant publications. Data were extracted and tabulated.
The heterogeneity in criteria evaluation and reporting, as well as the paucity of data precluded a meta-analysis and any statistical evaluation.
Pleomorphic adenoma draws its name from its architectural rather than cellular pleomorphism: epithelial and glandular elements intermingle with various amounts of mucoid, myxoid, or chondroid extracellular stroma. Seifert et al. (17) proposed a classification of PA based on relative composition of cellular and stroma components: hypocellular or stroma-rich or myxoid (50–80% of stroma), classical with balanced content (30–50% stroma), and hypercellular (<30% stroma). Myxoid PA account for more than half of cases, the classical subtype for a third, and the remaining 15% were hypercellular (Figure 1). The frequency distribution of these three subtypes varies in publications (Table 2), probably because their definition is not appreciated uniformly.
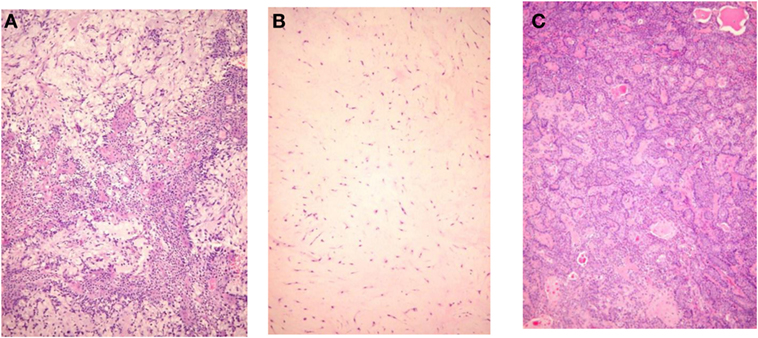
Figure 1. Histological subtypes of pleomorphic adenoma. (A) Classic type, (B) myxoid type, and (C) hypercellular type.
Seifert et al. (17) noticed that recurrences were more frequent in the hypocellular subtype, an idea previously advanced by others (73, 76). By examining 31 recurrent PA, Stennert et al. (77) found a predominance of myxoid histology (25/31). This tendency was confirmed (58/65) in an update report from the same group (78), as well as by others (21, 75, 77–79).
There is a consensus that, in the major salivary glands, PAs are enclosed by a layer of fibrous tissue termed a “capsule” (7). The presence of incomplete or bare capsule (Figure 2) as a source of recurrence was already discussed in the 1960s (10, 16).
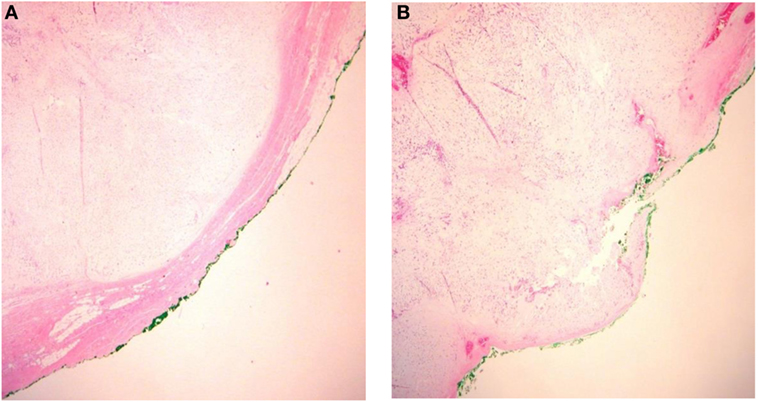
Figure 2. Integrity of the pleomorphic adenoma capsule. (A) Capsular integrity preserved and (B) ruptured capsula.
Absence of encapsulation of PA is found in 20–50% of pathology specimen. A somewhat related parameter is the amount of tumor surface with an incomplete capsule, and this was estimated by Stennert et al. (18) to be about 4%.
Naeim et al. (73) were probably the first to relate incompleteness of encapsulation to the pathological subtype of PA: 69% of myxoid PA had incomplete capsules vs. 30% of classic subtypes, and 18% of hypercellular subtypes. Most authors (18, 19, 74, 75, 79, 80) confirm a larger incidence of incomplete capsules in myxoid PA, although the statistical differences are not always significant. Stennert et al. (18) describe more extensive areas of incomplete capsule in the myxoid type, up to 28% of the whole tumor circumference.
The relationship between incomplete encapsulation and recurrence is summarized in Table 3. Although the data are sparse and often not statistically tested, in general, there is a trend for higher incidence of incomplete capsules in recurrent PA.
Thickness of the capsule of PA was found to vary from 5 to 250 µm by Stennert et al. (18). Although the details are not provided, myxoid PA is described as being at the lower end (5 μm) of the spectrum and cellular PA at the higher one (250 μm). Similar results were found by Naeim et al. (73), Webb and Eveson (19), and Zbären and Stauffer (74). There is no study examining capsular thickness and recurrence.
