- 1Population Health Sciences Institute, Newcastle University, Newcastle upon Tyne, United Kingdom
- 2Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, United Kingdom
- 3Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom
- 4Oxford University Hospitals NHS Foundation Trust and Medical Sciences Division, University of Oxford, Oxford, United Kingdom
- 5Peninsula Applied Research Collaboration (PenARC), University of Exeter, Exeter, United Kingdom
Introduction: Mechanical thrombectomy results in more favourable functional outcomes for patients with acute large vessel occlusion (LVO) stroke. Key clinical determinants of thrombectomy outcome include symptom severity, age and time from onset to treatment, but associations have also been reported with baseline physiological observations including systolic/diastolic blood pressure (SBP/DBP), blood/serum glucose, atrial fibrillation and conscious level. As these items are routinely available during initial emergency assessment, they might help to inform early prehospital and hospital triage decisions if evidence consistently shows associations with post-thrombectomy outcome. We undertook a meta-analysis of studies reporting pre-thrombectomy physiological observations and functional outcome.
Method: PRISMA guidelines were followed to search electronic bibliographies, select articles and extract data. Medline, PubMed, Cochrane HTA, Cochrane Central and Embase were searched. Included articles were observational or interventional thrombectomy studies published between 01/08/2004-19/04/2023 reporting 3-month modified Rankin Scale, split as favourable (0–2) and unfavourable (3–6). A modified version of the Quality in Prognostic Studies (QUIPS) tool was used to assess risk of bias. RevMan 5 was used to calculate Inverse Variance with Weighted Mean Differences (WMD) and Mantel-Haenszel Odds Ratios (OR) for continuous and categorical factors respectively.
Results: Thirty seven studies were eligible from 8,687 records. Significant associations were found between unfavourable outcome and higher blood/serum glucose as a continuous (WMD = 1.34 mmol/l (95%CI 0.97 to 1.72); 19 studies; n = 3122) and categorical (OR = 2.44 (95%CI 1.9 to 3.14) variable; 6 studies; n = 5481), higher SBP (WMD = 2.98 mmHg (95%CI 0.86 to 5.11); 16 studies; n = 4,400), atrial fibrillation (OR = 1.48 (95%CI 1.08 to 2.03); 3 studies; n = 736), and lower Glasgow Coma Scale (WMD = −2.72 (95%CI −4.01 to −1.44); 2 studies; n = 99). No association was found with DBP (WMD = 0.36 mmHg (95%CI −0.76 to 1.49); 13 studies; n = 3,614).
Conclusion: Basic physiological observations might assist early triage decisions for thrombectomy and could be used in combination with other information to avoid futile treatment and ambulance transfers. It is important to acknowledge that data were only from thrombectomy treated patients in hospital settings and it cannot be assumed that the predictors identified are independent or that modification can change outcome. Further work is needed to establish the optimal combination of prognostic factors for clinical care decisions.
Background
Stroke is a medical emergency requiring urgent assessment and treatment (National Institute for Health and Care Excellence, 2019; Zhou et al., 2022). Selected patients with ischaemic stroke due to large vessel occlusion (LVO) should receive mechanical thrombectomy, a highly effective procedure with a number needed to treat (NNT) of 3 patients to reduce disability by one point on the modified Rankin scale (mRS) (Goyal et al., 2016). The main determinants of thrombectomy outcomes are age, time from onset to treatment, baseline stroke severity and radiological variables such as ischaemic core volume and collateral blood supply (Sarraj et al., 2013; Goyal et al., 2016; Evans et al., 2017; Mokin et al., 2017, 2019; Nogueira et al., 2018; Cappellari et al., 2020; Diestro et al., 2020; Ramos et al., 2020; Wang and Zhou, 2020; Kremers et al., 2021; Venema et al., 2021). However, an increasing number of reports also describe potentially valuable relationships with basic clinical information, including physiological variables (e.g., blood pressure, glucose, oxygen saturation, temperature), and futile recanalisation (i.e., reperfusion with no clinical improvement) (Zhou et al., 2022). These factors are potentially of interest because they are routinely collected during initial clinical assessment by non-specialist emergency responders such as paramedics (Quinn et al., 2022) prior to specialist review and brain imaging, and could enhance early selection of patients for direct admission to a thrombectomy centre when this is not the closest hospital. They may also be useful in hospital to modify treatment decisions if there is uncertainty about the value of thrombectomy for patients at the margins of standard criteria [e.g., with mild stroke severity towards the end of treatment time windows (National Institute for Health and Care Excellence, 2019; Sentinel Stroke National Audit Programme, 2023)]. However, the evidence describing the relationships between physiological observations and treatment outcome is mixed and of variable quality (Nie et al., 2023).
