
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Stroke, 15 April 2024
Sec. Stroke in the Young
Volume 3 - 2024 | https://doi.org/10.3389/fstro.2024.1372949
This article is part of the Research TopicPrevention of Stroke and Vascular Cognitive Decline in the Pediatric PopulationView all 6 articles
 Paul Bangirana1,2
Paul Bangirana1,2 Amelia K. Boehme3
Amelia K. Boehme3 Annet Birabwa4
Annet Birabwa4 Robert O. Opoka2,5
Robert O. Opoka2,5 Deogratias Munube2,5
Deogratias Munube2,5 Ezekiel Mupere5
Ezekiel Mupere5 Phillip Kasirye6
Phillip Kasirye6 Grace Muwanguzi2
Grace Muwanguzi2 Maxencia Musiimenta2
Maxencia Musiimenta2 George Ru7
George Ru7 Nancy S. Green7*
Nancy S. Green7* Richard Idro2,5
Richard Idro2,5Introduction: The neurocognitive functions in Ugandan children aged 1–12 years with sickle cell anemia (SCA) were compared to their non-SCA siblings to identify risk factors for disease-associated impairment.
Methods: This cross-sectional study of the neurocognitive functions in children with SCA (N = 242) and non-SCA siblings (N = 127) used age- and linguistically appropriate standardized tests of cognition, executive function, and attention for children ages 1–4 and 5–12. Test scores were converted to locally derived age-normalized z-scores. The SCA group underwent a standardized stroke examination for prior stroke and transcranial Doppler ultrasound to determine stroke risk by arterial flow velocity.
Results: The SCA group was younger than their siblings (mean ages 5.46 ± 3.0 vs. 7.11 ± 3.51 years, respectively; p < 0.001), with a lower hemoglobin concentration (7.32 ± 1.02 vs. 12.06 ± 1.42, p < 0.001). The overall cognitive SCA z-scores were lower, −0.73 ± 0.98, vs. siblings, −0.25 ± 1.12 (p < 0.001), with comparable findings for executive function of −1.09 ± 0.94 vs. −0.84 ± 1.26 (p = 0.045), respectively. The attention z-scores for ages 5–12 for the SCA group and control group were similar: −0.37 ± 1.4 vs. −0.11 ± 0.17 (p = 0.09). The overall differences in SCA status were largely driven by the older age group, as the z-scores in the younger subsample did not differ from controls. Analyses revealed the strongest predictors of poor neurocognitive outcomes among the SCA sample to be the disease, age, and prior stroke (each p < 0.001). The impacts of anemia and SCA were indistinguishable.
Discussion: Neurocognitive testing in children with SCA compared to non-SCA siblings revealed poorer SCA-associated functioning in children older than age 4. The results indicate the need for trials assessing the impact of disease modification on children with SCA.
Sickle cell anemia (SCA) is a serious inherited blood condition affecting 0.5%−2% of births in Uganda and other high-prevalence countries in sub-Saharan Africa (Ndeezi et al., 2016; Ware et al., 2017; Ambrose et al., 2018; Uyoga et al., 2019; Nnodu et al., 2020). A high disease burden, compounded by health and health system challenges in low-income countries, exposes many affected children to early disease complications, including cerebrovascular injury (Makani et al., 2011; Bello-Manga et al., 2016; Uyoga et al., 2019; Nnodu et al., 2021; Ranque et al., 2022). SCA-associated cerebrovascular injury commonly results in overt and/or clinically “silent” infarcts, often in children younger than 10 years of age (Bernaudin et al., 2011; Brousse et al., 2015; DeBaun and Kirkham, 2016; Munube et al., 2016; Green et al., 2019). Infarcts can lead to impaired neurocognitive functions (Kawadler et al., 2016; Prussien et al., 2019a; Knight et al., 2021; Lee et al., 2022). In high-income countries where successful stroke prevention strategies are routinely practiced, the continued occurrence of silent infarcts remains a neurocognitive risk (Bernaudin et al., 2011; Brousse et al., 2015; DeBaun and Kirkham, 2016; Kawadler et al., 2016; Kwiatkowski et al., 2019; Estcourt et al., 2020; Longoria et al., 2022). Worldwide, children with SCA with or without imaging abnormalities have a heightened risk of intellectual deficits (Prussien et al., 2019b; Idro et al., 2022).
Severe anemia is a risk factor for SCA-associated cerebral infarcts and impaired neurocognition due to abnormal blood flow and reduced cerebral oxygen delivery (DeBaun et al., 2012; Quinn and Dowling, 2012; King et al., 2014; Ford et al., 2018; Ogunsile et al., 2018; Estcourt et al., 2020; Jacob et al., 2022). The risk of cognitive impairment from SCA in sub-Saharan Africa may be compounded by low parental education, a proxy for poverty, malnutrition, and endemic infections (Dhabangi et al., 2016; Oluwole et al., 2016; Macharia et al., 2018; Prussien et al., 2019a, 2020; Bello-Manga et al., 2020). Moreover, stroke reduction strategies are not generally available in the region (Noubiap et al., 2017; Marks et al., 2018; Green et al., 2019). Cerebrovascular injury among the many African children with SCA raises questions about the prevalence and types of neurocognitive risk in this population (Marks et al., 2018). To date, few pediatric studies of SCA in sub-Saharan Africa have assessed the associated neurocognitive effects compared to unaffected children (Ruffieux et al., 2013; Oluwole et al., 2016; Prussien et al., 2019a; Jacob et al., 2022). Only one of these studies compared results to sibling controls, a strategy that can better control for environmental and socioeconomic effects (Jacob et al., 2022).
We assessed the frequency of neurological and neurocognitive impairment in a cross-sectional study of Ugandan children with SCA ages 1–12 years, “Burden and Risk of Neurological and Cognitive Impairment in Pediatric Sickle Cell Anemia in Uganda (BRAIN SAFE)” (Green et al., 2019). The overall frequency of neurocognitive dysfunction was 11.2%, with older (ages 5–12) at a 3-fold higher risk of impairment compared to younger participants (ages 1–4). In this secondary analysis, we report detailed findings of the neurocognitive evaluation of participants compared to their non-SCA siblings to identify contributions from demographic and clinical factors beyond age. We hypothesized that, compared to non-SCA siblings, children with SCA had lower neurocognitive functioning and that age, malnutrition, adverse neurological outcomes of prior stroke, and elevated transcranial Doppler (TCD) ultrasound velocity were risk factors. In contrast to other sub-Saharan Africa studies of children with SCA, we assessed the detailed neurocognitive performance for cognition, executive function, and attention in a large sample of Ugandan children compared to non-SCA siblings, as well as the effects of key demographic and neurological risk factors.