Pseudopodia are tumor nodules bulging from at the tumor edges and separated by fibrous tissue from the main tumor mass but still localized within the main tumor capsule (Figure 3). Pseudopodia were recognized and advanced as a cause of PA recurrence already in the 1950s by Patey and Thackray (16).
Pseudopodia were found in 28% of PA in Stennert’s series (18), in 40% of PA by Zbären and Stauffer (74), and in 54% by Park et al. (75). No clear correlation was found between pseudopodia and PA subtype (18, 74, 75).
Henriksson et al. (13) observed pseudopodia in 5 of 9 (55%) PA with subsequent recurrence and in 16 of 197 (8%) without subsequent recurrence, although this series was not limited to the parotid gland. No such difference was found by Park et al. (75).
Satellite nodules as distinct tumor nodules in the vicinity of the main tumor lump but separated from it by salivary or fat tissue without any direct connection being traceable on serial histopathology sections (Figure 4). Orita et al. (83) and Li et al. (80) evaluated the distance between the main tumor and satellite nodules and found a maximum of 5.0 and 8.5 mm, respectively.
When satellite nodules extend to such a distance, one might wonder if PA could be multifocal (9). Most serial section studies have failed to confirm this hypothesis (14, 16, 19, 74, 84), although Orita et al. (83) found one case (1%) of multifocal PA.
These satellite nodules were found in 15% (74, 75) to 28% (18) of PA. There is no correlation between histological PA subtype and occurrence of satellite nodules (74, 75, 80).
Park et al. (75) found an increased prevalence of satellite nodules in patients with recurrent PA: 6 of the 10 patients (60%) vs. only 10% in non-recurrent PA.
Tumor size could be included as a pathological, surgery-related, or patient variable. More recently, this relation between PA tumor size and capsular integrity was examined by Li et al. (80): larger tumors (>4 cm) more often exhibited incomplete capsules, although the difference was not significant. Similar findings are described by Webb and Eveson (19) and Zbären and Stauffer (74).
A significant correlation between the size of tumor and satellite nodules was found, with larger PA exhibiting more often satellite nodules (74, 75, 80).
In Riad et al. (84) series, the mean PA diameter was 32.8 mm and recurrence of PA was related to tumor size, with a mean tumor diameter of 30 mm in non-recurrent PA and of 43 mm in the recurrent PA. Similarly, in Ghosh et al. (11) series, all recurrences occurred in tumors larger than 2.5 cm. On the other side, Henriksson et al. (13) could not correlate tumor size and recurrence.
Bankamp and Bierhoff (7) evaluated the proliferation index of recurrent and non-recurrent PA by the MIB-1 antibody immunohistochemistry against the cell proliferation-associated nuclear antigen (Ki-67 antigen): a statistically significant difference was present with a proliferation index of 4.67 ± 3.14 for recurrent PA and 2.34 ± 1.83 non-recurrent PA. Higher MIB-1 expression was also found by Glas et al. (85) and Stennert et al. (77) but the differences were not significant.
The presence of multiples nodules in recurrent PA prompted Stennert et al. (77) to suspect a lymphatic spread. This hypothesis was tested by immunohistochemistry for vascular endothelial factors C and D (VEGF-C and VEGF-D), which are ligands for endothelial tyrosine kinase receptor VEGFR-3, known to primarily mediate lymphangiogenesis. The results are inconclusive (20) and do not support the hypothesis of local lymphangiogenic spread.
A higher expression of mucin 1 (MUC1) glycoprotein and especially of the MUC1/DF3 glycoform by immunostaining was found in recurrent PA and was the only variable significant in multivariate analysis (21). Only 8% of non-recurrent PA showed some immunostaining for MUC1/DF3 (21). Other studies have confirmed the progressively increased staining for MUC1/DF3 from normal salivary gland to non-recurrent PA, recurrent PA, and carcinoma ex PA (22, 23). In addition, the staining pattern appears different in recurrent and non-recurrent PA (22).
Riad et al. (84) collected prospective data on 180 parotidectomies: 162 new tumors and 18 recurrent PA. The resected margins ranged between 0 and 26 mm, with a mean of 5.8 mm. Tumor recurrence was inversely related to the safety margin with an average of 1.3 mm in recurrent PA and of 6.0 mm in non-recurrent PA. Furthermore, the safety margin was inversely correlated with tumor size.
Ghosh et al. (11) reviewed the slides of 83 PA with prolonged follow-up (mean 12.5 years) and noted 6% recurrences. They classified the specimen margins (0; <1 mm; >1 mm) and amount of close margins relative to the specimen circumference (focal as <10% and wide as >10%). All recurrences were in the widely exposed capsule group, and most recurrences were found in margins either 0 or <1 mm (18%) vs. 1.8% if larger margins were obtained.
Positive margins were also seen as source of recurrence by Buchman et al. (86).
Tumor puncture is described as a breach in the tumor capsule during surgery and described in the pathological report. Spillage consists of spread in the surgical field of tumor content.