Previous reviews have considered the prognostic relationship between specific physiological observations and thrombectomy outcome. For example, a systematic review of blood pressure showed that patients with hypertension had significantly higher odds of an unfavourable (mRS 3–6) functional outcome (odds ratio 0.70; 95% CI 0.57–0.85) (Yuan et al., 2020). However, this review did not differentiate between a previous history of high blood pressure and actual pre-thrombectomy blood pressure values. It is uncertain whether these have equivalent effects. Another review found that higher blood glucose was significantly associated with lower odds of a favourable (mRS 0–2) functional outcome (odds ratio 0.92, 95% CI 0.09–0.95) (Chamorro et al., 2019), but only included data from seven randomised trials comparing thrombectomy with standard care between 2010 and 2017.
As there have been significant advances in thrombectomy and patient selection over the last six years, an up-to-date comprehensive review is required to consider the relationship between outcomes and pre-treatment physiological observations, including data from real-world populations as well as clinical trials with strict inclusion criteria. We aimed to synthesise all published evidence in a meta-analysis describing the association between a clinically important functional outcome post-thrombectomy (3-month mRS split as favourable (0–2) and unfavourable (3–6) functional outcome) and individual basic physiological observations, which could be collected pre-thrombectomy without specialist training.
Methodology
The review was conducted in accordance with PRISMA (Transparent Reporting of Systematic Reviews and Meta-Analyses) 2020 guidance from EQUATOR (Page et al., 2021) using a PICOTS research question format (Riley et al., 2019).
Search strategy
The following electronic databases were searched using a combination of MeSH terms and keywords: MEDLINE, PubMed, Cochrane HTA, Cochrane Central and EMBASE. The search strategy was developed in collaboration with an information scientist (see Supplementary Table S1). Searches only included research published after FDA approval of the first intra-arterial thrombectomy device for acute ischaemic stroke (01/08/2004). The most recent search was on 19/04/2023. No restrictions were placed on country of origin, although searches were restricted to papers with abstracts published in the English language. Only completed studies were included; study protocols were excluded. Literature reviews and individual case studies were excluded.
Study selection
Observational, interventional, prospective or retrospective research studies were selected for inclusion if they reported data describing the relationship between standard physiological observations collected pre-treatment and post-thrombectomy outcome reported as favourable (0–2) vs. unfavourable (3-6) mRS at 3 months. The search was not limited to this outcome to prevent excluding relevant papers with an alternative description of the same outcome.
Inclusion criteria were defined using a PICOTS approach (Table 1) and incorporated into a Study Selection Form (Supplementary Table S2). Studies were excluded if interventionists primarily used first generation devices which are no longer in routine use, e.g., MERCI (NICE, 2018). Patients were only included if they were treated with stentrievers, aspiration or a combined approach. Duplicate studies including the same patients and case reports were excluded. It was necessary for studies to present data which could be combined into a meta-analysis: i.e., group proportions and/or comparable/convertible measurements were reported.
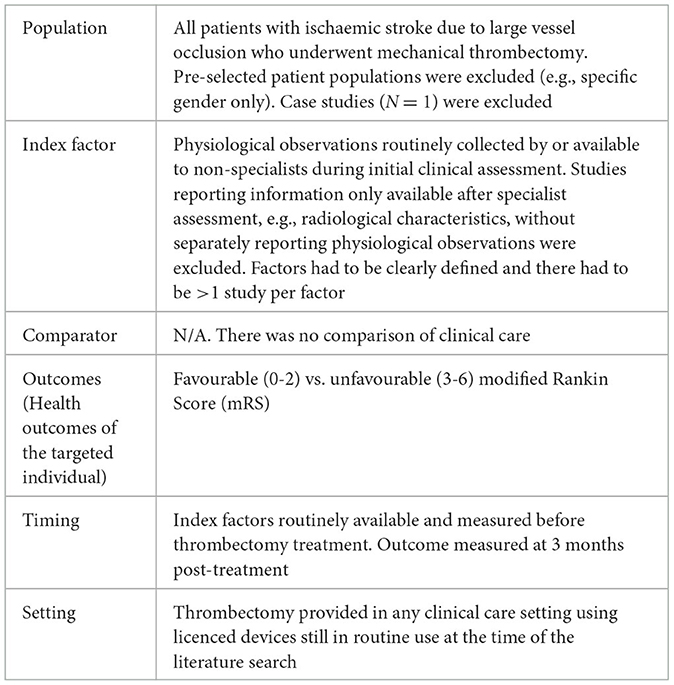
Table 1. Meta-analysis of physiological observations as thrombectomy prognostic factors PICOTS components.
