- 1Department of Spine Surgery, Sir Ganga Ram Hospital, New Delhi, India
- 2Department of Anesthesiology, Sir Ganga Ram Hospital, New Delhi, India
Introduction: Intraoperative neuromonitoring (IONM) has become a standard of care in spinal deformity surgeries to minimize the incidence of new onset neurological deficit. Stagnara wake up test and ankle clonus test are the oldest techniques described for spinal cord monitoring, but they cannot be solely relied upon as a neuromonitoring modality. Somatosensory evoked potentials monitor only dorsal tracts and give high false positive and negative alerts. Transcranial motor evoked potentials (TcMEPs) monitor the more useful motor pathways. The purpose of our study was to report the safety, efficacy, limitations of TcMEPs in spine deformity surgeries, and the role of a checklist.
Study design: Retrospective review of all spinal deformity surgeries performed with TcMEPs from 2011 to 2015.
Materials and methods: All patients were subjected to IONM by TcMEPs during the spinal deformity surgery. Patients were included in the study only if complete operative reports and neuromonitoring data and postoperative neurological data were available for review. An alert was defined as 80% or more decrement in the motor evoked potential amplitude, or increase in threshold of 100 V or more from baseline. The systemic and surgical causes of IONM alerts and the postoperative neurological status were recorded.
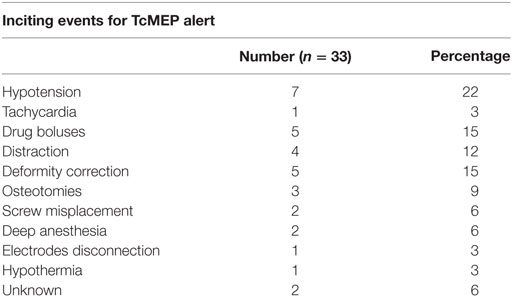
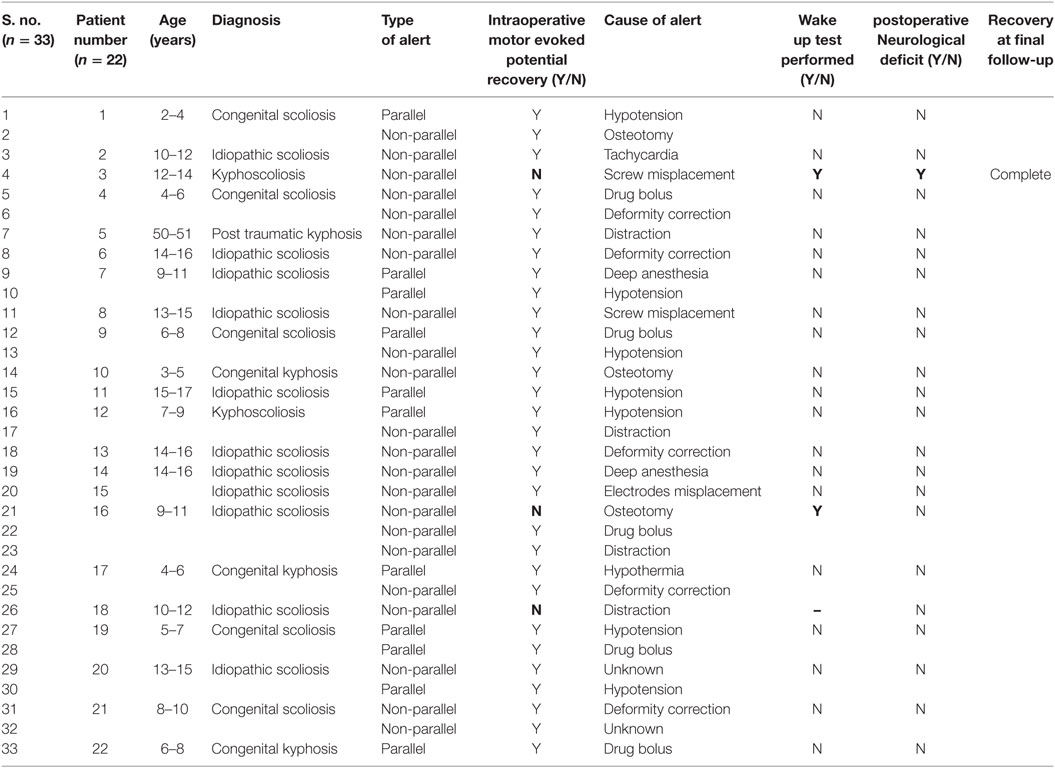
Results: In total, 61 patients underwent surgery for spinal deformities with TcMEPs. The average age was 12.6 years (6–36 years) and male:female ratio was 1:1.3. Diagnoses included idiopathic scoliosis (n = 35), congenital scoliosis (n = 13), congenital kyphosis (n = 7), congenital kyphoscoliosis (n = 4), post-infectious kyphosis (n = 1), and post-traumatic kyphosis (n = 1). The average kyphosis was 72° (45°–101°) and the average scoliosis was 84° (62°–128°). There were in total 33 alerts in 22 patients (36%). The most common causes were hypotension (n = 7), drug induced (n = 5), deformity correction (n = 5), osteotomies (n = 3), tachycardia (n = 1), screw placement (n = 2), and electrodes disconnection (n = 1). Reversal of the inciting event cause resulted in complete reversal of the alert in 90% of the times. Three patients showed persistent alerts, out of whom one had a positive wake up test and woke up with neurodeficit, which recovered over few weeks, while the other patients showed persistent alerts but woke up without any deficit. Sensitivity and specificity of TcMEP in deformity correction surgery were 100 and 96.6%, respectively, in our study.
Conclusion: IONM alerts are frequent during spinal deformity surgery. In our study, more than 50% of the alerts were associated with anesthetic management. IONM with TcMEPs is a safe and effective monitoring technique and wake up test still remains a valuable tool in cases of a persistent alert.
Introduction
Neurological deficit following surgical correction of deformity is a major concern for any spine surgeon (1, 2). Ankle clonus test (3, 4) and Stagnara wake up test (5) are the earliest tests described for assessing the spinal cord function. These tests assess only gross motor deficits and they also require emergence from anesthesia (cannot be applied multiple times) and hence these tests cannot be solely relied upon as a neuromonitoring modality. Role of somatosensory evoked potentials (SSEPs) in spinal cord monitoring was first demonstrated by Tamaki et al. (6) However, there can be a motor deficit without any concomitant sensory change due to vascular injury (7–12). SSEPs have high false positive (FP) and false negative (FN) alerts (12–16) and also need averaging before alerting the surgeon and are thus time consuming (12). Transcranial motor evoked potentials (TcMEPs) on the other hand monitor the more useful motor pathways and are easily administered with high reliability and validity (7). TcMEPs provide feedback almost instantaneously and thus have a good ease of applicability. One of the most important goals of any surgeon performing deformity correction is to maintain the preoperative neurological status (1, 17). Intraoperative neuromonitoring (IONM) system is the means to identify spinal cord injury at the time when corrective measures could reverse it and also to define the nature of insult allowing the surgeon to minimize further injury (18). Controversy still continues regarding the efficacy of TcMEPs alone and few authors prefer multimodality monitoring (19). The purpose of this study was to report the safety, efficacy, and limitations of TcMEPs in spine deformity correction surgeries, and also to establish the role of a checklist.