The incidence of tumor puncture during parotidectomy is unclear, although Henriksson et al. (13) describe an incidence of 14%. In the study of Riad et al. (84), all the 7 tumors that recurred were punctured (100%), while there was an incidence of 11% of puncture in non-recurrent PA. Spillage was reported in seven patients and four developed a recurrence (80%). Park et al. (75) conclude that the risk of recurrence is more than 14-fold higher (4 vs. 30%) if the tumor had ruptured during the surgery.
High incidence of recurrences with tumor puncture was also reported by Henriksson et al. (13) (7 vs. 4%), although the difference was not significant. High incidence of recurrences with tumor spillage were also reported by Ghosh et al. (11) (11 vs. 5%), although the difference was not significant. Buchman et al. (86) found that recurrence did not necessarily follow tumor spillage, although different treatment options including radiation were employed preventively to avoid recurrence.
The younger age at initial presentation of patients who later present a recurrence is well established (15, 21, 48, 78, 87, 88): patients who will later exhibit a recurrent PA tend to be 20 years younger at initial presentation. However, this relationship was not confirmed in some series (84).
Recurrence of PA is a complex problem, occurring 2–15 years after the initial parotidectomy (78, 87) and in relatively young patents (15, 48, 78, 87). The recurrence is often multinodular (50–100%) (77, 87) and associated with an increased rate of post-operative complications, especially facial nerve paralysis (2–20%) (78, 87), further recurrences (77, 78), and malignant degeneration (0–16%) (87–90). Even expert surgeons consider parotidectomy of recurrent PA as one of the most challenging head and neck procedures.
The relative low incidence of recurrent PA and the necessity of a prolong follow-up, associated with the lack of national or international databases, has hampered progress. A recent publication from a Dutch registry covering close to 5,500 patients followed for 15 years and treated with formal parotidectomy (3) puts the incidence of recurrent PA to 2.9% and the rate of malignant transformation to 3.3% (88).
Beyond incidence, the time frame between initial surgery and recurrence implies that the treatment of the recurrence is often performed by a different surgeon and often in a different institution. As a result, the pathology slides from the initial parotidectomy are often unavailable for analysis. Furthermore, surgeons tend to blame recurrence on previously inadequate procedure and little progress has been achieved on the exact causes of PA recurrence. Conceptually, tumor reappearance to inadequate initial resection could be viewed as PA persistence rather than PA recurrence.
Despite the lack of randomized studies and the paucity of prospective studies, the available data in this systematic review seem to point toward a series of coherent arguments. Myxoid subtypes of PA tend to have incomplete and thinner capsules and to recur more frequently. Larger PA tend not only to exhibit incomplete capsules but in addition are associated with more numerous satellite nodules. Larger tumors seem, in most studies, to be associated with more frequent recurrences. When surgical variables are examined, it seems that positive margins and tumor spillage are linked to recurrences. In possibly the only study with some statistical power, Park et al. (75) demonstrated significant differences for satellite nodules, positive margins, and tumor spillage in univariate analysis and for positive margins, and tumor spillage in multivariate analysis.
Because of the measured spread of satellite nodules, Li et al. (80) recommend margins of healthy parotid tissue of about 1 cm. Unfortunately, this requirement seems impossible to fulfill because tumors often abut the facial nerve or its branches as already demonstrated by Danovan and Conley (8) and others (see Table 3). If medial capsular exposure is sometimes unavoidable, there is no good reason to obtain an exposed capsule on the lateral, inferior, and even superior aspects of a PA. Sternocleidomastoid, SMAS, and even skin can be sacrificed to obtain adequate margins, and this can be performed without compromising both functional and esthetic aspects of parotidectomy.
We purposefully tried to avoid the ongoing controversy of the extent of parotidectomy in PA (4, 91–96). As expected, capsular exposure is much more frequent in extracapsular dissection (4, 91, 95). While low rates of recurrence with extracapsular dissection have been published by experienced groups in highly selected cases, series with elevated rate of recurrence also exist (90, 95–97) and a systematic review concluded to a higher recurrence rate of PA with this procedure (92). Probably limited resections, if recommendable at all, should be limited to selected cases with small tumor size and not of the myxoid subtype. If sufficient margins cannot be achieved, the procedure should be converted to a formal parotidectomy.
Recurrence of PAs seem to be increased in the myxoid PA subtype in which the capsule is more often thinner and incomplete. Recurrences seem also more frequent in larger tumors, which not only exhibit incomplete capsules but are also associated with more numerous satellite nodules. Surgical variables related to recurrence include positive margins and tumor spillage.