One reviewer (HL) independently assessed initial eligibility of the titles and abstracts retrieved via the search strategy using the literature review screening software, “Rayyan” (Mourad et al., 2016). The same reviewer further assessed eligibility of the retained full text studies. Any uncertainties were queried with another (medically qualified) member of the review team (CP/LS).
Data extraction
Three reviewers (HL, JM and AA) each independently extracted information from one third of the retained full text articles using a standard data extraction form (Supplementary Table S3) in Microsoft Excel. Extracted data included: Basic Study Information (e.g., Author, Year and Title), Methodological Information (e.g., Design, Criteria, Clinical Factor(s), Outcome measures, Timing; Clinical Treatment Context) and Study Results (e.g., Sample size, Predictive value; Statistics relevant to meta-analysis). All reviewers cross-checked 5% of their allocation to confirm agreement, and all extracted data were reconfirmed by HL. Any uncertainties regarding data extraction at any stage were discussed amongst the three reviewers and adjudicated by a further member of the review team when necessary. Authors were not contacted regarding missing data, and all data extracted reflected that contained in the text, tables, figures and/or Supplementary material of published reports. Studies in other languages included for having English abstracts were translated by Google Translate.
Quality assessment
Risk of Bias (RoB) was evaluated by a modified version of the Quality in Prognostic Studies (QUIPS) tool (Riley et al., 2019) (Supplementary Table S4). The tool was simplified to align with the review aims and the anticipated variable nature of the literature. Assessment domains include: (1) Study participation, (2) Study attrition, (3) Prognostic factor measurement, (4) Outcome measure, (5) Confounding factors, (6) Statistics and reporting. Studies were categorised into tertiles as having low (score of 10.5–14/14), moderate (4–10/14), or high (0–3.5/14) risk of bias.
Data synthesis
No transformations were performed on variable data apart from the conversion of glucose values reported in mg/dL into mmol/L for direct comparison. Binary glucose thresholds are reported in their original units in Supplementary Tables S6–S10 but following transformation it was possible to combine studies using a threshold of 7.8 mmol/L (140 mg/dL). Some categorical thresholds were negligibly different (<7.8 mmol/L vs. >/ = 7.8 mmol and </ = 7.8 mmol/L vs. >7.8 mmol/L) so were merged. Although serum and blood glucose values are reported to differ by ~1% due to the presence and absence of red blood cells, data were pooled without correction because this is a very small effect which would be very unlikely to influence the difference between favourable and unfavourable outcomes. Glasgow Coma Scale (GCS) scores could be included if reported as continuous/parametric data, although it was anticipated that this would limit study eligibility as it is typically an ordinal scale. Papers reporting AF were only included if this had been confirmed by ECG during the assessment for thrombectomy treatment, which ensured that its presence was temporally related to the procedure and detection was consistent across studies, thereby reducing heterogeneity in the analysis.
Review Manager 5 (Review Manager 2020) (Nordic Cochrane Centre, 2020; Jonathan et al., 2021) was used to produce forest plots assessing associations between prognostic factors and unfavourable (mRS 3–6) 3-month functional outcomes. For continuous factors, Inverse Variance was used as the statistical method and Weighted Mean Difference (WMD) was used as the effect measure as outcomes were measured in the same way across studies. For binary factors, the Mantel-Haenszel method was used with Odds Ratios (with 95% CIs) as the effect measure. Risk of bias (modified QUIPS) scores were added to the forest plots but were not adjusted for in the analysis.
We assessed the extent of heterogeneity between trial results for each factor using the I2 statistic, which measures the percentage of the variability in effect estimates attributable to heterogeneity rather than sampling error, in conjunction with Tau2 for measuring variance between studies (Higgins, 2020). We considered I2 >50% and Tau2 >0.5 as substantial heterogeneity indicating that studies do not share a common effect. Random effects models were used regardless of heterogeneity because it was assumed that there would always be differences between patient populations and clinical care in different study settings. Fixed effects models were used if a factor had fewer than 5 contributing studies (Tufanaru et al., 2015). Funnel plots were generated to examine the extent of publication bias.