Materials and Methods
After approval from Ethics committee (ID: EC/01/17/1107), retrospective review of all spine deformity surgeries performed in our institute during the period 2011–2015 was done. Our study included 67 deformity correction surgeries performed by three senior spine surgeons with a minimum experience of 15 years. Surgeries performed with TcMEP monitoring alone are included in our study. All the surgeries were performed under total intravenous anesthesia (TIVA) protocol developed by the institute, and a trained neurophysiologist who monitors IONM with TcMEPs. Age at the time of surgery, gender, diagnosis, duration of surgery, preoperative neurology, type of instrumentation, blood loss and the number of alerts during surgery, nature of insult, corrective measures done, and postoperative neurology were reviewed. From anesthesia records, depth of anesthesia and mean arterial pressure (MAP) at the time of alert plus anesthesia drug bolus usage were noted.
Anesthesia Protocol
Before induction, the Stagnara wake up test is explained to each patient; TIVA was employed for induction and maintenance in all the patients. Anesthesia is induced with propofol 1–2 mg/kg i.v., fentanyl 2–3 μg/kg i.v., and dexmedetomidine 1 μg/kg i.v. Intubation is facilitated with only a small, single, short-acting dose of muscle relaxant. The patient’s eyes are taped shut and padded for protection from injury in the prone position. A urinary catheter is placed, an arterial line inserted, two large bore i.v. lines are secured, a temperature probe inserted, and appropriate sized bite blocks are wedged in place between the molars to prevent injury to the contents of the oral cavity (the teeth, tongue, and endotracheal tube). Intraoperative depth of anesthesia was judged by the bispectral index. All used sponges were weighed and saline washes measured, so that accurate assessment of intraoperative blood loss is made. Arterial blood gas analysis and hemoglobin estimations are done as and when required. Anesthesia maintenance is done with i.v. propofol 100–150 μg/kg/h, fentanyl 1–2 μg/kg/h i.v., and dexmedetomidine 0.5 μg/kg/h i.v.
IONM Technique
Potentials were elicited by transcranial stimulation using corkscrew electrodes placed subcutaneously over the motor cortex (Nim-Eclipse, Medtronic). Motor evoked potentials (MEPs) were obtained from intramuscular electrodes (13 mm, 27G, dual electrodes) placed in four (sometimes five) bilateral muscle groups. One muscle group above the level of surgery was always used as a control (thenar muscles). Other electrodes were placed in rectus abdominis, vastus lateralis, tibialis anterior, and abductor hallucis. The most distal electrodes were placed in the anal sphincter in one case with S2 hemivertebrae. Ultrasound guided placement of electrodes into the rectus abdominis muscle was done in six patients.
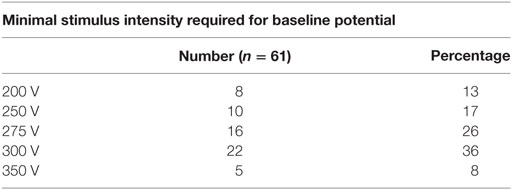
Biphasic stimuli were given starting at three pulse, 200 V, and 0.5 ms duration with 2.0 ms interval between stimuli, and if needed increments were done each time by 25 V (up to 400 V) and at five- or seven-pulse train till a satisfactory baseline amplitude (50 μV) was obtained. The same protocol was followed during intraoperative monitoring and the maximal stimulus intensity needed was noted. The initiation of MEP stimulation and recording was done after intubation while the patient was in supine position and once again after patient was placed prone. The MEP recording obtained just before incision was taken as the baseline for future reference. The final MEP was obtained after the closure of wound but before application of dressing.
An “alert” was defined as a decrease in amplitude by 80% or more, or 100 V increase in threshold, or latency prolongation >10% from baseline in one or more electrodes. This need not necessarily be due to a surgical maneuver.
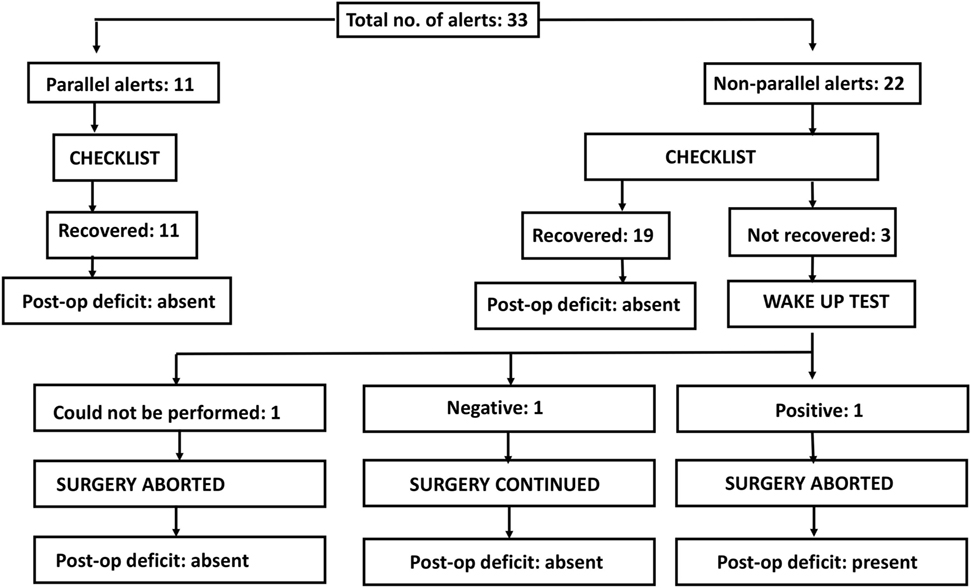
Parallel alert: a similar change (increase/decrease) seen in all the recording electrodes.
Non-parallel alert: a decrease or loss seen in only one or few recording electrodes.