A random cross-sectional sample of 265 children with SCA ages 1–12 years attending the Mulago Hospital Sickle Cell Clinic in Kampala, Uganda, and a sample of their non-SCA siblings were enrolled in BRAIN SAFE 1 (2016–2018) (Green et al., 2019). The sample size was determined from previously reported frequencies and impacts of cerebral infarction on neurological and neurocognitive functions (Kawadler et al., 2016; Prussien et al., 2019a; Knight et al., 2021; Lee et al., 2022). Routine SCA pediatric care did not include disease-modifying therapy at that time. The study was approved by the Makerere University School of Medicine Research and Ethics Committee, the Uganda National Council for Science and Technology, and the Columbia University Institutional Review Board.
As previously reported, inclusion criteria were (a) SCA confirmed by hemoglobin electrophoresis (HbSS or HbS-B0 thalassemia) and (b) having attended the Mulago SCA clinic (Green et al., 2019). To focus on SCA-related neurological complications, we excluded those with a history of neurological abnormalities before 4 months of age (Bainbridge et al., 1985). Caregiver written informed consent was obtained, with assent from participants aged 8 years or older. Non-SCA participants (N = 127) were also enrolled, with inclusion criteria of (a) aged 1–12 years and (b) hemoglobin electrophoresis demonstrating a lack of SCA (i.e., HbAA or HbAS). Among these controls, 119 (93.7%) were siblings; the rest were other close relatives or neighbors. Hence, we refer to them as “siblings.”
The World Health Organization (WHO) standards were used for the anthropometric assessments for malnutrition of the SCA and sibling participants to detect malnutrition, defined as low weight-for-height (“wasting”), as previously reported (Duggan, 2010; Green et al., 2019). The assessments of SCA participants at enrollment were a medical history and physical examination, an examination for prior stroke using the National Institutes of Health (NIH) Pediatric Stroke Scale, and stroke risk stratification by intracranial arterial flow velocity identified by TCD as elevated to ≥170 cm/s (“conditional” or “abnormal”) (Green et al., 2019). Caregiver educational attainment was scored as previously performed, reported as none, primary school, secondary school, more than secondary education, or unknown (Bangirana et al., 2009).
Overall neurocognitive functioning, including behavioral measures, attention, and executive function, was assessed using age-appropriate tests by experienced testers in both SCA and non-SCA siblings. All assessment tools had previously been translated into the predominant local language, validated, and used to establish age-specific community norms for healthy children in Kampala (Green et al., 2019). These tools have also been used to assess cognitive outcomes after severe malaria and pediatric HIV within the same community (Familiar et al., 2015; Hickson et al., 2019).
For children aged 1–4 years, the Mullen Scales of Early Learning (Mullen) and the Behavioral Rating Inventory for Executive Function–Preschool version (BRIEF-P) assessed cognitive functioning and executive function, respectively (Gioia et al., 2002; Boivin et al., 2013). The Mullen subtests assess gross and fine motor, visual reception, receptive language, and expression language. A summation of fine motor, visual reception, receptive language, and expressive language scores constitute the Early Learning Composite for measuring overall neurocognitive ability, the primary outcome for the Mullen. The BRIEF-P is a caregiver assessment of the child's executive functioning using 63 items for which the caregiver endorses child behaviors exhibited over the prior 6 months. The summation of these items gives a Global Executive Composite for measuring executive function, the primary outcome of the BRIEF-P. The subtests were for self-control, flexibility, and metacognition.
Children aged 5–12 years were tested using the Kaufman Assessment Battery for Children, Second Edition (KABC-II) (Tumwine et al., 2018), the BRIEF school-age version (Gioia et al., 2002), and the Test of Variables of Attention (TOVA) (Bangirana et al., 2009) to assess overcall neurocognitive functioning, executive function, and attention, respectively. The KABC-II subscales assessed working memory, visual-spatial ability, learning ability, and reasoning. A summation of these four scales generates a composite value, the Global Mental Processing Index. The BRIEF for school-age participants uses caregiver responses on 86 items. Here, the composite score, computed from the subtests of behavioral regulation and metacognition, generated the General Executive Composite. The TOVA, a computerized test for which children are instructed to press a switch whenever a specific target appears on the screen, assesses attention and inhibitory control. The composite score, D-prime, is calculated from subtest scores for omission errors, commission errors, response time, and attention-deficit/hyperactivity disorder.
SCA and non-SCA participants were grouped into two age ranges according to the tests used. The raw scores for all neurocognitive assessments were converted to age-normalized z-scores using the established standards for unaffected healthy children, as previously described (Green et al., 2019). Within each age range, the z-scores were analyzed and compared, by group, using means and standard deviations. Negative values correspond to scores below the age-normalized z-scores. In contrast, negative z-scores for the BRIEF and the BRIEF-P indicate better function. Hence, the positive signs for those two BRIEF tests were flipped to negative for a consistent directionality in reporting the results (Hickson et al., 2019). Data were analyzed using means and standard deviation. For categorical data, an analysis of variation and a Pearson chi-square test were used for analyses. Continuous data were analyzed using the Pearson correlation. The factors associated with impaired neurocognition were assessed using linear regression. No missing participant data were imputed.
Neurocognitive assessment was performed on 242 of 265 (91.3%) SCA participants and all 127 non-SCA siblings. The parents of the 23 SCA participants without neurocognitive assessments were unable to schedule testing (Green et al., 2019). The mean age of SCA participants tested was 5.46 ± 2.98 years vs. 7.11 ± 3.51 in the non-SCA siblings (p < 0.001; Table 1). The mean hemoglobin concentration was also highly different by group: 7.32 ± 1.02 vs. 12.06 ± 1.42 in the SCA vs. control group, respectively (p < 0.001). Close to half (48.4%) of the SCA sample was female compared to 43.9% of the non-SCA siblings (p = 0.33). Malnutrition, defined as weight-for-age at −2 z-scores or below using WHO global norms by age and sex, was found in 37 (15.3%) children with SCA and 11 (8.7%) controls (p = 0.20). Caregiver education differed between the two study groups (p = 0.018), with a higher proportion of caregivers in the control group having little or no education.