Results
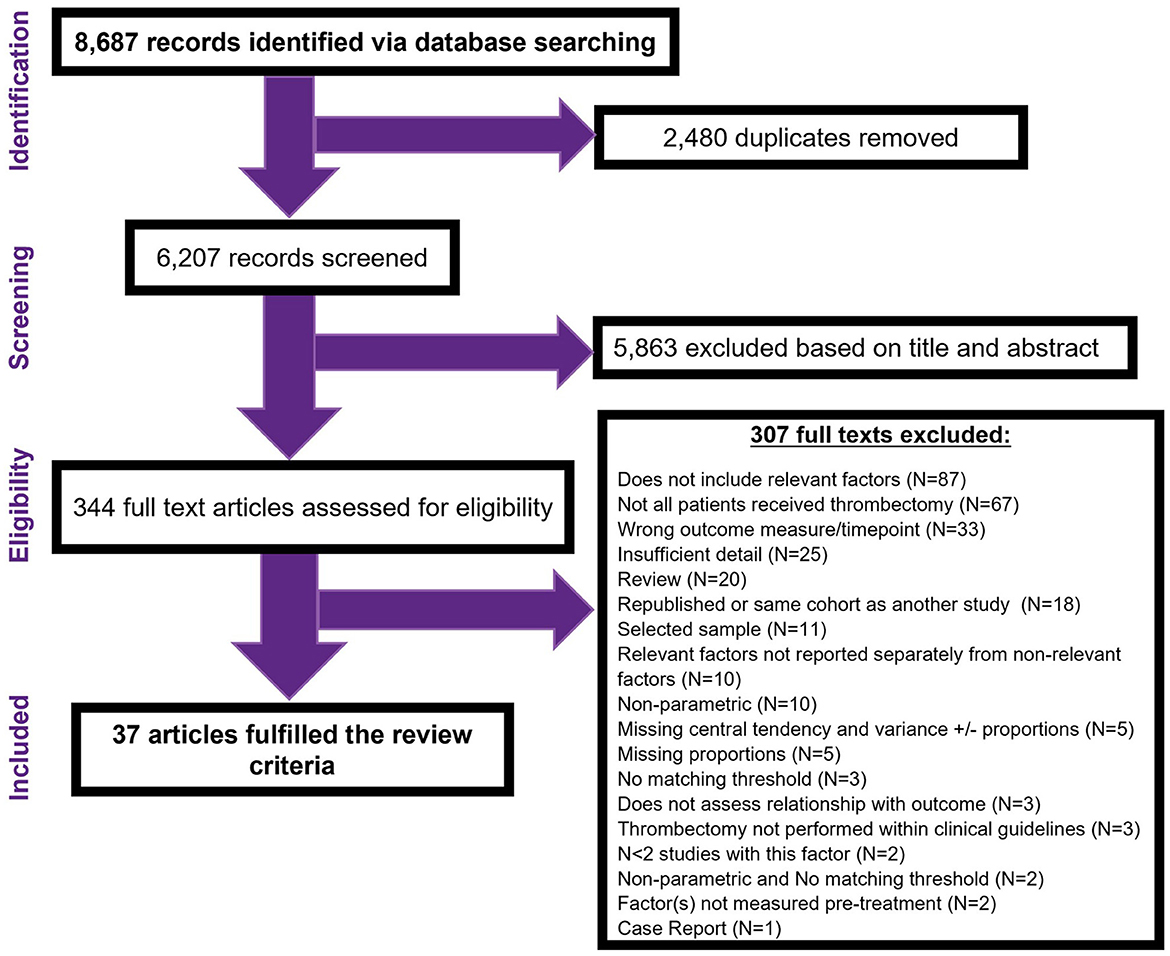
The search strategy identified 8,687 records. After removing 2,480 database duplicates, the title and abstract of 6,207 records were screened. This led to examination of 344 full text articles, 37 of which were eligible for inclusion (Figure 1).
Of the 307 excluded full-text articles, the primary reasons for ineligibility were as follows: Ninety-Nine (32%) studies did not include or individually report relevant factors/measurement timepoints. Not all patients received thrombectomy/thrombectomy within clinical guidelines in 70 (23%) studies. Forty-seven (15%) studies did not present sufficient detail to judge eligibility or include in the analysis. Thirty-three (11%) studies did not include our selected primary outcome measure/timepoint. Twenty (7%) studies were review articles. Eighteen (6%) studies were duplicate reports (e.g., conference abstracts of full texts articles or reports of the same factor(s) on the same cohort of patients). Eleven (4%) studies reported selected patient subgroups only. Five (2%) studies were excluded for having unique prognostic factors (N < 2). Three (1%) studies did not directly assess the relationship between the prognostic factor(s) and outcome measure. One (1%) paper was a single case study.
Summary of included studies
Study summary information is detailed in Table 2.
Basic study information
Of 37 included studies, the corresponding author of each was based in the following countries: China [N = 14 (38%)], United States [N = 9 (24%)], France [N = 3 (8%)], Germany [N = 2 (5%)], South Korea [N = 2 (5%)], Italy (N = 1), Netherlands (N = 1), New Zealand (N = 1), Poland (N = 1), Switzerland (N = 1), Turkey (N = 1) and Vietnam (N = 1). All studies were published after 2012, with the majority from 2019-2022 [N = 25 (68%)].