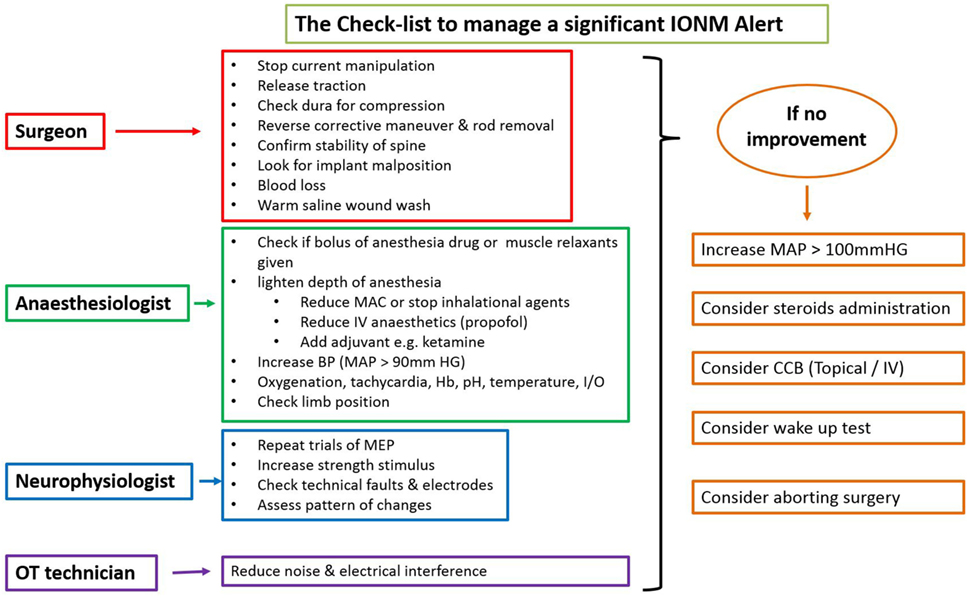
There is an ongoing protocol in the hospital as a part of neuromonitoring program in Department of Spine Surgery that defined these alerts and also a protocol taken in response to an alert (Figure 1). MEPs were obtained at periodic intervals (10–20 min) during the entire procedure, at lesser intervals during instrumentation, and also immediately after any high-risk maneuver (pedicle breach, distraction, derotation, osteotomy). Once an alert was elicited, the teams performed their set protocols developed by the authors as shown in the checklist (Figure 1).
When an alert was noted immediately following a high-risk maneuver and if recovery of amplitudes was noted after undoing that maneuver, rest of the parameters in the checklist being normal, then it was considered to be the cause. However, if an alert was noted during a routine monitoring protocol, respective teams evaluated all the parameters and the corrective maneuver by which the amplitudes were restored was taken as the most probable cause of an alert.
If the alert persisted even after all the corrective measures were undertaken for up to 30 min, the Stagnara wake up test was done. If the test was negative, the surgery was continued while MEPs were obtained at regular short intervals and if the wake up test was positive, surgery was aborted and the attendants were explained regarding the same.
Outcome Parameters
The success of IONM (TcMEPs in our study) in determining cord compression at an early stage is expressed with true positive (TP), true negative (TN), false positive (FP), and negative (FN).
TP: an alert that persisted despite corrective measures or returned to baseline after corrective measures, but patient had a positive wake up test (if performed) or postoperative new neurological deficit.
FP: an alert that persisted during surgery despite corrective measures, but patient had a negative wake up test (if performed) or developed no new postoperative deficit.
TN: no alert was recorded during surgery and patient developed no new neurological deficit following surgery.
FN: no alert was recorded during surgery, but patient developed neurological deficit following surgery.
Indeterminate: An alert that returned to baseline value following corrective measures and patient had no new postoperative neurological deficit.
Specificity (Sp), sensitivity (Sn), negative predictive value (NPV), and positive predictive value (PPV) were calculated in our study. Sp and Sn give the percentage of negative and positive outcomes correctly indicated by the technique. PPV and NPV describe the probability that a patient has an injury if the test is positive and does not if the test is negative, respectively. PPV and NPV describe the performance of the technique (chance of a positive or negative neurological event).
Safety was evaluated by observation for scalp burns, arrhythmias, or injuries due to movements induced by TcMEPs like tongue or lip lacerations, seizures, and whether these movements interfered with surgery.
Results
A total of 67 patients underwent deformity correction surgery with TcMEP monitoring, 6 patients had preoperative neurological deficit and were excluded. A total of 61 patients are included in this study, with an average age of 12.8 years (6–36 years). Most common cause of deformity was idiopathic scoliosis (n = 35) (Table 1), other causes being congenital scoliosis (n = 13), congenital kyphosis (n = 7), congenital kyphoscoliosis (n = 4), post-infection kyphosis (n = 1), and post-traumatic kyphosis (n = 1). Only posterior instrumentation was done in all the cases. Voltage required for obtaining a baseline MEP was generally between 200 and 300 V in our study. Maximal stimulus intensity needed was 200 V in 8 patients (13%), 250 V in 10 patients (17%), 275 V in 16 patients (26%), 300 V in 22 patients (36%), and 350 V in 5 patients (8%) (Table 2). Average kyphosis and scoliosis were 72° (45°–101°) and 84° (62°–128°), respectively.
We had a total of 33 alerts in 22 patients (36%) (Table 3), 86% (19 out of 22) of alerts returned to baseline values following corrective measures. Eight (25%) alerts were due to altered hemodynamics (hypotension-7, tachycardia-1) (Figure 2). Other causes were anesthetic drug boluses (5, 15%) and distraction of spinal cord (4, 12%) (Figure 3); derotation or deformity correction (5, 15%), osteotomy (3, 9%), hypothermia (1, 3%), screw misplacement (2, 6%), deep stage of anesthesia (2, 6%), and electrode disconnection (1, 3%) (Figure 4). Out of these 33 alerts, 11 were parallel and the rest 22 were non-parallel (Figure 5). We had incidental dural tear in four patients (6.5%), but the authors noted no relation to the alerts.