All the authors: design of the work; data acquisition, analysis, interpretation, draft contribution, and approval of the final version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Eveson JW, Cawson RA. Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol (1985) 146(1):51–8. doi: 10.1002/path.1711460106
2. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg (1986) 8(3):177–84. doi:10.1002/hed.2890080309
3. Quer M, Guntinas-Lichius O, Marchal F, Vander Poorten V, Chevalier D, Leon X, et al. Classification of parotidectomies: a proposal of the European Salivary Gland Society. Eur Arch Otorhinolaryngol (2016) 273(10):3307–12. doi:10.1007/s00405-016-3916-6
4. Witt RL. The significance of the margin in parotid surgery for pleomorphic adenoma. Laryngoscope (2002) 112(12):2141–54. doi:10.1097/00005537-200212000-00004
5. Benedict EG, Meigs JV. Tumors of the parotid gland. A study of 225 cases with complete end-results in 80 cases. Surg Gynecol Obstet (1930) 51:626–47.
6. McFarland J. Three hundred mixed tumors of the salivary glands, of which sixty-nine recurred. Surg Gynecol Obstet (1936) 63:457–68.
7. Bankamp DG, Bierhoff E. [Proliferative activity in recurrent and nonrecurrent pleomorphic adenoma of the salivary glands]. Laryngorhinootologie (1999) 78(2):77–80. doi:10.1055/s-2007-996835
8. Danovan DT, Conley JJ. Capsular significance in parotid tumor surgery: reality and myths of lateral lobectomy. Laryngoscope (1984) 94:324–9.
9. Delarue J. Les tumeurs mixtes plurifocales de la glande parotide. Ann Anat Pathol (Paris) (1956) 1(1):34–58.
10. Eneroth CM. Mixed tumors of major salivary glands: prognostic role of capsular structure. Ann Otol Rhinol Laryngol (1965) 74(4):944–53. doi:10.1177/000348946507400403
11. Ghosh S, Panarese A, Bull PD, Lee JA. Marginally excised parotid pleomorphic salivary adenomas: risk factors for recurrence and management. A 12.5-year mean follow-up study of histologically marginal excisions. Clin Otolaryngol Allied Sci (2003) 28(3):262–6. doi:10.1046/j.1365-2273.2003.00704.x
12. Harney MS, Murphy C, Hone S, Toner M, Timon CV. A histological comparison of deep and superficial lobe pleomorphic adenomas of the parotid gland. Head Neck (2003) 25(8):649–53. doi:10.1002/hed.10281
13. Henriksson G, Westrin KM, Carlsoo B, Silfversward C. Recurrent primary pleomorphic adenomas of salivary gland origin: intrasurgical rupture, histopathologic features, and pseudopodia. Cancer (1998) 82(4):617–20. doi:10.1002/(SICI)1097-0142(19980215)82:4<617::AID-CNCR1>3.0.CO;2-I
14. Lam KH, Wei WI, Ho HC, Ho CM. Whole organ sectioning of mixed parotid tumors. Am J Surg (1990) 160(4):377–81. doi:10.1016/S0002-9610(05)80547-1
15. McGregor AD, Burgoyne M, Tan KC. Recurrent pleomorphic salivary adenoma – the relevance of age at first presentation. Br J Plast Surg (1988) 41(2):177–81. doi:10.1016/0007-1226(88)90048-3
16. Patey DH, Thackray AC. The treatment of parotid tumours in the light of a pathological study of parotidectomy material. Br J Surg (1958) 45(193):477–87. doi:10.1002/bjs.18004519314
17. Seifert G, Langrock I, Donath K. Pathomorphologische Subklassifikation der pleomorphen Speicheldrusenadenome. Analyse von 310 pleomorphen Parotisadenomen. HNO (1976) 24(12):415–26.
18. Stennert E, Guntinas-Lichius O, Klussmann JP, Arnold G. Histopathology of pleomorphic adenoma in the parotid gland: a prospective unselected series of 100 cases. Laryngoscope (2001) 111(12):2195–200. doi:10.1097/00005537-200112000-00024
19. Webb AJ, Eveson JW. Pleomorphic adenomas of the major salivary glands: a study of the capsular form in relation to surgical management. Clin Otolaryngol Allied Sci (2001) 26(2):134–42. doi:10.1046/j.1365-2273.2001.00440.x
20. Salzman R, Starek I, Kucerova L, Skalova A, Hoza J. Neither expression of VEGF-C/D nor lymph vessel density supports lymphatic invasion as the mechanism responsible for local spread of recurrent salivary pleomorphic adenoma. Virchows Arch (2014) 464(1):29–34. doi:10.1007/s00428-013-1502-5
21. Hamada T, Matsukita S, Goto M, Kitajima S, Batra SK, Irimura T, et al. Mucin expression in pleomorphic adenoma of salivary gland: a potential role for MUC1 as a marker to predict recurrence. J Clin Pathol (2004) 57(8):813–21. doi:10.1136/jcp.2003.014043
22. Gao P, Zhou GY, Song XR, Hou JX, Zhang CJ, Ma C. [The relationship of abnormal expression of cell glucoprotein with recurrence of pleomorphic adenoma in salivary gland]. Hua Xi Kou Qiang Yi Xue Za Zhi (2005) 23(2):164–6.