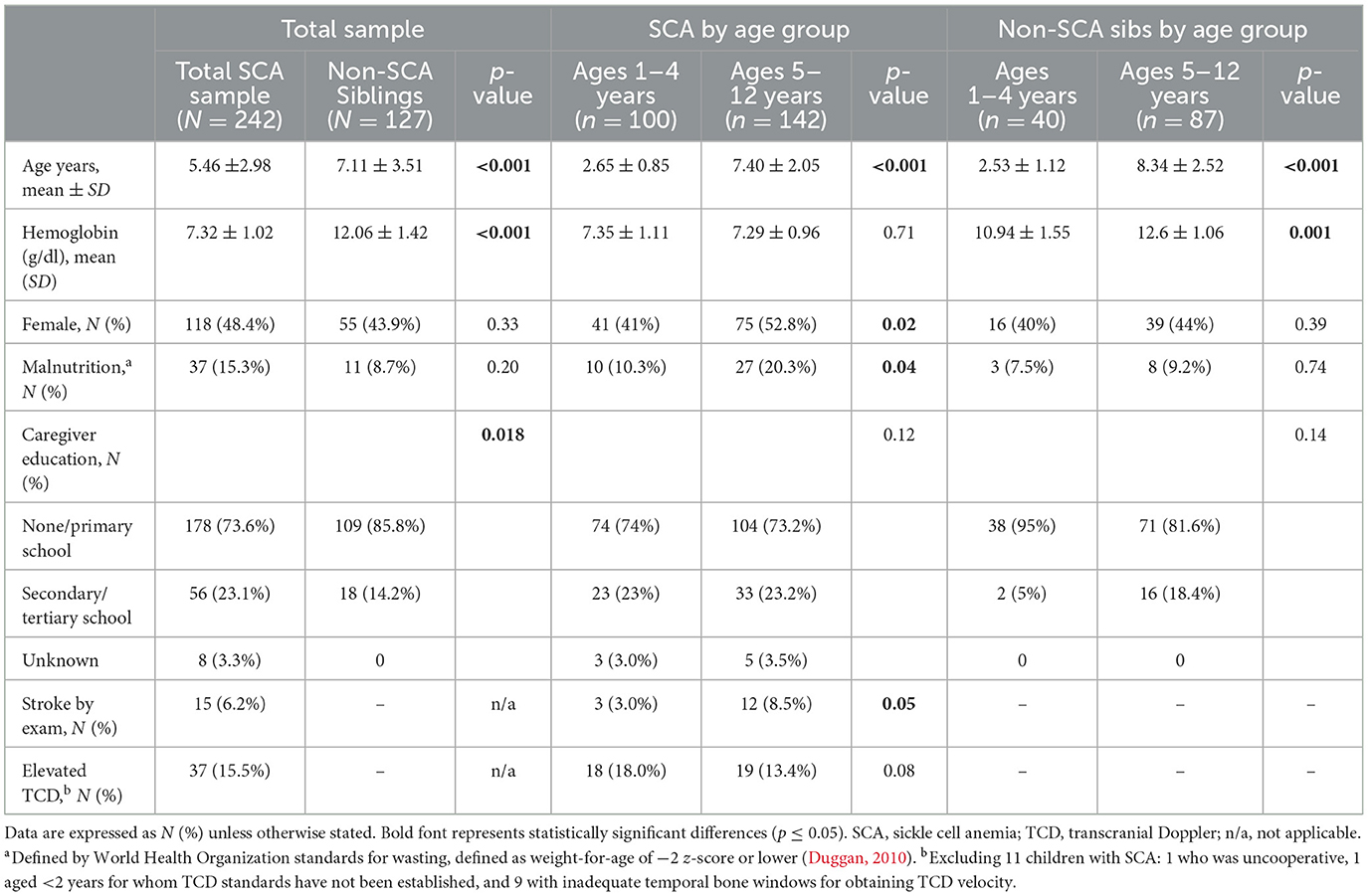
Table 1. Demographic and neurologic characteristics stratified by SCA status and age group, 1–4 or 5–12 years of age.
As the neurocognitive testing platforms used differed by the two age groups, ages 1–4 and 5–12, key variables were compared within the group of children with SCA and the controls (Table 1). For the SCA sample, the older group had higher proportions of females and malnutrition compared to the younger group. Among neurological outcomes in the SCA sample, a higher proportion of the older group had a prior stroke and a marginally lower proportion had an elevated TCD velocity. Among the controls, the older group differed only in having a significantly higher mean hemoglobin concentration.
Supplementary Table S1 compares each age group by SCA status. As in the overall sample, higher hemoglobin levels were found in the control group for each age group. No other significant differences were found between the younger groups. In contrast, the older SCA group was younger than the control group, had a larger proportion of malnutrition, and higher caregiver education.
The mean scores for the groups with SCA and non-SCA siblings were normally distributed for all three neurocognitive domains tested. The overall sample with SCA performed significantly worse for cognition in the standardized age-appropriate tests than the non-SCA controls, −0.73 ± 0.98 vs. −0.25 ± 1.12 (p < 0.001; Table 2, Figure 1). Similarly, the SCA sample scored lower for executive function than their unaffected siblings: −1.09 ± 0.94 vs. −0.84 ± 1.26 (p = 0.045), respectively.
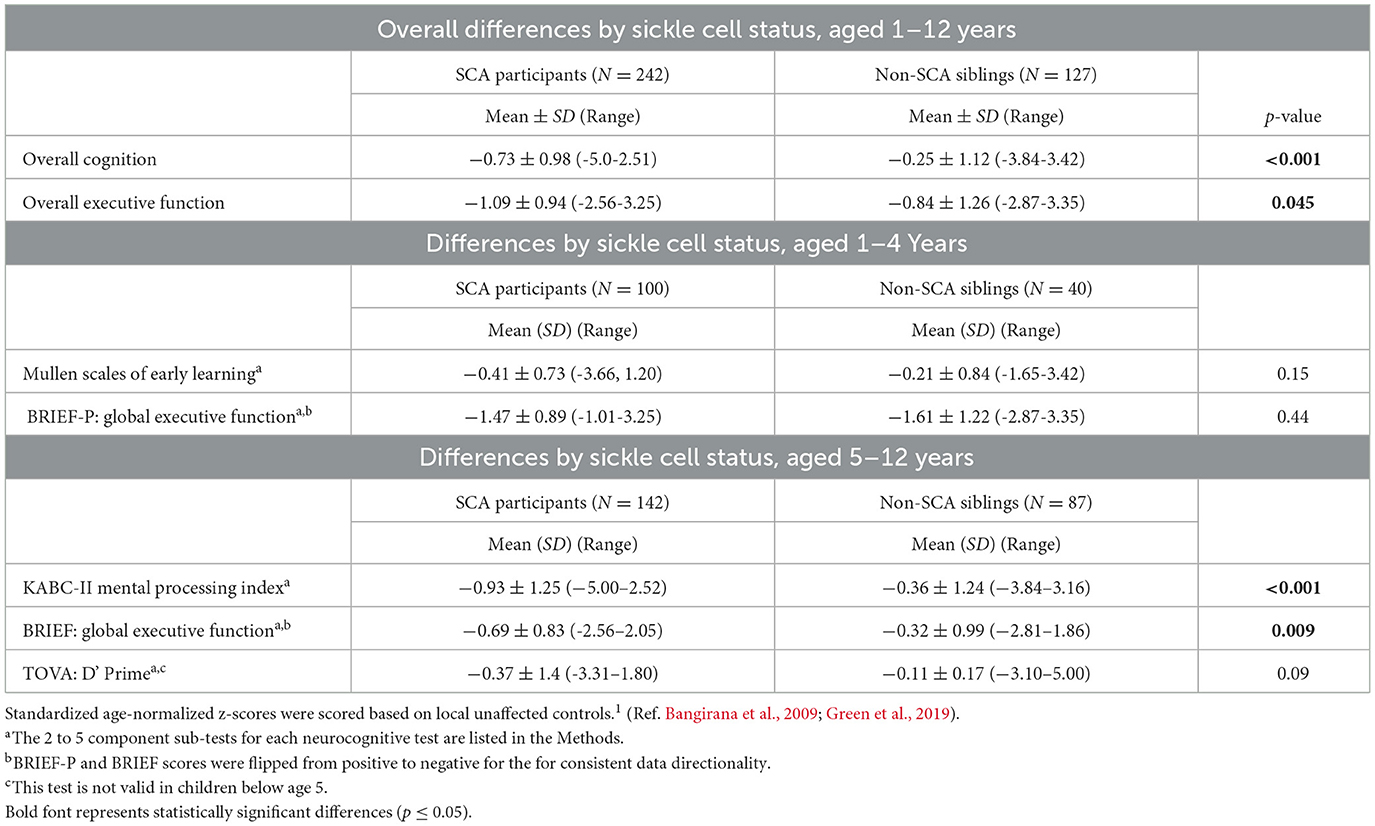
Table 2. Composite neurocognitive test results, by z-scores, for children aged 1–4 and 5–12 years, assessed by tests for cognition, executive function and attention (the last test was used only with the older group).