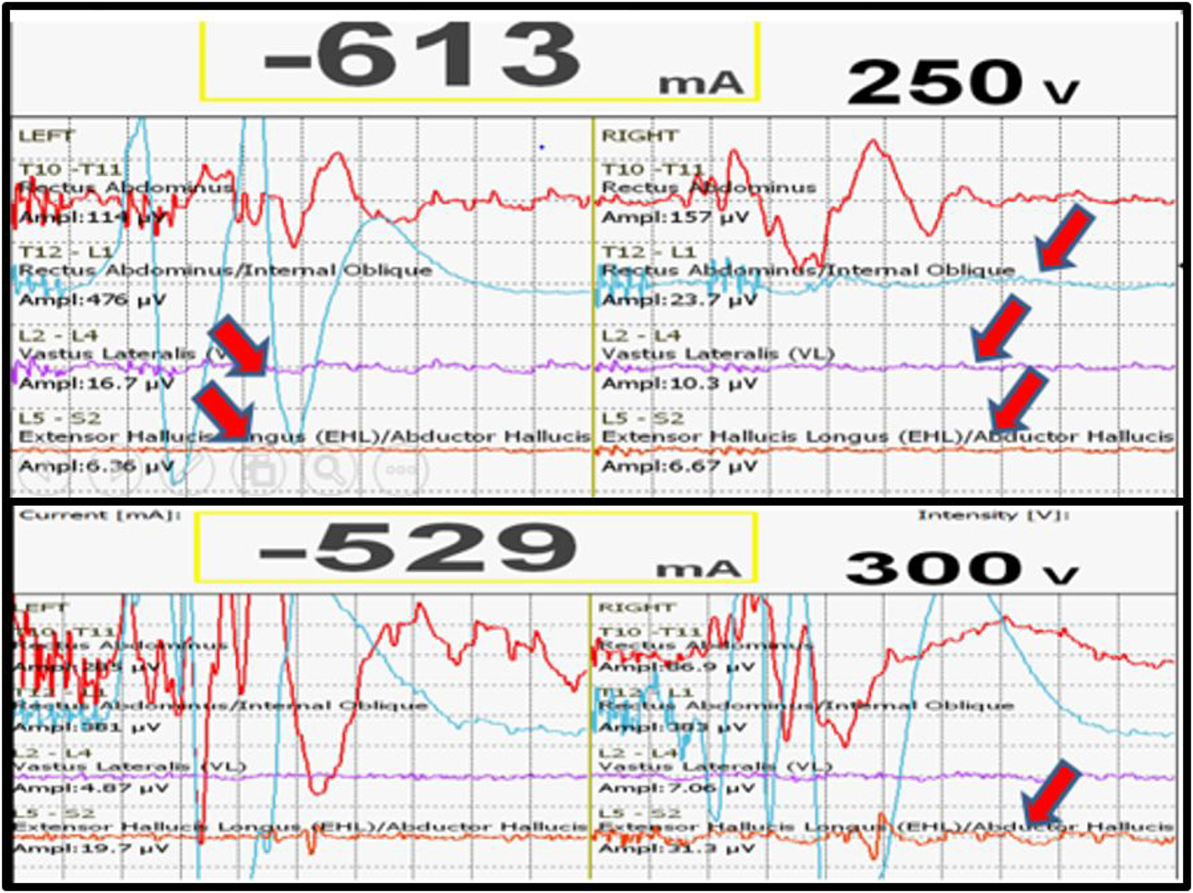
Figure 2. Loss of MEPs following hypotension (above), recovery of MEPs following correction of hypotension.
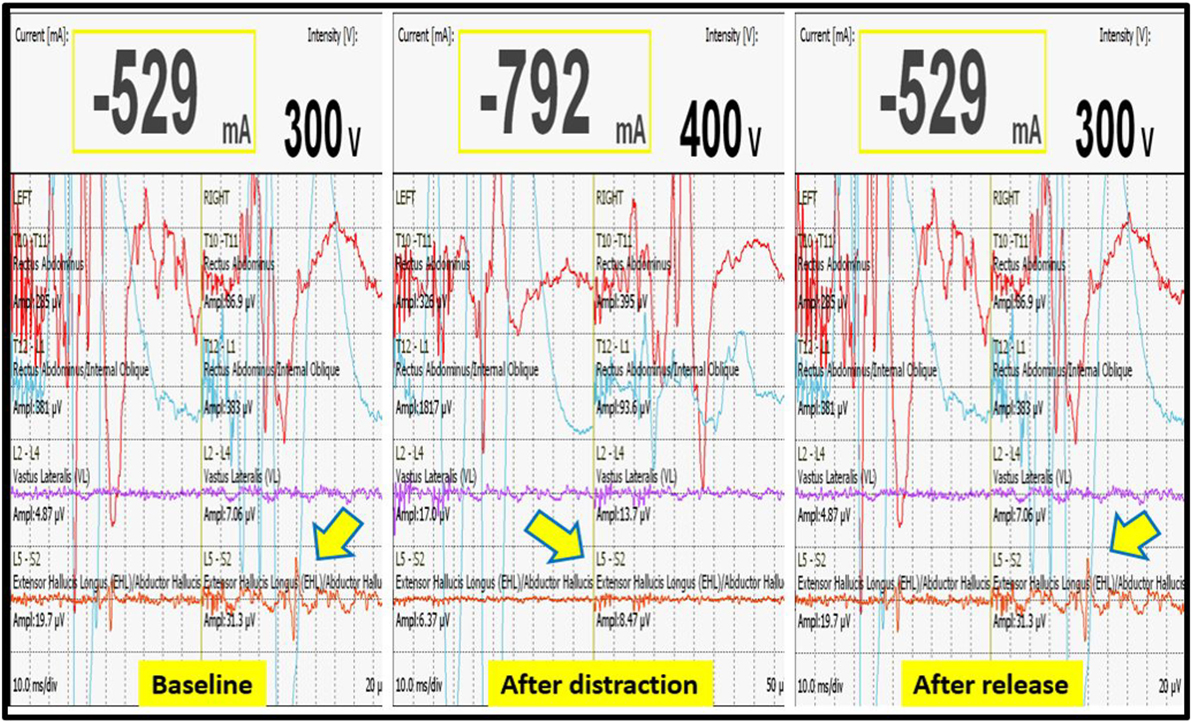
Figure 3. Decrease in MEP following distraction when compared to baseline value, restoration of MEP after release of distraction.
Three patients (13.6%) had persistent alerts; sudden loss of MEPs in both lower limbs was seen in one patient following accidental injury to the spinal cord by pedicle sound through a misplaced screw tract and two patients had decreased MEPs from both lower limbs following distraction. Stagnara wake up test was performed in two patients, out of whom one patient had negative and one had a positive result, while the wake up test could not be performed in one patient. The surgeon decided to continue with surgery in the patient with negative wake up test and MEPs were taken at more frequent intervals; the MEPs restored to baseline value after 50 min and patient woke up with no new neurologic deficit. In the patient with positive wake up test, it was decided to abort the case at that stage and the patient woke up with postoperative deficit. Neurodeficit resolved after a duration of 4 months. Surgery was also aborted in third patient in whom the wake up test couldn’t be done, as the MEPs were persistently low even after all corrective measures had been instituted (Figure 5).
No significant differences were noted in age and gender between patients with no alerts and those who had alerts with or without postoperative deficits. Electrodes were displaced during surgery in three patients, in one case electrodes were reinserted, while in other two cases, surgery was continued without reinsertion. We had no complications with TcMEPs during or after the surgery. All the alerts are shown in Table 4.
Sensitivity and Sp of TcMEP in deformity correction surgery were 100 and 96.6%, respectively, in our study. NPV and PPV were 100 and 33.3%, respectively (Table 5).
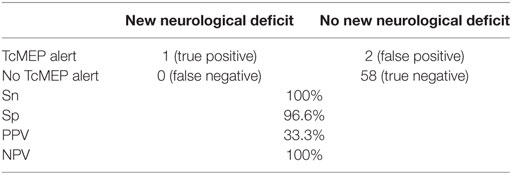
Table 5. Sensitivity (Sn), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) of TcMEP alerts.
There were no instances of tongue or lip lacerations, seizures, or any other complications during or after the surgery.