23. Soares AB, Demasi AP, Altemani A, de Araujo VC. Increased mucin 1 expression in recurrence and malignant transformation of salivary gland pleomorphic adenoma. Histopathology (2011) 58(3):377–82. doi:10.1111/j.1365-2559.2011.03758.x
24. Maynard JD. Enucleated parotid tumours. Br J Surg (1988) 75(8):764–6. doi:10.1002/bjs.1800750814
25. Musy JP, Gotzos V. [Histochemical study of a recurrent mixed tumor of the parotid gland]. Ann Anat Pathol (Paris) (1973) 18(3):347–56.
26. Altini M, Coleman H, Kienle F. Intra-vascular tumour in pleomorphic adenomas – a report of four cases. Histopathology (1997) 31(1):55–9. doi:10.1046/j.1365-2559.1997.5790821.x
27. Brieger J, Duesterhoeft A, Brochhausen C, Gosepath J, Kirkpatrick CJ, Mann WJ. Recurrence of pleomorphic adenoma of the parotid gland – predictive value of cadherin-11 and fascin. APMIS (2008) 116(12):1050–7. doi:10.1111/j.1600-0463.2008.01088.x
28. Gentile R, Zeppa P, Zabatta A, Vetrani A. [The role of morphometry in the cytology of pleomorphic adenomas of the salivary glands]. Pathologica (1994) 86(2):167–9.
29. Matturri L, Lavezzi AM, Biondo B, Mantovani M. Cell kinetics of pleomorphic adenomas of the parotid gland. Eur J Cancer B Oral Oncol (1996) 32B(3):154–7. doi:10.1016/0964-1955(95)00090-9
30. Ryan RE Jr, DeSanto LW, Weiland LH, Devine KD, Beahrs OH. Cellular mixed tumors of the salivary glands. Arch Otolaryngol (1978) 104(8):451–3. doi:10.1001/archotol.1978.00790080033008
31. Skalova A, Altemani A, Di Palma S, Simpson RH, Hosticka L, Andrle P, et al. Pleomorphic adenoma of the salivary glands with intravascular tumor deposits: a diagnostic pitfall. Am J Surg Pathol (2012) 36(11):1674–82. doi:10.1097/PAS.0b013e3182690afe
32. Soares AB, de Araujo VC, Juliano PB, Altemani A. Angiogenic and lymphangiogenic microvessel density in recurrent pleomorphic adenoma. J Oral Pathol Med (2009) 38(8):623–9. doi:10.1111/j.1600-0714.2009.00794.x
33. Takahashi M, Hokunan K, Unno T. [Immunohistochemical study of basement membrane in pleomorphic adenomas of the parotid gland: comparison between primary treated tumor and recurrent tumor]. Nihon Jibiinkoka Gakkai Kaiho (1992) 95(11):1759–64. doi:10.3950/jibiinkoka.95.1759
34. Yang S, Wang HP, Wang XY, Guo LJ, Tang XF, Gao QH, et al. Expression of CD44V6 in parotid pleomorphic adenoma and carcinoma ex pleomorphic adenoma. Expert Opin Investig Drugs (2010) 19(Suppl 1):S101–8. doi:10.1517/13543781003718866
35. Huber A, Schmid S, Fisch U. [Pleomorphic adenoma of the parotid gland. Results of surgical treatment]. HNO (1994) 42(9):553–8.
36. Papadogeorgakis N. Partial superficial parotidectomy as the method of choice for treating pleomorphic adenomas of the parotid gland. Br J Oral Maxillofac Surg (2011) 49(6):447–50. doi:10.1016/j.bjoms.2010.06.012
37. Szymczyk K, Czecior E, Misiolek M, Poninska-Polanczuk J, Kubik P. [Parotid tumors treated at the II Department of Laryngology, Silesian Medical Academy, Zabrze]. Przegl Lek (1992) 49(5):157–8.
38. Duroux S, Ballester M, Michelet V, Majoufre C, Siberchicot F, Pinsolle J. [Surgical treatment of pleomorphic adenoma of the parotid gland. Apropos of 192 cases]. Rev Stomatol Chir Maxillofac (1998) 98(6):336–8.
39. Guntinas-Lichius O, Kick C, Klussmann JP, Jungehuelsing M, Stennert E. Pleomorphic adenoma of the parotid gland: a 13-year experience of consequent management by lateral or total parotidectomy. Eur Arch Otorhinolaryngol (2004) 261(3):143–6. doi:10.1007/s00405-003-0632-9
40. Guntinas-Lichius O, Klussmann JP, Wittekindt C, Stennert E. Parotidectomy for benign parotid disease at a university teaching hospital: outcome of 963 operations. Laryngoscope (2006) 116(4):534–40. doi:10.1097/01.mlg.0000200741.37460.ea
41. Laccourreye H, Laccourreye O, Cauchois R, Jouffre V, Menard M, Brasnu D. Total conservative parotidectomy for primary benign pleomorphic adenoma of the parotid gland: a 25-year experience with 229 patients. Laryngoscope (1994) 104(12):1487–94. doi:10.1288/00005537-199412000-00011
42. Laccourreye O, Brasnu D, Cauchoix R, Jouffre V, Naudo P, Laccourreye H. [Long-term results of total conservative parotidectomy for pleomorphic adenoma]. Presse Med (1995) 24(33):1563–5.