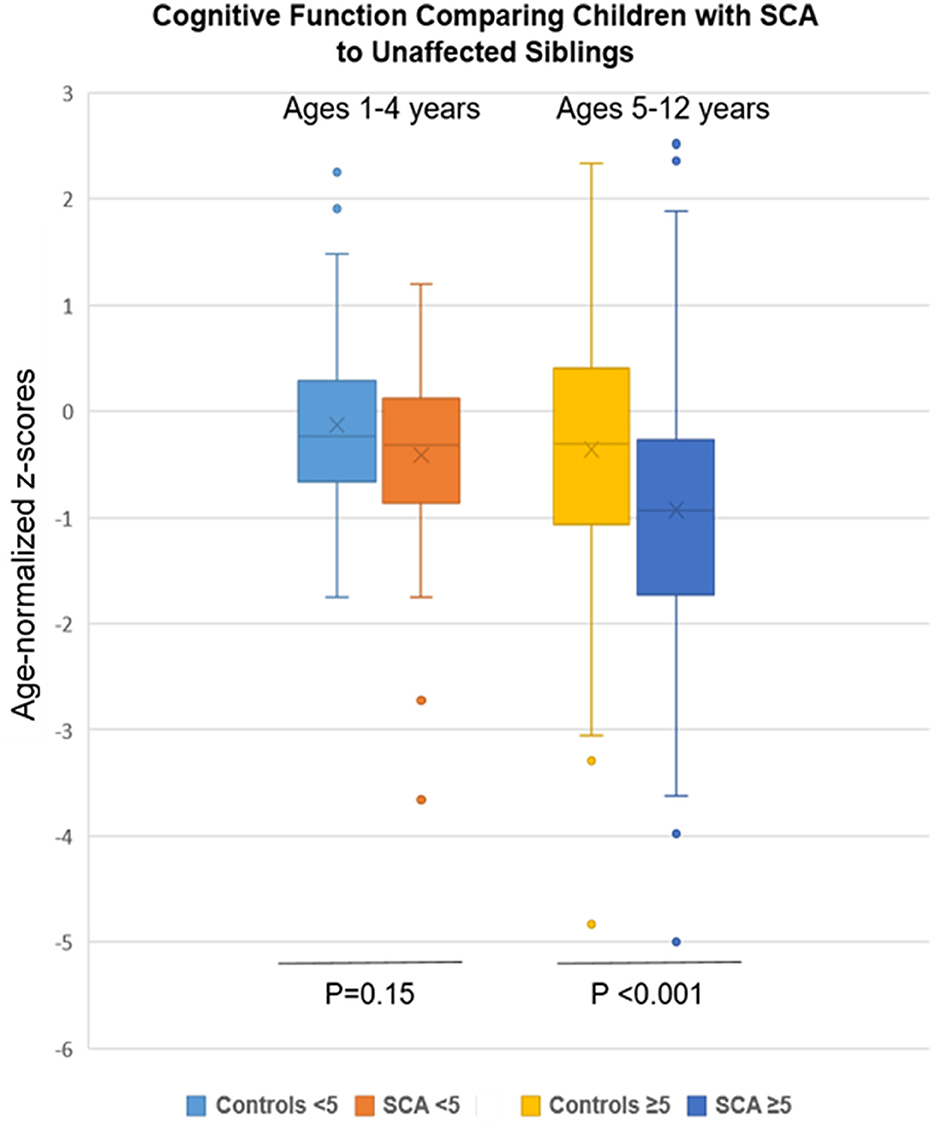
Figure 1. Cognitive findings by SCA status and age group (1–4 and 5–12 years) compared to unaffected siblings. Age-normalized z-scores for mean cognitive testing were lower in the SCA group but only in the older age group (p < 0.001).
Among those in the younger age group, ages 1–4 years, who were tested, no differences according to SCA status were found in cognition or executive function. Cognitive function, tested using the Mullen, was −0.41 ± 0.73 for SCA and −0.21 ± 0.84 (p = 0.15) for non-SCA samples. Similarly, executive function, tested using the BRIEF-P in these younger participants comparing SCA vs. non-SCA was −1.47 ± 0.89 vs. −1.61 ± 1.22 (p = 0.44), respectively. Hence, neurocognitive function was retained in the younger subsample of children with SCA through age 4.
In contrast, lower z-scores were found according to SCA status for the older children for neurocognition, tested using the KABC-II, −0.93 ± 1.25 vs. −0.36 ± 1.24 (p < 0.001), and for executive function, tested using the BRIEF, −0.69 ± 0.83 vs. −0.32 ± 0.99 (p = 0.009). However, testing for attention, using TOVA, which was possible only among the older age group, demonstrated a similar performance between the two groups: −0.37 ± 1.4 vs. −0.11 ± 0.17 (p = 0.09) for the SCA and control groups, respectively. Two of the three areas of assessment, cognition and executive function, were lower in the older subsample of children with SCA compared to controls.
To remove the potential of excess influence on cognition from prior stroke within the SCA sample, we reanalyzed the mean z-scores after removing 15 affected SCA participants (mean age 6.0 ± 2.59 years). As expected, the mean z-score for the SCA subsample with prior stroke vs. no stroke was much lower −2.18 ± 1.53 than the overall scores (p < 0.001). However, removing this small subset affected by stroke from the SCA sample had no significant effects on the mean SCA z-scores for cognition or executive function compared to controls.