Discussion
The purpose of IONM is to provide real-time assessment of spinal cord function during surgery that involves cord manipulation. Various mechanisms of spinal cord injury in deformity correction surgery are distraction, ischemia, and compression (20). IONM should alert the surgeon of spinal cord injury at a time when corrective measures could reverse it. Patients with congenital scoliosis, kyphosis, and preoperative deficits have a higher chance of neurological injury (1). Stagnara wake up test (5) and postoperative clonus test (3, 4) are the earliest techniques described for knowing the spinal cord integrity during complex deformity corrections. Both tests monitor only gross motor deficits, need emergence from anesthesia and hence they are not real time and cannot be used multiple number of times. There is the risk of self-extubation, loss of patient positioning (21), and is also difficult to perform in some patients (22–24). Despite all these disadvantages, wake up test still has a significant role in certain circumstances; wake up test was done in two patients who had persistent alerts despite corrective measures while wake up test couldn’t be performed in one patient. We routinely do not perform wake up test in all patients, though all except those with low understanding levels, like in very young patients, are counseled for the same. Tamaki et al. in year 1984 first reported the role of SSEPs in deformity corrections (6). SSEPs monitor only dorsal tracts and ventral column can be compromised without a concomitant sensory change (5, 7, 9–12). There are numerous reports of new postoperative deficit in absence of SSEP alerts (12, 16, 25–30). SSEPs require averaging of potentials before an alert is issued and hence lag behind TcMEPs (14, 31, 32). For monitoring with direct spinal cord stimulation (D-wave), electrodes have to be introduced into the dura, and the stimulus unavoidably activates sensory tracts, producing antidromic and peripheral nerve sensory potentials (33). Hence potentials after spinal cord stimulation cannot be attributed to motor tracts alone (34, 35).
In TcMEPs, stimulus is delivered to the motor cortex from subcutaneously placed corkscrew electrodes, and potentials are recorded from electrodes placed in various bilateral muscle groups. The purpose of recording potentials from maximum number of possible muscles is to increase the Sn (36). We used four or five bilateral muscle groups in all cases. Electrodes placed in thenar muscles were always used as a control. In one patient with S2 hemivertebrae excision, the most distal electrodes were placed in anal sphincter on both sides, but during surgery electrodes on one side were displaced, but no attempt was made to reinsert these electrodes. In six patients, ultrasound guided insertion of electrodes was done as rectus abdominis muscle was not easily palpable for direct insertion. Multi-pulse stimulus was used in all cases, as it produces a short train of high frequency stimuli that summate to depolarize motor neurons, thus achieving specific responses (9, 37–42). We used three-, five- or seven-pulse stimulus in all the cases. TcMEPs do not use supramaximal stimuli; hence as depth of anesthesia increases, suppression of lower motor neurons occurs, this may cause disappearance or fading of evoked potentials. Therefore, increasing the pulse number or stimulus intensity may be necessary sometimes to maintain responses (14). In one patient, MEPs were lost from all the electrodes on one side as the patient was at deeper stage of anesthesia. Lack of antidromic contamination in TcMEPs provides Sp and thus is effective and practical in intraoperative period (43). In a direct comparison of TcMEPs with SSEPs, MacDonald and Janusz (44) showed that the former technique provides a rapid feedback.
An 80% or greater decrease in the MEP amplitude to be taken as a criteria for “alert” was introduced by Langeloo et al. (45), while present or absent criteria as an alert was proposed by Sala et al. (46) Various other criteria defined for an “alert” were MEP amplitude changes of 50% (47), 60% (13, 48), 70% (49), and even complete loss (50–53). In our study, alert was defined as a decrease in amplitude by 80% or more, or 100 V increase in threshold, or latency prolongation >10% from the baseline in one or more electrodes.
In all our cases, IONM with TcMEPs was done with strict adherence to anesthesia protocol (TIVA) and checklist. An alert not synchronous with any high-risk surgical maneuver could be likely due to various non-surgical factors and such an alert when not quickly identified and corrected could mislead and compel the surgeon to take unreasonable risk or to change the surgery plan. A checklist places emphasis on all the likely surgical and non-surgical factors that cause an alert and thus a checklist might not allow any potential risk factor to be missed and to mark an alert due to any cause as a FP alert. As the systemic state varies from time to time, baseline potentials obtained at the beginning of surgery may no longer be appropriate at later point. In the intraoperative period, MEP amplitude has high trial-by-trial variability (40) and even a mild drop in MAP can affect MEPs and produce an alert (54–56). These systemic alterations can be identified by parallel alerts, regardless of degree of change in the amplitude. In our study, out of a total 33 alerts, 11 (33%) were parallel and all of these alerts returned to baseline values after restoration of blood volume, increasing the MAP, changing depth of anesthesia, and increasing core temperature. Out of 22 non-parallel alerts seen, 2 (9%) were due to systemic alterations. In our study, all parallel alerts were due to systemic alterations, while non-parallel alerts were due to systemic and focal alterations.
Skinner et al. (57) reported that in some cases, free-running EMGs were the only findings in patients with postoperative neurological deficits. Free-running EMGs were not used in our study, and authors have no experience with these. Relative contraindications to TcMEPs are skull defects, cardiac pacing, epilepsy, and presence of any implantable device (58). Although Schwartz et al. (59) reported no episodes of seizure in 35 patients with a history of epilepsy; TcMEPs were used in only one patient, and epilepsy still remains a contraindication for TcMEPs use in our institute. Wake up test alone cannot be relied as an only monitoring technique as it doesn’t provide real-time assessment of the spinal cord. An alert that persists even after protocol completion could be a FP alert and a negative wake up test can reassure the surgeon of no significant neurological injury (60–62). In three patients who had persistent alerts despite corrective measures, wake up test was performed and out of whom one patient had a negative result. Hence, surgery was continued and the patient woke up with no postoperative deficit. Surgery was abandoned in a patient with positive wake up test, and woke up with deficit in the lower limbs. Wake up test could not be performed in one patient of double major scoliosis due to anesthesia reasons; hence, we decided to abort the case. However, the patient woke up with no new postoperative neurological deficit. Wilson-Holden et al. (63) and Thuet et al. (64) defined alert that normalized after corrective measures and associated with no new postoperative deficit as FP, while Tamkus et al. (65) defined it as TP and Kim et al. (66) as indeterminate. We adopted the definition given by Kim et al. (66) in our study.
We had no FN alerts in our study, although a few case reports exist in literature (52, 67, 68). Sn and Sp of TcMEPs were 100 and 96.6%, while NPV and PPV were 100 and 33.3%, respectively, in our study. According to a meta-analysis by Fehlings et al. (19), many spine centers routinely use multimodality neuromonitoring (TcMEPs and SSEPs) and they also recommend multimodality monitoring in complex deformity surgeries, but FP cases are reported by Hyun et al. even with multimodality neuromonitoring. Absence of any complications or adverse events during or after the surgery, suggest that TcMEPs can be applied safely. In our study, we had high Sn and reasonable Sp with TcMEPs alone; hence, TcMEPs are efficacious for detection of any spinal cord injury in deformity correction surgeries. In patients with neuromuscular scoliosis and in patients with history of epilepsy, we use SSEPs in our institute.