43. Cristofaro MG, Allegra E, Giudice A, Colangeli W, Caruso D, Barca I, et al. Pleomorphic adenoma of the parotid: extracapsular dissection compared with superficial parotidectomy – a 10-year retrospective cohort study. Scientific World Journal (2014) 2014:564053. doi:10.1155/2014/564053
44. Dell’Aversana Orabona G, Bonavolonta P, Iaconetta G, Forte R, Califano L. Surgical management of benign tumors of the parotid gland: extracapsular dissection versus superficial parotidectomy – our experience in 232 cases. J Oral Maxillofac Surg (2013) 71(2):410–3. doi:10.1016/j.joms.2012.05.003
45. Hancock D. Pleomorphic adenomas of the parotid salivary gland. Br J Surg (1989) 76(10):1101–2. doi:10.1002/bjs.1800761043
46. McMullen CP, Smith RV, Ow TJ, Tassler A, Schiff BA. Minimal margin extracapsular dissection: a viable alternative technique for benign parotid lesions? Ann Otol Rhinol Laryngol (2016) 125(11):912–7. doi:10.1177/0003489416661344
47. Vaiman M, Abuita R, Jabarin B. Selective deep lobe parotid surgery for benign tumors. Acta Otolaryngol (2015) 135(12):1319–22. doi:10.3109/00016489.2015.1076170
48. Laskawi R, Schott T, Schroder M. Recurrent pleomorphic adenomas of the parotid gland: clinical evaluation and long-term follow-up. Br J Oral Maxillofac Surg (1998) 36(1):48–51. doi:10.1016/S0266-4356(98)90748-3
49. Ott PM, Grob U. [Recurring tumors of the parotid gland with special reference to pleomorphic adenoma]. Schweiz Med Wochenschr (1982) 112(21):757–60.
50. Strek P, Reron E, Modrzejewski M, Trabka-Zawicki P, Olszewski E. [Analysis of causes of the parotid pleomorphic adenoma recurrence]. Otolaryngol Pol (1998) 52(4):431–4.
51. Chilla R, Schneider K, Droese M. [Recurrence tendency and malignant transformation of pleomorphic adenomas]. HNO (1986) 34(11):467–9.
52. Clairmont AA, Richardson GS, Hanna DC. The pseudocapsule of pleomorphic adenomas (benign mixed tumors): the argument against enucleation. Am J Surg (1977) 134(2):242–3. doi:10.1016/0002-9610(77)90354-3
53. Dutescu N. [Potential malignancy of the pleomorphic adenoma of the parotid gland]. Minerva Stomatol (1971) 20(6):256–8.
54. Fliss DM, Rival R, Gullane P, Mock D, Freeman JL. Pleomorphic adenoma: a preliminary histopathologic comparison between tumors occurring in the deep and superficial lobes of the parotid gland. Ear Nose Throat J (1992) 71(6):254–7.
55. Friedrich RE, Li L, Knop J, Giese M, Schmelzle R. Pleomorphic adenoma of the salivary glands: analysis of 94 patients. Anticancer Res (2005) 25(3A):1703–5.
56. Gandon J, Trotoux J, Peynegre R, Andre J, Brasnu D. [Study of a series of 158 parotidectomies and histological problems in mixed tumors of salivary glands (author’s transl)]. Ann Otolaryngol Chir Cervicofac (1979) 96(4–5):261–80.
57. Gao M, Chen Y, Gao Y, Peng X, Yu GY. [Clinicopathologic characteristics and management of parotid pleomorphic adenomas closely abutting the facial nerve]. Beijing Da Xue Xue Bao (2012) 44(1):43–6. doi:10.3969/j.issn.1671-167X.2012.01.009
58. Guerra G, Testa D, Montagnani S, Tafuri D, Salzano FA, Rocca A, et al. Surgical management of pleomorphic adenoma of parotid gland in elderly patients: role of morphological features. Int J Surg (2014) 12(Suppl 2):S12–6. doi:10.1016/j.ijsu.2014.08.391
60. Kiseleva ES, Dar’ialova SL. [Long-term results of treatment of the so-called mixed tumors of the parotid salivary gland (according to the records of the P. A. Herzen State Oncological Institute for 1945-1962)]. Vopr Onkol (1965) 11(10):100–5.
61. Lacomme Y. [Recurrence of pleomorphic adenomas of the parotid gland]. Rev Laryngol Otol Rhinol (Bord) (1988) 109(1):17–24.
62. Laniukh SV, Kremenetskaia LE, Shipkova TP. [Recurrence of polymorphic adenoma of the salivary glands]. Stomatologiia (Mosk) (1990) 69(2):40–3.