The overall neurocognitive test results for each of the domains for SCA were compared to controls for each variable collected. We first asked whether hemoglobin concentration for the SCA group compared to controls had effects that were separable from SCA in neurocognitive outcomes. Using linear regression with both variables in the model—SCA status and hemoglobin concentration—the difference was p = 0.004, with the main effect largely driven by SCA. No effects from sex, malnutrition, or elevated TCD velocity were found in any of the three neurocognitive domains tested (Table 3).
We then examined overall test outcomes for factors contributing to the outcomes (Table 3). For overall cognition or executive function, age-normalized z-scores declined with age. Adjusting for other variables of hemoglobin concentration, caregiver education, and prior stroke demonstrated their impact on effect sizes. Despite those changes, the outcomes were unchanged. These findings demonstrated that the poorer function of the SCA group was attributed to the impact of the disease.
By the TOVA test, for attention in the older participants in both SCA and controls, age also negatively impacted z-scores. The influence of age for the SCA group was assessed as r2 = −0.58 and −0.72 for the controls (both p < 0.001). Unexpectedly, neither prior stroke nor elevated TCD was associated with reduced attention.
Finally, we examined the SCA sample for impact from prior stroke or elevated TCD velocity on each of the three outcomes. Prior stroke strongly affected cognition (p < 0.001) but had no significant effects on executive function or attention (Table 4). Elevated TCD velocity had a borderline impact on cognition, but it, like prior stroke, had no impact on executive function or attention.
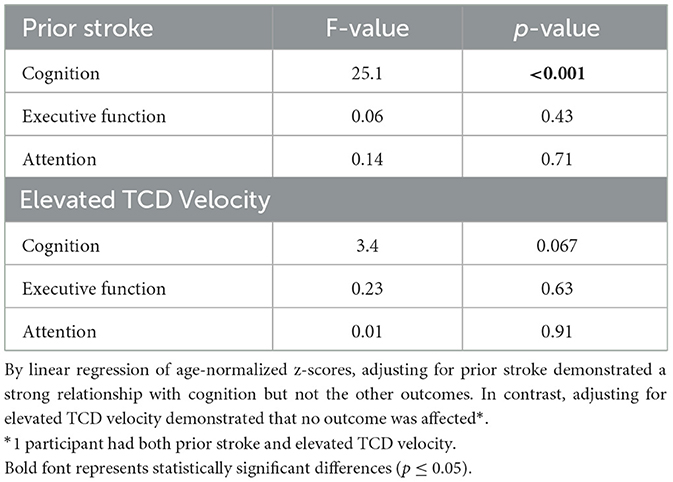
Table 4. Examining the role of prior stroke or elevated TCD velocity on neurocognitive outcomes within the SCA sample.
Children with SCA in sub-Saharan Africa are at risk for disease-associated cerebrovascular injury as well as environmental challenges (Oron et al., 2020; Nnodu et al., 2021). In a large clinic-based sample of Ugandan children with SCA in Kampala compared to their non-SCA siblings, our cross-sectional neurocognitive assessment revealed these main findings: (1) The mean test z-scores for cognition and executive function were substantially lower in the SCA sample, even after accounting for age, hemoglobin concentration, caregiver education, and prior stroke. No discernable effects were seen regarding sex, malnutrition, or elevated TCD velocity. These findings confirm SCA as the main cause of impaired neurocognition in this study. Lower scores in cognition testing of approximately 0.5 z-scores in the children with SCA correspond to approximately 8 IQ points below that of their siblings. (2) The differences by hemoglobinopathy diagnosis were driven by the older subsample with SCA, ages 5–12 (Gur et al., 2021). In contrast, children with SCA aged 1–4 were not different in cognition or executive function from unaffected siblings in that age group. (3) Age and clinically evident prior stroke, but not elevated TCD arterial velocity, were the largest drivers of impaired cognition. Nonetheless, excluding a modest number of SCA participants with prior stroke from the analyses did not significantly affect the results of the remaining sample. (4) Among those aged 5–12, attention was not significantly different from the controls unless accounting for differences in age and/or prior stroke. (5) The non-SCA siblings in our study scored below healthy Kampala-based age-normalized controls. What social, economic, and/or educational opportunities affected neurocognitive performance in the siblings was not assessed.
Similar findings of impaired neurocognitive function and attention of older SCA children compared to non-SCA siblings were observed in a prior Tanzanian report of a smaller sample (Jacob et al., 2022). Similar to prior reports of children with SCA in Africa, the United States, and elsewhere, our study's participants with SCA had lower executive functioning (Ruffieux et al., 2013; Prussien et al., 2019a; Jacob et al., 2022). This consistent finding was seen despite our use of a test by parental report, which may under-report functional deficits, rather than direct testing used by other studies (Prussien et al., 2019a; Trpchevska et al., 2022).
Unlike our findings here, the Tanzanian study did not observe a decline in performance in older vs. younger SCA participants (Jacob et al., 2022). As that study tested children aged 6 years and older, taken together, these findings support our findings of the sparing and/or resilience of the younger age group with SCA. Consistent with this observation, the cumulative effects of SCA cerebrovascular injury over time are considered to be primarily responsible for the association between age and neurocognitive impairment in children with SCA (Wang et al., 2001; Schatz and McClellan, 2006). Collectively, these observations suggest that low caregiver education, alone and/or as a surrogate for low socioeconomic status (SES), and/or educational disadvantages may contribute to—but are not the main drivers of—the age effects seen in children with SCA in sub-Saharan Africa (Bangirana et al., 2009). Similar findings were reported in a U.S. study, in which SCA and social factors both influenced neurocognition (King et al., 2014). The effect of age on the neurocognitive outcome in our study could also be a consequence of “growing into deficit,” whereby effects of brain injury become apparent as the child grows older (Zhuo et al., 2022).
A relatively high proportion of neurocognitive impairment in the older group of non-SCA siblings may be at least attributable to social issues, as all neurocognitively impaired siblings had caregivers with low education. Our group has previously reported this association among healthy children in Kampala (Bangirana et al., 2009).
This study's limitations include potential biases from SCA-associated survival and the cross-sectional study design. These issues may have affected the relationships seen with age. Nonetheless, our data reflect results from a substantial number of children receiving SCA care at a large urban center. Executive function was based on parental report rather than direct child assessment; hence, it could have been biased. More direct measures of executive function may provide clearer insights. A test for attention comparable to the TOVA for children ages 1–4 years was not available. Executive function was not tested directly, as the BRIEF and BRIEF-P use parental reports. At that time, we had no other option for which local translated platforms and age-normalized standards were available. We have previously used these tests to assess executive function in local studies on childhood malaria and HIV infection (Familiar et al., 2015; Hickson et al., 2019). Additional limitations include potential differential influences from illness-associated school absences adversely impacting test results and no assessment of attention in the younger age group (Olatunya et al., 2018). The contributions from hemoglobin concentration could not be discerned from SCA as they were tightly linked. Unlike the random SCA clinic-based selection, sibling participation may have been biased, for example, from possible parental concerns. The potential for downward socioeconomic pressure associated with having a child affected by SCA may have contributed to subnormal scores among the siblings (Amarachukwu et al., 2022). Low caretaker education level, a marker of poverty, may have adversely affected neurocognitive scores, although the sibling assessment would have modulated those effects (King et al., 2014; Bello-Manga et al., 2020; Jacob et al., 2022). No adjustments were made for multiple comparisons.