Study design
All but one of the studies were retrospective cohort design [N = 36 (97%)] and the remaining study was a combined cohort and randomised controlled trial (RCT) [N = 1 (3%)]. Settings were described as Comprehensive Stroke Centres [N = 29 (81%)] or Tertiary Hospitals [N = 8 (19%)]. Blinding was not reported in 28 (76%) studies, and it was only stated that outcome assessors were blinded to baseline information in 7 (18%) or were not blinded in 2 (5%) studies. The average risk of bias was low overall [N = 34 (92%), mean 12.23/14] and was similar in reports of significant [N = 25 (43.1%), mean 12.68] and non-significant [N = 32 (55.2%), mean 11.86] findings (studies reported on multiple factors). The three (8%) remaining studies had a moderate risk of bias.
Treatment context
Ten (27%) studies included patients treated within traditionally standard thrombectomy time windows (i.e. <6 h stroke onset for anterior, <24 h for posterior circulation stroke), whilst 14 (38%) included patients treated within the extended time window (i.e. 6–24 h for anterior circulation stroke with eligibility informed by advanced imaging) (National Institute for Health and Care Excellence, 2019; Sentinel Stroke National Audit Programme, 2023). The treatment time window was not reported in 13 (35%) studies. Twenty (54%) studies included patients with an occlusion in the anterior vascular territory only, five (14%) included posterior circulation occlusions only and 12 (32%) included both territories. The majority of studies reported either a mix of thrombectomy devices, usually individualised to the case [N = 10 (27%)], or included only the use of stent-retrievers [N = 9(24%)]. Three (8%) studies reported on the primary combined approach (aspiration-catheter and stent-retriever) with balloon catheter and three (8%) without balloon-catheter. Eight (22%) and four (11%) did not report or were unclear (respectively) about the use of thrombectomy devices. Bridging thrombolytic treatment was reported as available by 32 (86%) studies, however, use varied greatly [Median 51.5% (range 7.8%−80%)]. Five (14%) studies did not report the proportion of patients receiving thrombolysis.
Prognostic factors
Within the included studies, there were 58 reports of the relationship between physiological observations and our primary outcome measure (Supplementary Table S5), reflecting the fact that studies often reported on multiple factors. Some studies also included both categorical and continuous analyses of the same factor. Although many papers examining pre-treatment atrial fibrillation (AF) were identified in the literature search, the majority either included it only as a previously recorded comorbidity or mixed patients with comorbid and acutely Electrocardiogram (ECG) detected AF. The following pre-treatment physiological observations were included: continuous (mmol/L) blood glucose (BG) (N = 19), categorical blood/serum glucose (hyperglycaemia >/ = 7.8mmol/L) (N = 6), continuous (mmHg) systolic blood pressure (SBP) (N = 16), continuous (mmHg) diastolic blood pressure (DBP) (N = 13), categorical ECG evidence of atrial fibrillation (AF) (N = 3) and continuous conscious level using the Glasgow Coma Scale (GCS) (N = 2). In 35 (95%) studies, the pre-thrombectomy prognostic factor measurement timepoint was described as “admission” and the remaining 2 (5%) as “pre-operative.”
Outcome measures
Included studies were required to report favourable (0–2) vs. unfavourable (3–6) mRS at 3 months post-thrombectomy, but many also described multiple thrombectomy outcome measures at multiple timepoints. Other variations of mRS (i.e., ordinal mRS and different dichotomisations) and other non-mRS outcome measures were reported alongside our defined primary mRS outcome measure, leading to a total of 89 individual outcome results across factors. The frequency of all outcome measures is listed in Supplementary Table S5, with timepoints and results reported in Supplementary Tables S6-S10.
Glucose
Of 24 studies (8,405 total patients) assessing the relationship between pre-treatment glucose and outcome, there were 19 reports (3,122 total patients) of glucose as a continuous variable (10 blood glucose, four serum glucose and five not specified) and six reports (5,481 total patients) of glucose as a categorical variable with a binary threshold of hyperglycaemia (>/ = 7.8 mmol/L) vs. no hyperglycaemia (<7.8 mmol/L) (four serum glucose, two not specified and one blood glucose). One study was included in both categorical and continuous analyses (Huo et al., 2019). Within the included continuous studies, three had ineligible categorical data due to missing proportions (N = 1), no matching threshold (N = 1) and no defined threshold (N = 1). Continuous data could not be included from three included categorical studies due to missing parametric data. The average number of patients per study was 350 [450 for 17 (70.83%) studies with significant findings and 107 for 7 (29.17%) non-significant studies]. The risk of bias (Modified QUIPS) was low overall (mean 11.96/14) regardless of whether results showed a significant difference (mean 12/14) or were non-significant (mean 11.86/14). Further details on glucose studies, as well as alternative outcomes, are reported in Supplementary Table S6.