63. Lawson HH. Capsular penetration and perforation in pleomorphic adenoma of the parotid salivary gland. Br J Surg (1989) 76(6):594–6. doi:10.1002/bjs.1800760622
64. McGurk M, Renehan A, Gleave EN, Hancock BD. Clinical significance of the tumour capsule in the treatment of parotid pleomorphic adenomas. Br J Surg (1996) 83(12):1747–9. doi:10.1002/bjs.1800831227
65. Robertson BF, Robertson GA, Shoaib T, Soutar DS, Morley S, Robertson AG. Pleomorphic adenomas: post-operative radiotherapy is unnecessary following primary incomplete excision: a retrospective review. J Plast Reconstr Aesthet Surg (2014) 67(12):e297–302. doi:10.1016/j.bjps.2014.09.030
66. Silvoniemi A, Pulkkinen J, Grenman R. Parotidectomy in the treatment of pleomorphic adenoma – analysis of long-term results. Acta Otolaryngol (2010) 130(11):1300–5. doi:10.3109/00016489.2010.488248
67. Takahashi M, Kumai M, Kamito T, Uehara M, Unno T. [Clinico-pathological findings of recurrent pleomorphic adenomas of the parotid gland]. Nihon Jibiinkoka Gakkai Kaiho (1991) 94(4):489–94. doi:10.3950/jibiinkoka.94.489
68. Tarakji B, Nassani MZ. Survey of opinions on the management of pleomorphic adenoma among United Kingdom oral and maxillofacial surgeons. Kulak Burun Bogaz Ihtis Derg (2010) 20(3):129–36.
69. Touquet R, Mackenzie IJ, Carruth JA. Management of the parotid pleomorphic adenoma, the problem of exposing tumour tissue at operation. The logical pursuit of treatment policies. Br J Oral Maxillofac Surg (1990) 28(6):404–8. doi:10.1016/0266-4356(90)90040-R
70. Witt RL, Eisele DW, Morton RP, Nicolai P, Poorten VV, Zbaren P. Etiology and management of recurrent parotid pleomorphic adenoma. Laryngoscope (2015) 125(4):888–93. doi:10.1002/lary.24964
71. Witt RL, Nicolai P. Recurrent benign salivary gland neoplasms. Adv Otorhinolaryngol (2016) 78:63–70. doi:10.1159/000442126
72. Zhan KY, Khaja SF, Flack AB, Day TA. Benign parotid tumors. Otolaryngol Clin North Am (2016) 49(2):327–42. doi:10.1016/j.otc.2015.10.005
73. Naeim F, Forsberg MI, Waisman J, Coulson WF. Mixed tumors of the salivary glands. Growth pattern and recurrence. Arch Pathol Lab Med (1976) 100(5):271–5.
74. Zbären P, Stauffer E. Pleomorphic adenoma of the parotid gland: histopathologic analysis of the capsular characteristics of 218 tumors. Head Neck (2007) 29(8):751–7. doi:10.1002/hed.20569
75. Park GC, Cho KJ, Kang J, Roh JL, Choi SH, Kim SY, et al. Relationship between histopathology of pleomorphic adenoma in the parotid gland and recurrence after superficial parotidectomy. J Surg Oncol (2012) 106(8):942–6. doi:10.1002/jso.23202
76. Krolls SO, Boyers RC. Mixed tumors of salivary glands. Long-term follow-up. Cancer (1972) 30(1):276–81. doi:10.1002/1097-0142(197207)30:1<276::AID-CNCR2820300138>3.0.CO;2-V
77. Stennert E, Wittekindt C, Klussmann JP, Arnold G, Guntinas-Lichius O. Recurrent pleomorphic adenoma of the parotid gland: a prospective histopathological and immunohistochemical study. Laryngoscope (2004) 114(1):158–63. doi:10.1097/00005537-200401000-00030
78. Wittekindt C, Streubel K, Arnold G, Stennert E, Guntinas-Lichius O. Recurrent pleomorphic adenoma of the parotid gland: analysis of 108 consecutive patients. Head Neck (2007) 29(9):822–8. doi:10.1002/hed.20613
79. Goudot P, Auriol M, Chomette G, Vaillant JM, Guilbert F. [Pleomorphic adenoma of the salivary glands. Impact of the myxoid component on the prognosis]. Rev Stomatol Chir Maxillofac (1989) 90(2):119–22.