In conclusion, comparing a sample of Ugandan children with SCA to their non-SCA siblings aged 1–12 years, we demonstrated that children with SCA had worse cognitive impairment and executive function than the unaffected siblings and that these differences were attributable to the older age group, aged 5–12. The younger children with SCA were not different from their non-SCA siblings. Age 5–12 and prior stroke were most strongly associated with neurocognitive impairment, with some contribution from caregiver educational attainment. Low neurocognitive z-scores by age among non-SCA siblings suggest environmental influences, for example, SES and education, among all participants, with potential parental selection bias for the siblings tested. Given the increased risk of impairment with age, interventions in early childhood may more likely provide benefits. Disease-modifying therapies, for example, hydroxyurea, should be tested for stabilizing or improving neurocognitive functions in young sub-Saharan children through amelioration of modifiable risk factors with SCA, including anemia (Tshilolo et al., 2019; Opoka et al., 2020).
The data supporting the conclusions of this article will be made available by the authors, in accordance with appropriate protection of the privacy of participants.
The studies involving humans were approved by the Makerere University School of Medicine Research and Ethics Committee and the Columbia University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
PB: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Supervision, Writing – review & editing. AKB: Formal analysis, Writing – original draft, Writing – review & editing. AB: Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing. RO: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. DM: Conceptualization, Supervision, Writing – review & editing. EM: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing. PK: Resources, Supervision, Writing – review & editing. GM: Data curation, Investigation, Project administration, Supervision, Writing – review & editing. MM: Data curation, Investigation, Project administration, Supervision, Writing – review & editing. GR: Investigation, Writing – review & editing. NG: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing. RI: Conceptualization, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
The authors declare that financial support was received for the research, authorship and/or publication of this article. This study was supported by grants from the U.S. National Institutes of Health (NIH) R21HD089791 (Idro, Green) and R01HD096559 (Idro, Green). Its contents are solely the responsibility of the authors and do not represent the official views of NIH. The funding source had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
We acknowledge the NIH Fogarty program of Global Brain Disorders Research. We thank the outstanding Makerere-based BRAIN SAFE team and Global Health Uganda. Most of all, we thank the study participants and families for their contributions. We acknowledge the parents and children who participated in the study and the outstanding study staff.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fstro.2024.1372949/full#supplementary-material
Amarachukwu, C. N., Okoronkwo, I. L., Nweke, M. C., and Ukwuoma, M. K. (2022). Economic burden and catastrophic cost among people living with sickle cell disease, attending a tertiary health institution in south-east zone, Nigeria. PLoS ONE 17:e0272491. doi: 10.1371/journal.pone.0272491
Ambrose, E. E., Makani, J., Chami, N., Masoza, T., Kabyemera, R., Peck, R. N., et al. (2018). High birth prevalence of sickle cell disease in Northwestern Tanzania. Pediatr Blood Cancer. 65:e26735. doi: 10.1002/pbc.26735
Bainbridge, R., Higgs, D. R., Maude, G. H., and Serjeant, G. R. (1985). Clinical presentation of homozygous sickle cell disease. J. Pediatr. 106, 881–885. doi: 10.1016/S0022-3476(85)80230-4
Bangirana, P., John, C. C., Idro, R., Opoka, R. O., Byarugaba, J., Jurek, A. M., et al. (2009). Socioeconomic predictors of cognition in Ugandan children: implications for community interventions. PLoS ONE 4:e7898. doi: 10.1371/journal.pone.0007898
Bello-Manga, H., DeBaun, M. R., and Kassim, A. A. (2016). Epidemiology and treatment of relative anemia in children with sickle cell disease in sub-Saharan Africa. Expert. Rev. Hematol. 9, 1031–1042. doi: 10.1080/17474086.2016.1240612
Bello-Manga, H., Galadanci, A. A., Abdullahi, S., Ali, S., Jibir, B., Gambo, S., et al. (2020). Low educational level of head of household, as a proxy for poverty, is associated with severe anaemia among children with sickle cell disease living in a low-resource setting: evidence from the SPRING trial. Br. J. Haematol. 190, 939–944. doi: 10.1111/bjh.16746
Bernaudin, F., Verlhac, S., Arnaud, C., Kamdem, A., Chevret, S., Hau, I., et al. (2011). Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood 117, 1130–1140. doi: 10.1182/blood-2010-06-293514
Boivin, M. J., Bangirana, P., Nakasujja, N., Page, C. F., Shohet, C., Givon, D., et al. (2013). A year-long caregiver training program improves cognition in preschool Ugandan children with human immunodeficiency virus. J. Pediatr. 163, 1409–1416. e1401-1405. doi: 10.1016/j.jpeds.2013.06.055
Brousse, V., Kossorotoff, M., and de Montalembert, M. (2015). How I manage cerebral vasculopathy in children with sickle cell disease. Br. J. Haematol. 170, 615–625. doi: 10.1111/bjh.13477
DeBaun, M. R., and Kirkham, F. J. (2016). Central nervous system complications and management in sickle cell disease. Blood 127, 829–838. doi: 10.1182/blood-2015-09-618579
DeBaun, M. R., Sarnaik, S. A., Rodeghier, M. J., Minniti, C. P., Howard, T. H., Iyer, R. V., et al. (2012). Associated risk factors for silent cerebral infarcts in sickle cell anemia: low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood 119, 3684–3690. doi: 10.1182/blood-2011-05-349621