Continuous (ratio) glucose (mmol/L)
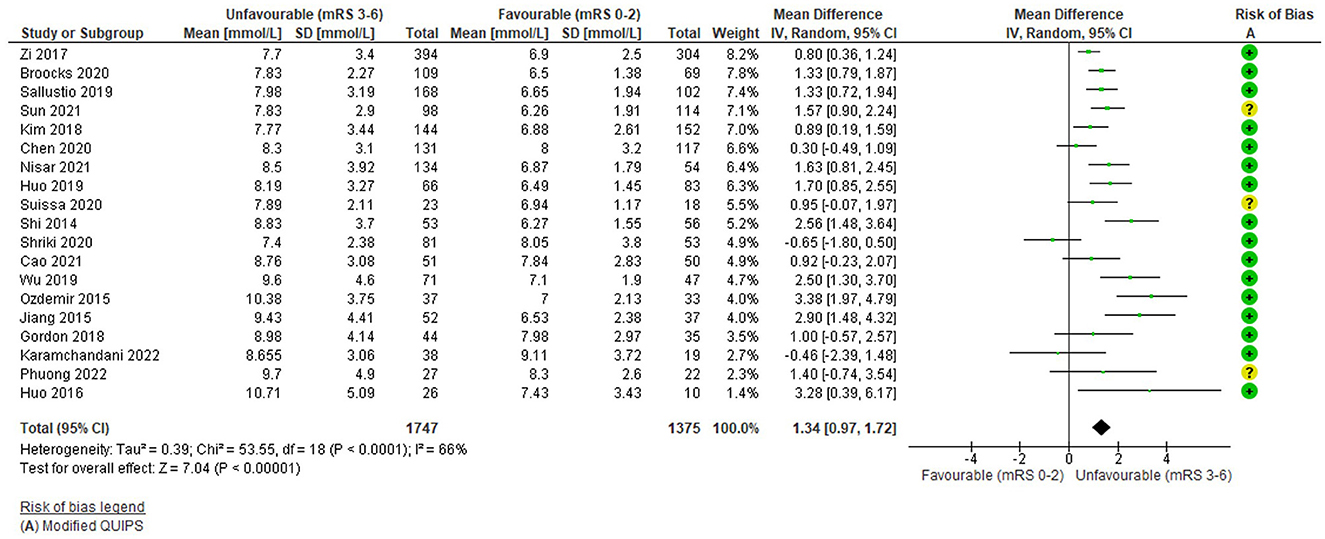
The funnel plot (Supplementary Figure S1) was fairly symmetrical; however, studies were significantly heterogeneous (I2 = 66%, Tau2 = 0.39, Chi2 = 53.55 (df = 18), p < 0.0001) and a random effects model was applied.
Overall, higher pre-thrombectomy blood/serum glucose was significantly associated with an unfavourable functional outcome: WMD = 1.34 mmol/L (95%CI 0.97 to 1.72; Z = 12.3) p < 0.00001 (Figure 2). Most [N = 13/19 (68%)] individual studies reported significantly higher glucose in unfavourable outcome groups (Shi et al., 2014; Jiang et al., 2015; Ozdemir et al., 2015; Zi et al., 2017; Kim et al., 2018; Huo et al., 2019; Sallustio et al., 2019; Wu et al., 2019; Broocks et al., 2020; Chen et al., 2020; Suissa et al., 2020; Nisar et al., 2021; Sun et al., 2021) (Supplementary Table S6), including the highest weighted study [(Zi et al., 2017); N = 698], although the WMD was not large. This was the case for most high weighted studies, apart from Chen et al. (2020). Shriki et al. (2020) and Karamchandani et al. (2022) displayed non-significant differences in the opposite direction but were small, low weighted studies. Risk of bias was low overall (mean 11.94/14) and studies with a moderate risk of bias were evenly distributed in terms of their weights. Other outcomes were generally consistent with the overall effect, with significantly greater mortality, higher ordinal mRS and fewer favourable outcomes (Supplementary Table S6).
Categorical (Binary) Glucose (Hyperglycaemia >/ = 7.8mmol/L vs. No Hyperglycaemia <7.8 mmol/L)
The funnel plot (Supplementary Figure S2) was fairly symmetrical; however, studies were significantly heterogeneous (I2 = 58%, Tau2 = 0.05, Chi2 = 11.81 (df = 5), p = 0.04) and a random effects model was applied.