IONM with TcMEPs alone is not without limitations; anesthetic and systemic changes produce high variability in amplitudes, inhalational agents decrease the effectiveness of stimulation, and muscle relaxants inhibit amplitudes from muscles and thus adherence to strict anesthesia protocol is important. Reliability of MEPs diminishes in patients with preoperative neurological deficits. IONM cannot detect abrupt loss of signals as in anterior spinal artery syndrome because this is an acute process. Although TcMEPs have been used without any complications by Schwartz et al. (59) in patients cardiac pace makers, epilepsy, and cardiac disease; they remain contraindications in our institute as patient safety is always paramount. And finally, a FP alert may compel the surgeon to change the surgery plan or to take unreasonable risk. All these limitations have to be kept in mind and also explained to the patient relatives that even in most ideal situations, TcMEPs do not eliminate all adverse neurological events.
Limitations of Our Study
This study was not a prospective study, study population may not reflect all the deformities (in patients with NM scoliosis and history of epilepsy only SSEPs were used) and no comparison was done with multimodality monitoring. Following an alert, respective teams performed their roles almost simultaneously; hence, the exact cause of an alert may not have been identified all the times. But, as we have an ongoing protocol; with checklist, we think that the point mentioned in the records would most probably represent the cause of an alert.
Conclusion
In neurologically normal patients, IONM with TcMEPs is a safe and efficacious real-time monitoring system to warn of impending neurological injury at a reversible stage, thus providing a window of opportunity for intervention. Type of alert (parallel or non-parallel) can differentiate systemic and focal compromise. Following the checklist helps in systematically analyzing the potential cause of alert and appropriate action to be taken. Wake up test still remains a valuable monitoring tool in situations of persistent alerts and can help the surgeon in decision-making. Finally, prompt action and close coordination amongst surgeon, anesthetist, neurophysiologist, and operating room staff are required to reduce neurologic mishaps.
Ethics Statement
This is the standard operating practice; only a standard methodology is assessed in our study.
Author Contributions
SA – Senior Consultant, Department of Spine Surgery; NP, PG – Spine Fellow, MK – Consultant, Department of Anesthesia.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. MacEwen GD, Bunnell WP, Sriram K. Acute neurological complication in the treatment of scoliosis. A report of the Scoliosis Research Society. J Bone Joint Surg (1975) 57:404–8. doi:10.2106/00004623-197557030-00020
2. Ahn H, Fehlings MG. Prevention, identification, and treatment of perioperative spinal cord injury. Neurosurg Focus (2008) 25:E15. doi:10.3171/FOC.2008.25.11.E15
4. Hoppenfeld S, Gross A, Andrews C, Lonner B. The ankle clonus test for assessment of the integrity of the spinal cord during operations for scoliosis. J Bone Joint Surg Am (1997) 79:208–12. doi:10.2106/00004623-199702000-00007
5. Vauzelle C, Stagnara P, Jouvinroux P. Functional monitoring of spinal cord activity during spinal surgery. Clin Orthop Relat Res (1973) 93:173–8. doi:10.1097/00003086-197306000-00017
6. Tamaki T, Noguchi T, Takano H, Tsuji H, Nakagawa T, Imai K, et al. Spinal cord monitoring as a clinical utilization of the spinal-evoked potential. Clin Orthop Relat Res (1984) (184):58–64.
7. Owen JH. The application of intraoperative monitoring during surgery for spinal deformity. Spine (Phila Pa 1976) (1999) 24:2649–62. doi:10.1097/00007632-199912150-00012
8. Ben-David B, Haller G, Taylor P. Anterior spinal fusion complicated by paraplegia. A case report of a false-negative somatosensory-evoked potential. Spine (Phila Pa 1976) (1987) 12:536–9. doi:10.1097/00007632-198707000-00005
9. Calancie B, Harris W, Broton JG, Alexeeva N, Green BA. “Threshold-level” multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: description of method and comparison to somatosensory-evoked potential monitoring. J Neurosurg (1998) 88:457–70. doi:10.3171/jns.1998.88.3.0457
10. Dawson EG, Sherman JE, Kanim LE, Nuwer MR. Spinal cord monitoring: results of the Scoliosis Research Society and the European Spinal Deformity Society survey. Spine (Phila Pa 1976) (1991) 16(8 Suppl):S361–4. doi:10.1097/00007632-199108001-00011
11. Dinner DS, Lüders H, Lesser RP, Morris HH, Barnett G, Klem G. Intraoperative spinal somatosensory evoked potential monitoring. J Neurosurg (1986) 65:807–14. doi:10.3171/jns.1986.65.6.0807
12. Lesser RP, Raudzens P, Lüders H, Nuwer MR, Goldie WD, Morris HH III, et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol (1986) 19:22–5. doi:10.1002/ana.410190105
13. Hilibrand AS, Schwartz DM, Sethuraman V, Vaccaro AR, Albert TJ. Comparison of transcranial electric motor and somatosensory evoked potential monitoring during cervical spine surgery. J Bone Joint Surg Am (2004) 86:1248. doi:10.2106/00004623-200406000-00018
14. MacDonald DB, Al Zayed Z, Khoudeir I, Stigsby B. Monitoring scoliosis surgery with combined multiple pulse transcranial electric motor and cortical somatosensory-evoked potentials from the lower and upper extremities. Spine (Phila Pa 1976) (2003) 28:194–203. doi:10.1097/00007632-200305150-00028
15. Gunnarsson T, Krassioukov AV, Sarjeant R, Fehlings MG. Real-time continuous intraoperative electromyographic and somatosensory evoked potential recordings in spinal surgery: correlation of clinical and electrophysiologic findings in a prospective, consecutive series of 213 cases. Spine (Phila Pa 1976) (2004) 29:677. doi:10.1097/01.BRS.0000115144.30607.E9
16. Minahan RE, Sepkuty JP, Lesser RP, Sponseller PD, Kostuik JP. Anterior spinal cord injury with preserved neurogenic ‘motor’ evoked potentials. Clin Neurophysiol (2001) 112:1442. doi:10.1016/S1388-2457(01)00567-3