Dhabangi, A., Ainomugisha, B., Cserti-Gazdewich, C., Ddungu, H., Kyeyune, D., Musisi, E., et al. (2016). Cerebral oximetry in ugandan children with severe anemia: clinical categories and response to transfusion. JAMA Pediatr. 170, 995–1002. doi: 10.1001/jamapediatrics.2016.1254
Duggan, M. B. (2010). Anthropometry as a tool for measuring malnutrition: impact of the new WHO growth standards and reference. Ann. Trop. Paediatr. 30, 1–17. doi: 10.1179/146532810X12637745451834
Estcourt, L. J., Kimber, C., Hopewell, S., Trivella, M., Doree, C., Abboud, M. R., et al. (2020). Interventions for preventing silent cerebral infarcts in people with sickle cell disease. Cochr. Datab. Syst. Rev. 4:CD012389. doi: 10.1002/14651858.CD012389.pub3
Familiar, I., Ruisenor-Escudero, H., Giordani, B., Bangirana, P., Nakasujja, N., Opoka, R., et al. (2015). Use of the behavior rating inventory of executive function and child behavior checklist in ugandan children with HIV or a history of severe malaria. J. Dev. Behav. Pediatr. 36, 277–284. doi: 10.1097/DBP.0000000000000149
Ford, A. L., Ragan, D. K., Fellah, S., Binkley, M. M., Fields, M. E., Guilliams, K. P., et al. (2018). Silent infarcts in sickle cell disease occur in the border zone region and are associated with low cerebral blood flow. Blood 132, 1714–1723. doi: 10.1182/blood-2018-04-841247
Gioia, G. A., Isquith, P. K., Retzlaff, P. D., and Espy, K. A. (2002). Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychol. 8, 249–257. doi: 10.1076/chin.8.4.249.13513
Green, N. S., Munube, D., Bangirana, P., Buluma, L. R., Kebirungi, B., Opoka, R., et al. (2019). Burden of neurological and neurocognitive impairment in pediatric sickle cell anemia in Uganda (BRAIN SAFE): a cross-sectional study. BMC Pediatr. 19:381. doi: 10.1186/s12887-019-1758-2
Gur, R. C., Moore, T. M., Weinberger, R., Mekori-Domachevsky, E., Gross, R., Emanuel, B. S., et al. (2021). Relationship between intelligence quotient measures and computerized neurocognitive performance in 22q11.2 deletion syndrome. Brain Behav. 11:e2221. doi: 10.1002/brb3.2221
Hickson, M. R., Conroy, A. L., Bangirana, P., Opoka, R. O., Idro, R., Ssenkusu, J. M., et al. (2019). Acute kidney injury in Ugandan children with severe malaria is associated with long-term behavioral problems. PLoS ONE 14:e0226405. doi: 10.1371/journal.pone.0226405
Idro, R., Boehme, A. K., Kawooya, M., Lubowa, S. K., Munube, D., Bangirana, P., et al. (2022). Brain magnetic resonance imaging and angiography in children with sickle cell anaemia in uganda in a cross-sectional sample. J. Stroke Cerebrovasc. Dis. 31:106343. doi: 10.1016/j.jstrokecerebrovasdis.2022.106343
Jacob, M., Stotesbury, H., Kija, E., Saunders, D., Mtei, R. J., Tutuba, H., et al. (2022). Effect of age, cerebral infarcts, vasculopathy and haemoglobin on cognitive function, in Tanzanian children with sickle cell anaemia. Eur. J. Paediatr. Neurol. 37, 105–113. doi: 10.1016/j.ejpn.2022.01.010
Kawadler, J. M., Clayden, J. D., Clark, C. A., and Kirkham, F. J. (2016). Intelligence quotient in paediatric sickle cell disease: a systematic review and meta-analysis. Dev. Med. Child Neurol. 58, 672–679. doi: 10.1111/dmcn.13113
King, A. A., Strouse, J. J., Rodeghier, M. J., Compas, B. E., Casella, J. F., McKinstry, R. C., et al. (2014). Parent education and biologic factors influence on cognition in sickle cell anemia. Am. J. Hematol. 89, 162–167. doi: 10.1002/ajh.23604
Knight, L. M. J., King, A. A., Strouse, J. J., and Tanabe, P. (2021). Pediatric neurodevelopmental delays in children 0 to 5 years of age with sickle cell disease: a systematic literature review. J. Pediatr. Hematol. Oncol. 43, 104–111. doi: 10.1097/MPH.0000000000002091
Kwiatkowski, J. L., Voeks, J. H., Kanter, J., Fullerton, H. J., Debenham, E., Brown, L., et al. (2019). Ischemic stroke in children and young adults with sickle cell disease in the post-STOP era. Am. J. Hematol. 94, 1335–1343. doi: 10.1002/ajh.25635
Lee, S., Lucas, S., Proudman, D., Nellesen, D., Paulose, J., Sheehan, V. A., et al. (2022). Burden of central nervous system complications in sickle cell disease: a systematic review and meta-analysis. Pediatr. Blood Cancer 69:e29493. doi: 10.1002/pbc.29493
Longoria, J. N., Heitzer, A. M., Hankins, J. S., Trpchevska, A., and Porter, J. S. (2022). Neurocognitive risk in sickle cell disease: utilizing neuropsychology services to manage cognitive symptoms and functional limitations. Br. J. Haematol. 197, 260–270. doi: 10.1111/bjh.18041
Macharia, A. W., Mochamah, G., Uyoga, S., Ndila, C. M., Nyutu, G., Makale, J., et al. (2018). The clinical epidemiology of sickle cell anemia in Africa. Am. J. Hematol. 93, 363–370. doi: 10.1002/ajh.24986
Makani, J., Cox, S. E., Soka, D., Komba, A. N., Oruo, J., Mwamtemi, H., et al. (2011). Mortality in sickle cell anemia in Africa: a prospective cohort study in Tanzania. PLoS ONE 6:e14699. doi: 10.1371/journal.pone.0014699
Marks, L. J., Munube, D., Kasirye, P., Mupere, E., Jin, Z., LaRussa, P., et al. (2018). Stroke prevalence in children with sickle cell disease in sub-Saharan Africa: a systematic review and meta-analysis. Glob. Pediatr. Health. 5:2333794X.18774970. doi: 10.1177/2333794X18774970
Munube, D., Katabira, E., Ndeezi, G., Joloba, M., Lhatoo, S., Sajatovic, M., et al. (2016). Prevalence of stroke in children admitted with sickle cell anaemia to Mulago Hospital. BMC Neurol. 16:175. doi: 10.1186/s12883-016-0704-2
Ndeezi, G., Kiyaga, C., Hernandez, A. G., Munube, D., Howard, T. A., Ssewanyana, I., et al. (2016). Burden of sickle cell trait and disease in the Uganda Sickle Surveillance Study (US3): a cross-sectional study. Lancet Glob. Health. 4, e195–200. doi: 10.1016/S2214-109X(15)00288-0
Nnodu, O. E., Oron, A. P., Sopekan, A., Akaba, G. O., Piel, F. B., Chao, D. L., et al. (2021). Child mortality from sickle cell disease in Nigeria: a model-estimated, population-level analysis of data from the 2018 Demographic and Health Survey. Lancet Haematol. 8, e723–e731. doi: 10.1016/S2352-3026(21)00216-7