Figure 3 demonstrates that hyperglycaemia >/ = 7.8mmool/L had a significant association with unfavourable functional outcome: OR = 2.44 (95%CI 1.9 to 3.14, Z = 6.96), p < 0.00001. This was consistent for all six studies (Bouslama et al., 2018; Goyal et al., 2018; Huo et al., 2019; Rinkel et al., 2020; Genceviciute et al., 2022; Lasek-Bal et al., 2022) (Supplementary Table S6). The highest weighted studies (Bouslama et al., 2018; Rinkel et al., 2020; Genceviciute et al., 2022) showed a clear association and lower weighted studies had greater mean differences (Huo et al., 2019; Lasek-Bal et al., 2022). Risk of bias was low (mean 12.08/14). Other outcomes reported by these studies were consistent with this direction of effect, with significantly greater mortality, higher ordinal mRS and fewer favourable (mRS 0–2) functional outcomes (Supplementary Table S6).
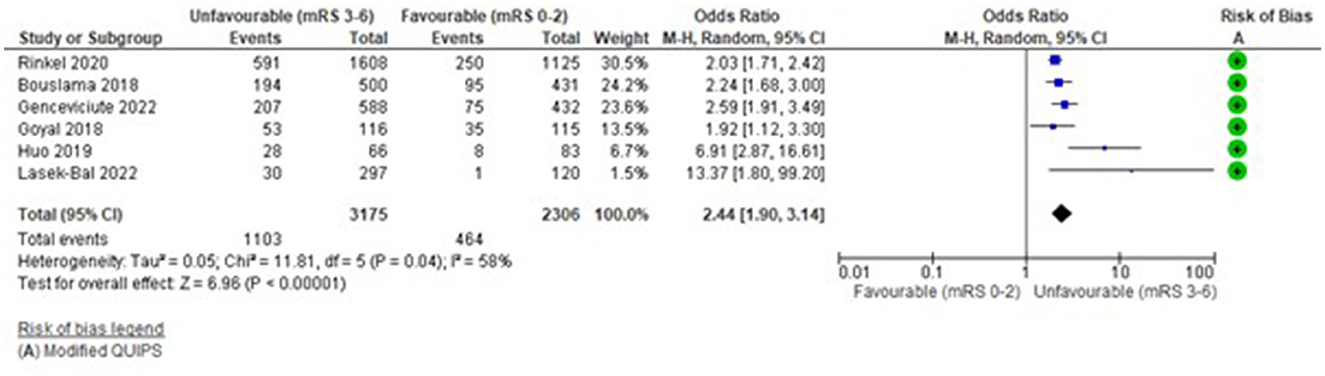
Figure 3. Effect of pre-treatment hyperglycaemia (Glucose >/ = 7.8 mmol/L) vs. no hyperglycaemia (<7.8 mmol/L) on outcome (mRS).
Systolic blood pressure
Sixteen studies (4,400 total patients) assessed the relationship between pre-treatment SBP (mmHg) as a continuous variable and 3-month functional outcome. The average number of patients per study was 276 (209 for 5 studies with significant findings and 293 for 10 studies with non-significant findings). The risk of bias (Modified QUIPS) for the SBP studies was low overall (mean 12.5/14), and similar for studies with significant (mean 12.4/14) and non-significant (mean 12.6/14) differences in functional outcome. One study had a moderate risk of bias (Sun et al., 2021). Further details on SBP studies, as well as alternative outcomes, are reported in Supplementary Table S7.
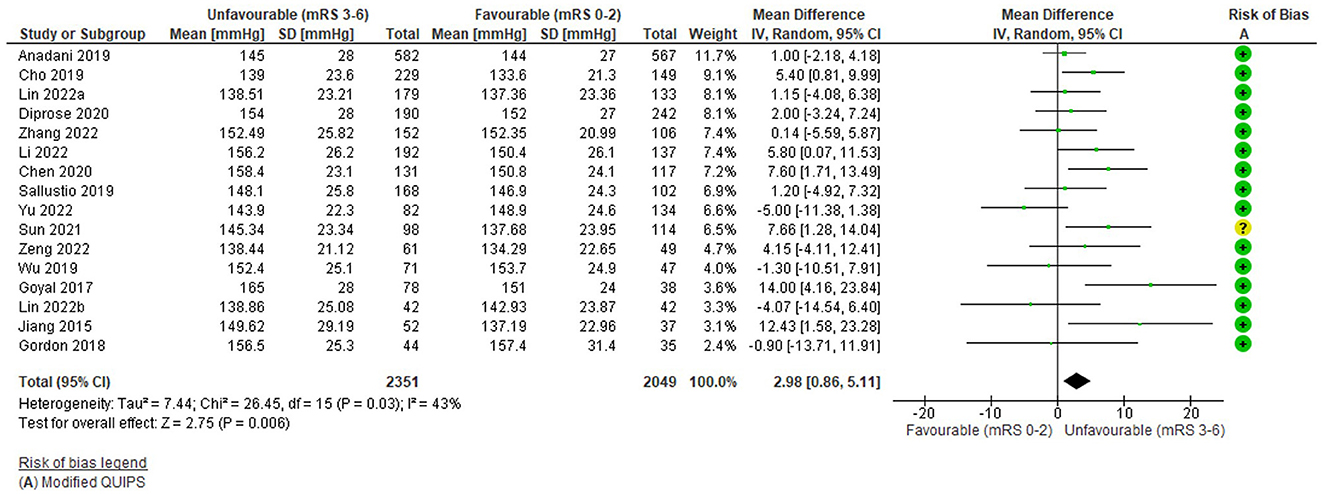
The funnel plot (Supplementary Figure S3) was fairly symmetrical; however, studies were significantly heterogeneous [I2 = 43%, Tau2 = 7.44, Chi2 = 26.45 (df = 15), p = 0.03] and a random effects model was applied.