17. Apel DM, Marrero G, King J, Tolo VT, Bassett GS. Avoiding paraplegia during anterior spinal surgery. The role of somatosensory evoked potential monitoring with temporary occlusion of segmental spinal arteries. Spine (Phila Pa 1976) (1991) 16(Suppl):S365–70. doi:10.1097/00007632-199108001-00012
18. Pajewski TN, Arlet V, Phillips LH. Current approach on spinal cord monitoring: the point of view of the neurologist, the anesthesiologist and the spine surgeon. Eur Spine J (2007) 16:10. doi:10.1007/s00586-007-0419-6
19. Fehlings MG, Brodke DS, Norvell DC, Dettori JR. The evidence for intraoperative neurophysiological monitoring in spine surgery. Does it make a difference? Spine (Phila Pa 1976) (2010) 35(9 Suppl):S37–46. doi:10.1097/BRS.0b013e3181d8338e
20. Bridwell KH, Lenke LG, Baldus C, Blanke K. Major intraoperative neurologic deficits in pediatric and adult spinal deformity patients: incidence and etiology at one institution. Spine (Phila Pa 1976) (1998) 23:324–31. doi:10.1097/00007632-199802010-00008
21. Padberg AM, Bridwell KH. Spinal cord monitoring: current state of the art. Orthop Clin North Am (1999) 30:407–33. doi:10.1016/S0030-5898(05)70095-X
22. Winter RB. Neurologic safety in spinal deformity surgery. Spine (Phila Pa 1976) (1997) 22:1527–33. doi:10.1097/00007632-199707010-00022
23. Cronin AJ. Spinal cord monitoring. Curr Opin Orthop (2002) 13:188–92. doi:10.1097/00001433-200206000-00006
24. Hall JE, Levine CR, Sudhir KG. Intraoperative awakening to monitor spinal cord function during Harrington instrumentation and spine fusion. J Bone Joint Surg Am (1978) 60:533–6. doi:10.2106/00004623-197860040-00017
25. Mendiratta A, Emerson RG. Neurophysiologic intraoperative monitoring of scoliosis surgery. J Clin Neurophysiol (2009) 26:62–9. doi:10.1097/WNP.0b013e31819f9049
26. Ginsburg HH, Shetter AG, Raudzens PA. Postoperative paraplegia with preserved intraoperative somatosensory evoked potentials: case report. J Neurosurg (1985) 63:296–300. doi:10.3171/jns.1985.63.2.0296
27. Chatrian GE, Berger MS, Wirch AL. Discrepancy between intraoperative SSEP’s and postoperative function: case report. J Neurosurg (1988) 69:450–4. doi:10.3171/jns.1988.69.3.0450
28. Zornow MH, Grafe MR, Tybor C, Swenson MR. Preservation of evoked potentials in a case of anterior spinal artery syndrome. Electroencephalogr Clin Neurophysiol (1990) 77:137–9. doi:10.1016/0168-5597(90)90028-C
29. Jones SJ, Buonamassa S, Crockard HA. Two cases of quadriparesis following anterior cervical discectomy, with normal perioperative somatosensory evoked potentials. J Neurol Neurosurg Psychiatry (2003) 74:273–6. doi:10.1136/jnnp.74.2.273
30. Pelosi L, Jardine A, Webb JK. Neurological complications of anterior spinal surgery for kyphosis with normal somatosensory evoked potentials (SEPs). J Neurol Neurosurg Psychiatry (1999) 66:662–4. doi:10.1136/jnnp.66.5.662
31. Schwartz DM, Auerbach JD, Dormans JP, Flynn J, Drummond DS, Bowe JA, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am (2007) 89:2440–9. doi:10.2106/JBJS.F.01476
32. Pelosi L, Blumhardt LD. Effects of age on working memory: an event-related potential study. Brain Res Cogn Brain Res (1999) 7:321–34. doi:10.1016/S0926-6410(98)00035-4
33. Su CF, Haghighi SS, Oro JJ, Gaines RW. “Backfiring” in spinal cord monitoring. High thoracic spinal cord stimulation evokes sciatic response by antidromic sensory pathway conduction, not motor tract conduction. Spine (Phila Pa 1976) (1992) 17:504–8. doi:10.1097/00007632-199205000-00006
34. Mochida K, Komori H, Okawa A, Shinomiya K. Evaluation of motor function during thoracic and thoracolumbar spinal surgery based on motor-evoked potentials using train spinal stimulation. Spine (Phila Pa 1976) (1997) 22:1385–93. doi:10.1097/00007632-199706150-00018
35. Nagle KJ, Emerson RG, Adams DC, Heyer EJ, Roye DP, Schwab FJ, et al. Intraoperative monitoring of motor-evoked potentials: a review of 116 cases. Neurology (1996) 47:999–1004. doi:10.1212/WNL.47.4.999
36. Dillingham TR, Pezzin LE, Lauder TD. Relationship between muscle abnormalities and symptom duration in lumbosacral radiculopathies. Am J Phys Med Rehabil (1998) 77:103–7. doi:10.1097/00002060-199803000-00003
37. Cioni B, Meglio M, Rossi GF. Intraoperative motor-evoked potentials monitoring in spinal neurosurgery. Arch Ital Biol (1999) 137:115–26.
38. Deletis V, Isgum V, Amassian VE. Neurophysiological mechanisms underlying motor-evoked potentials in anesthetized humans: part 1. Recovery time of corticospinal tract direct waves elicited by pairs of transcranial electrical stimuli. Clin Neurophysiol (2001) 112:438–44. doi:10.1016/S1388-2457(00)00557-5
39. Deletis V, Rodi Z, Amassian VE. Neurophysiological mechanisms underlying motor-evoked potentials in anesthetized humans: part 2. Relationship between epidurally and muscle recorded MEPs in man. Clin Neurophysiol (2001) 112:445–52. doi:10.1016/S1388-2457(00)00557-5
40. Jones SJ, Harrison R, Koh KF, Mendoza N, Crockard HA. Motor-evoked potential monitoring during spinal surgery: responses of distal limb muscles to transcranial cortical stimulation with pulse trains. Electroencephalogr Clin Neurophysiol (1996) 100:375–83. doi:10.1016/0168-5597(96)95728-7
41. Pechstein U, Cedzich C, Nadstawek J, Schramm J. Transcranial high-frequency repetitive electrical stimulation for recording myogenic motor-evoked potentials with the patient under general anesthesia. Neurosurgery (1996) 39:335–43. doi:10.1097/00006123-199608000-00020
42. Rodi Z, Deletis V, Morota N, Vodusek DB. Motor-evoked potentials during brain surgery. Pflugers Arch (1996) 431(6 Suppl 2):R291–2. doi:10.1007/BF02346383
43. Ubags LH, Kalkman CJ, Been HD, Koelman JH, Ongerboer de Visser BW. A comparison of myogenic motor evoked responses to electrical and magnetic transcranial stimulation during nitrous oxide/opioid anesthesia. Anesth Analg (1999) 88:568–72. doi:10.1213/00000539-199903000-00019
44. MacDonald DB, Janusz M. An approach to intraoperative monitoring of thoracoabdominal aneurysm surgery. J Clin Neurophysiol (2002) 19:43–54. doi:10.1097/00004691-200201000-00006