Nnodu, O. E., Sopekan, A., Nnebe-Agumadu, U., Ohiaeri, C., Adeniran, A., Shedul, G., et al. (2020). Implementing newborn screening for sickle cell disease as part of immunisation programmes in Nigeria: a feasibility study. Lancet Haematol. 7, e534–e540. doi: 10.1016/S2352-3026(20)30143-5
Noubiap, J. J., Mengnjo, M. K., Nicastro, N., and Kamtchum-Tatuene, J. (2017). Neurologic complications of sickle cell disease in Africa: a systematic review and meta-analysis. Neurology. 89, 1516–1524. doi: 10.1212/WNL.0000000000004537
Ogunsile, F. J., Currie, K. L., Rodeghier, M., Kassim, A., DeBaun, M. R., Sharma, D., et al. (2018). History of parvovirus B19 infection is associated with silent cerebral infarcts. Pediatr. Blood Cancer. 65:e26767. doi: 10.1002/pbc.26767
Olatunya, O. S., Oke, O. J., Kuti, B. P., Ajayi, I. A., Olajuyin, O., Omotosho-Olagoke, O., et al. (2018). Factors influencing the academic performance of children with sickle cell anaemia in ekiti, south west Nigeria. J. Trop. Pediatr. 64, 67–74. doi: 10.1093/tropej/fmx034
Oluwole, O. B., Noll, R. B., Winger, D. G., Akinyanju, O., and Novelli, E. M. (2016). Cognitive functioning in children from Nigeria with sickle cell anemia. Pediatr. Blood Cancer. 63, 1990–1997. doi: 10.1002/pbc.26126
Opoka, R. O., Hume, H. A., Latham, T. S., Lane, A., Williams, O., Tymon, J., et al. (2020). Hydroxyurea to lower transcranial Doppler velocities and prevent primary stroke: the Uganda NOHARM sickle cell anemia cohort. Haematologica 105, e272–e275. doi: 10.3324/haematol.2019.231407
Oron, A. P., Chao, D. L., Ezeanolue, E. E., Ezenwa, L. N., Piel, F. B., Ojogun, O. T., et al. (2020). Caring for Africa's sickle cell children: will we rise to the challenge? BMC Med. 18:92. doi: 10.1186/s12916-020-01557-2
Prussien, K. V., Jordan, L. C., DeBaun, M. R., and Compas, B. E. (2019a). Cognitive function in sickle cell disease across domains, cerebral infarct status, and the lifespan: a meta-analysis. J. Pediatr. Psychol. 44, 948–958. doi: 10.1093/jpepsy/jsz031
Prussien, K. V., Salihu, A., Abdullahi, S. U., Galadanci, N. A., Bulama, K., Belonwu, R. O., et al. (2019b). Associations of transcranial doppler velocity, age, and gender with cognitive function in children with sickle cell anemia in Nigeria. Child Neuropsychol. 25, 705–720. doi: 10.1080/09297049.2018.1526272
Prussien, K. V., Siciliano, R. E., Ciriegio, A. E., Anderson, A. S., Sathanayagam, R., DeBaun, M. R., et al. (2020). Correlates of cognitive function in sickle cell disease: a meta-analysis. J. Pediatr. Psychol. 45, 145–155. doi: 10.1093/jpepsy/jsz100
Quinn, C. T., and Dowling, M. M. (2012). Cerebral tissue hemoglobin saturation in children with sickle cell disease. Pediatr. Blood Cancer. 59, 881–887. doi: 10.1002/pbc.24227
Ranque, B., Kitenge, R., Ndiaye, D. D., Ba, M. D., Adjoumani, L., Traore, H., et al. (2022). Estimating the risk of child mortality attributable to sickle cell anaemia in sub-Saharan Africa: a retrospective, multicentre, case-control study. Lancet Haematol. 9, e208–e216. doi: 10.1016/S2352-3026(22)00004-7
Ruffieux, N., Njamnshi, A. K., Wonkam, A., Hauert, C.-A., Chanal, J., Verdon, V., et al. (2013). Association between biological markers of sickle cell disease and cognitive functioning amongst Cameroonian children. Child Neuropsychol. 19, 143–160. doi: 10.1080/09297049.2011.640932
Schatz, J., and McClellan, C. B. (2006). Sickle cell disease as a neurodevelopmental disorder. Ment Retard. Dev. Disabil. Res. Rev. 12, 200–207. doi: 10.1002/mrdd.20115
Trpchevska, A., Longoria, J., Okhomina, V., Raches, D., Potter, B., Kang, G., et al. (2022). Adaptive functioning in children and adolescents with sickle cell disease. J. Pediatr. Psychol. 47, 939–951. doi: 10.1093/jpepsy/jsac024
Tshilolo, L., Tomlinson, G., Williams, T. N., Santos, B., Olupot-Olupot, P., Lane, A., et al. (2019). Hydroxyurea for children with sickle cell anemia in Sub-Saharan Africa. N. Engl. J. Med. 380, 121–131. doi: 10.1056/NEJMoa1813598
Tumwine, J. K., Nankabirwa, V., Diallo, H. A., Engebretsen, I. M. S., Ndeezi, G., Bangirana, P., et al. (2018). Exclusive breastfeeding promotion and neuropsychological outcomes in 5-8 year old children from Uganda and Burkina Faso: Results from the PROMISE EBF cluster randomized trial. PLoS ONE 13:e0191001. doi: 10.1371/journal.pone.0191001
Uyoga, S., Macharia, A. W., Mochamah, G., Ndila, C. M., Nyutu, G., Makale, J., et al. (2019). The epidemiology of sickle cell disease in children recruited in infancy in Kilifi, Kenya: a prospective cohort study. Lancet Glob. Health. 7, e1458–e1466. doi: 10.1016/S2214-109X(19)30328-6
Wang, W., Enos, L., Gallagher, D., Thompson, R., Guarini, L., Vichinsky, E., et al. (2001). Neuropsychologic performance in school-aged children with sickle cell disease: a report from the cooperative study of sickle cell disease. J. Pediatr. 139, 391–397. doi: 10.1067/mpd.2001.116935
Ware, R. E., de Montalembert, M., Tshilolo, L., and Abboud, M. R. (2017). Sickle cell disease. Lancet. 390, 311–323. doi: 10.1016/S0140-6736(17)30193-9
Keywords: sickle cell anemia, neurocognition, neurocognitive impairment, pediatric sickle cell, sub-Saharan Africa
Citation: Bangirana P, Boehme AK, Birabwa A, Opoka RO, Munube D, Mupere E, Kasirye P, Muwanguzi G, Musiimenta M, Ru G, Green NS and Idro R (2024) Neurocognitive impairment in Ugandan children with sickle cell anemia compared to sibling controls: a cross-sectional study. Front. Stroke 3:1372949. doi: 10.3389/fstro.2024.1372949
Received: 18 January 2024; Accepted: 25 March 2024;
Published: 15 April 2024.
Edited by:
Fenella Jane Kirkham, University College London, United KingdomReviewed by:
Dilek Necioglu Orken, Istanbul Arel University, TürkiyeCopyright © 2024 Bangirana, Boehme, Birabwa, Opoka, Munube, Mupere, Kasirye, Muwanguzi, Musiimenta, Ru, Green and Idro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nancy S. Green, TlNHMTFAY3VtYy5jb2x1bWJpYS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.