80. Li C, Xu Y, Zhang C, Sun C, Chen Y, Zhao H, et al. Modified partial superficial parotidectomy versus conventional superficial parotidectomy improves treatment of pleomorphic adenoma of the parotid gland. Am J Surg (2014) 208(1):112–8. doi:10.1016/j.amjsurg.2013.08.036
81. Natvig K, Soberg R. Relationship of intraoperative rupture of pleomorphic adenomas to recurrence: an 11-25 year follow-up study. Head Neck (1994) 16(3):213–7. doi:10.1002/hed.2880160302
82. Paris J, Facon F, Chrestian MA, Giovanni A, Zanaret M. [Pleomorphic adenoma of the parotid: histopathological study]. Ann Otolaryngol Chir Cervicofac (2004) 121(3):161–6. doi:10.1016/S0003-438X(04)95504-1
83. Orita Y, Hamaya K, Miki K, Sugaya A, Hirai M, Nakai K, et al. Satellite tumors surrounding primary pleomorphic adenomas of the parotid gland. Eur Arch Otorhinolaryngol (2010) 267(5):801–6. doi:10.1007/s00405-009-1149-7
84. Riad MA, Abdel-Rahman H, Ezzat WF, Adly A, Dessouky O, Shehata M. Variables related to recurrence of pleomorphic adenomas: outcome of parotid surgery in 182 cases. Laryngoscope (2011) 121(7):1467–72. doi:10.1002/lary.21830
85. Glas AS, Hollema H, Nap RE, Plukker JT. Expression of estrogen receptor, progesterone receptor, and insulin-like growth factor receptor-1 and of MIB-1 in patients with recurrent pleomorphic adenoma of the parotid gland. Cancer (2002) 94(8):2211–6. doi:10.1002/cncr.10445
86. Buchman C, Stringer SP, Mendenhall WM, Parsons JT, Jordan JR, Cassisi NJ. Pleomorphic adenoma: effect of tumor spill and inadequate resection on tumor recurrence. Laryngoscope (1994) 104(10):1231–4. doi:10.1288/00005537-199410000-00008
87. Abu-Ghanem Y, Mizrachi A, Popovtzer A, Abu-Ghanem N, Feinmesser R. Recurrent pleomorphic adenoma of the parotid gland: institutional experience and review of the literature. J Surg Oncol (2016) 114(6):714–8. doi:10.1002/jso.24392
88. Andreasen S, Therkildsen MH, Bjorndal K, Homoe P. Pleomorphic adenoma of the parotid gland 1985-2010: a Danish nationwide study of incidence, recurrence rate, and malignant transformation. Head Neck (2016) 38(Suppl 1):E1364–9. doi:10.1002/hed.24228
89. Mann W, Beck C, Karatay MC. [Recurrent benign tumors of the parotid gland and their tendency for becoming malignant]. Laryngol Rhinol Otol (Stuttg) (1985) 64(3):133–5. doi:10.1055/s-2007-1008101
90. Chang EZ, Lee WC. Surgical treatment of pleomorphic adenoma of the parotid gland: report of 110 cases. J Oral Maxillofac Surg (1985) 43(9):680–2. doi:10.1016/0278-2391(85)90193-4
91. Witt RL, Iacocca M. Comparing capsule exposure using extracapsular dissection with partial superficial parotidectomy for pleomorphic adenoma. Am J Otolaryngol (2012) 33(5):581–4. doi:10.1016/j.amjoto.2012.03.004
92. Witt RL, Rejto L. Pleomorphic adenoma: extracapsular dissection versus partial superficial parotidectomy with facial nerve dissection. Del Med J (2009) 81(3):119–25.
93. Xu YQ, Li C, Fan JC, Zhang B, Chen JC, Wang ZH, et al. [Evidence for determining the safe surgical margin for pleomorphic adenoma of parotid gland]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi (2012) 47(2):137–41. doi:10.3760/cma.j.issn.1673-0860.2012.02.011
94. Zbaren P, Vander Poorten V, Witt RL, Woolgar JA, Shaha AR, Triantafyllou A, et al. Pleomorphic adenoma of the parotid: formal parotidectomy or limited surgery? Am J Surg (2013) 205(1):109–18. doi:10.1016/j.amjsurg.2012.05.026
95. Wierzbicka M, Kopec T, Szyfter W. [Analysis of recurrence and treatment results of parotid gland non-malignant tumors with particular focus on pleomorphic adenoma]. Otolaryngol Pol (2012) 66(6):392–6. doi:10.1016/j.otpol.2012.06.011
96. Piekarski J, Nejc D, Szymczak W, Wronski K, Jeziorski A. Results of extracapsular dissection of pleomorphic adenoma of parotid gland. J Oral Maxillofac Surg (2004) 62(10):1198–202. doi:10.1016/j.joms.2004.01.025
Keywords: pleomorphic adenoma, recurrence, parotidectomy, pathology, parotid tumors
Citation: Dulguerov P, Todic J, Pusztaszeri M and Alotaibi NH (2017) Why Do Parotid Pleomorphic Adenomas Recur? A Systematic Review of Pathological and Surgical Variables. Front. Surg. 4:26. doi: 10.3389/fsurg.2017.00026
Received: 28 February 2017; Accepted: 19 April 2017;
Published: 15 May 2017
Edited by:
Narayanan Prepageran, University of Malaya, MalaysiaReviewed by:
Yves Brand, University of Basel, SwitzerlandCopyright: © 2017 Dulguerov, Todic, Pusztaszeri and Alotaibi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pavel Dulguerov, cGRnaG5zQG91dGxvb2suY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.