45. Langeloo DD, Journee HL, de Kleuver M, Grotenhuis JA. Criteria for transcranial electrical motor evoked potential monitoring during spinal deformity surgery—a review and discussion of the literature. Neurophysiol Clin (2007) 37:431–9. doi:10.1016/j.neucli.2007.07.007
46. Sala F, Bricolo A, Faccioli F, Lanteri P, Gerosa M. Surgery for intramedullary spinal cord tumors: the role of intraoperative (neurophysiological) monitoring. Eur Spine J (2007) 16:S130–9. doi:10.1007/s00586-007-0423-x
47. Hsu B, Cree AK, Lagopoulos J, Cummine JL. Transcranial motor-evoked potentials combined with response recording through compound muscle action potential as the sole modality of spinal cord monitoring in spinal deformity surgery. Spine (Phila Pa 1976) (2008) 33:1100–6. doi:10.1097/BRS.0b013e31816f5f09
48. Lee JY, Hilibrand AS, Lim MR, Zavatsky J, Zeiller S, Schwartz DM, et al. Characterization of neurophysiologic alerts during anterior cervical spine surgery. Spine (Phila Pa 1976) (2006) 31:1916–22. doi:10.1097/01.brs.0000228724.01795.a2
49. Schwartz DM, Sestokas AK, Hilibrand AS, Vaccaro AR, Bose B, Li M, et al. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput (2006) 20:437–44. doi:10.1007/s10877-006-9032-1
50. Kempton LB, Nantau WE, Zaltz I. Successful monitoring of transcranial electrical motor evoked potentials with isoflurane and nitrous oxide in scoliosis surgeries. Spine (Phila Pa 1976) (2010) 35:E1627–9. doi:10.1097/BRS.0b013e3181cc8dba
51. Sakaki K, Kawabata S, Ukegawa D, Hirai T, Ishii S, Tomori M, et al. Warning thresholds on the basis of origin of amplitude changes in transcranial electrical motor-evoked potential monitoring for cervical compression myelopathy. Spine (Phila Pa 1976) (2012) 37:E913–21. doi:10.1097/BRS.0b013e31824caab6
52. Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery (2006) 58:1129–43; discussion 1129–43. doi:10.1227/01.NEU.0000215948.97195.58
53. Nordwall A, Wikkelso C. A late neurologic complication of scoliosis surgery in connection with syringomyelia. Acta Orthop Scand (1979) 50:407–10. doi:10.3109/17453677908989783
54. Jacobs MJ, Elenbass TW, Schurink GWH, Mess WH, Mochtar B. Assessment of spinal cord integrity during thoracoabdominal aortic aneurysm repair. Ann Thorac Surg (2000) 74:S1864–6. doi:10.1016/S0003-4975(02)04154-1
55. Sloan T, Heyer EJ. Anesthesia for intraoperative neurophysiologic monitoring of the spinal cord. J Clin Neurophysiol (2002) 19:430–43. doi:10.1097/00004691-200210000-00006
56. Wiedemayer H, Fauser B, Sandalcioglu IE, Schäfer H, Stolke D. The impact of neurophysiological intraoperative monitoring on surgical decisions: a critical analysis of 423 cases. J Neurosurg (2002) 96:255–62. doi:10.3171/jns.2002.96.2.0255
57. Skinner SA, Nagib M, Bergman TA, Maxwell RE, Msangi G. The initial use of free-running electromyography to detect early motor tract injury during resection of intramedullary spinal cord lesions. Neurosurgery (2005) 56(2 Suppl):299–314.
58. Macdonald DB. Intraoperative motor evoked potential monitoring: overview and update. J Clin Monit Comput (2006) 20:347–77. doi:10.1007/s10877-006-9033-0
59. Schwartz DM, Sestokas AK, Dormans JP, Vaccaro AR, Hilibrand AS, Flynn JM, et al. Transcranial electric motor evoked potential monitoring during spine surgery: is it safe? Spine (Phila Pa 1976) (2011) 36(13):1046–9. doi:10.1097/BRS.0b013e3181ecbe77
60. York DH, Chabot RJ, Gaines RW. Response variability of somatosensory evoked potentials during scoliosis surgery. Spine (Phila Pa 1976) (1987) 12:864–76.
61. Toleikis JR, Carlvin AO, Shapiro DE, Schafer MF. The use of dermatomal evoked responses during surgical procedures that use intrapedicular fixation of the lumbosacral spine. Spine (Phila Pa 1976) (1993) 18:2401–7.
62. Kearse LA Jr, Lopez-Bresnahan M, McPeck K, Tambe V. Loss of somatosensory evoked potentials during intramedullary spinal cord surgery predicts postoperative neurologic deficits in motor function [corrected]. J Clin Anesth (1993) 5:392–8. doi:10.1016/0952-8180(93)90103-L
63. Wilson-Holden TJ, Padberg AM, Lenke LG, Larson BJ, Bridwell KH, Bassett GS. Efficacy of intraoperative monitoring for pediatric patients with spinal cord pathology undergoing spinal deformity surgery. Spine (Phila Pa 1976) (1999) 24:1685–92. doi:10.1097/00007632-199908150-00010
64. Thuet ED, Winscher JC, Padberg AM, Bridwell KH, Lenke LG, Dobbs MB, et al. Validity and reliability of intraoperative monitoring in pediatric spinal deformity surgery: a 23-year experience of 3436 surgical cases. Spine (Phila Pa 1976) (2010) 35:1880–6. doi:10.1097/BRS.0b013e3181e53434
65. Tamkus AA, Rice KS, Kim HL. Differential rates of false-positive findings in transcranial electric motor evoked potential monitoring when using inhalational anesthesia versus total intravenous anesthesia during spine surgeries. Spine J (2013) 14(8):1440–6. doi:10.1016/j.spinee.2013.08.037
66. Kim DH, Zaremski J, Kwon B, Jenis L, Woodard E, Bode R, et al. Risk factors for false-positive transcranial motor evoked potential monitoring alerts during surgical treatment of cervical myelopathy. Spine (Phila Pa 1976) (2007) 32:3041–6. doi:10.1097/BRS.0b013e31815d0072
67. Manninen PH. Monitoring evoked potentials during spinal surgery at one institution. Can J Anaesth (1998) 45:460–5. doi:10.1007/BF03012582
Keywords: motor evoked potentials, neuromonitoring, spine deformities, transcranial evoked potentials, deformity correction
Citation: Acharya S, Palukuri N, Gupta P and Kohli M (2017) Transcranial Motor Evoked Potentials during Spinal Deformity Corrections—Safety, Efficacy, Limitations, and the Role of a Checklist. Front. Surg. 4:8. doi: 10.3389/fsurg.2017.00008
Received: 19 November 2016; Accepted: 26 January 2017;
Published: 13 February 2017
Edited by:
Jaimo Ahn, Hospital of the University of Pennsylvania, USAReviewed by:
Konstantinos Markatos, Henry Dunant Hospital, GreeceHarish Hosalkar, The Hosalkar Institute for Joint Preservation and Injury Care, USA
Copyright: © 2017 Acharya, Palukuri, Gupta and Kohli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nagendra Palukuri, bmFnZW5kcmE1NThAZ21haWwuY29t
 Shankar Acharya
Shankar Acharya Nagendra Palukuri
Nagendra Palukuri Pravin Gupta1
Pravin Gupta1