Figure 4 demonstrates that higher admission SBP had a small, significant association with unfavourable functional outcome: WMD = 2.98 mmHg (95%CI 0.86 to 5.11, Z = 2.75), p = 0.006. Reflected in the forest plot, four highly weighted studies and one low weighted study demonstrated a small but significant difference in outcome (Jiang et al., 2015; Goyal et al., 2017; Cho et al., 2019; Chen et al., 2020; Sun et al., 2021) (Supplementary Table S7). However, this small effect was not evenly distributed across studies and the highest weighted studies (Anadani et al., 2019; Diprose et al., 2020; Lin et al., 2022a), reported non-significant associations (Supplementary Table S7). Four studies demonstrated non-significant effects in the opposite direction (Gordon et al., 2018; Wu et al., 2019; Lin et al., 2022b; Yu et al., 2022; Zhang et al., 2022). However, the overall direction of the effect was consistent with other outcomes reported by these studies, including mortality and final infarct volume (Supplementary Table S7).
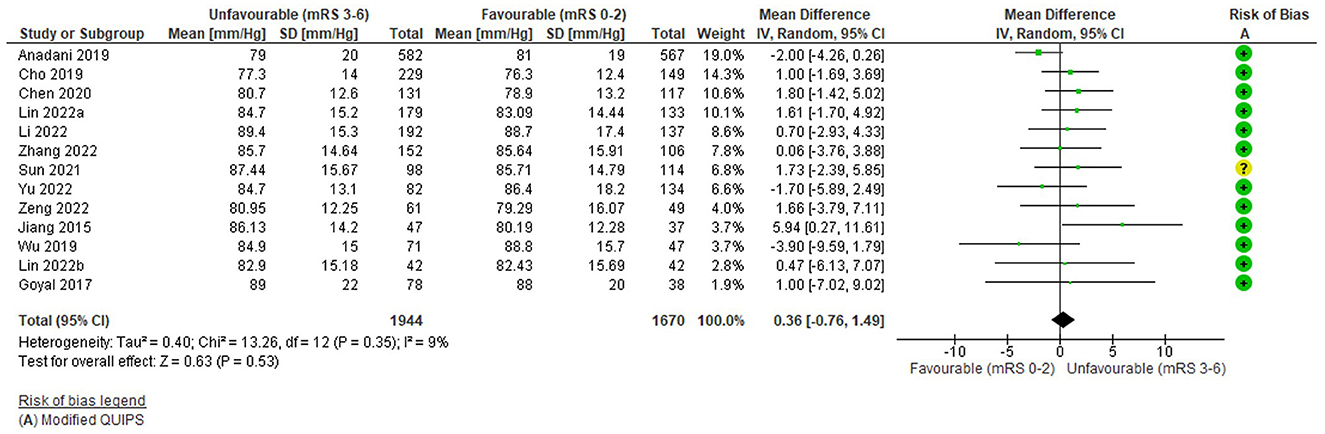
Diastolic blood pressure
Thirteen studies (3,614 total patients) assessed the relationship between pre-treatment DBP (mmHg) as a continuous variable and 3-month functional outcome (mRS 0–2 vs. 3–6). The average number of patients per study was 271 (169 for 2 studies with significant findings and 290 for 11 studies with non-significant findings). The risk of bias (Modified QUIPS) for the DBP studies was low overall (mean 12.6/14) and similar for studies with significant (mean 12.8/14) and non-significant (mean 12.5/14) differences; only one study had a moderate risk of bias (Sun et al., 2021). Further detail on DBP studies, as well as alternative outcomes, is reported in Supplementary Table S8.
The funnel plot (Supplementary Figure S4) was fairly symmetrical and there was no significant heterogeneity (Tau2 = 0.04, i2 = 9%, Chi2 = 13.26 (df = 12), p = 0.35) but as heterogeneity was anticipated and study results were bidirectional, a random effects model was still applied.
Overall, there was no significant association between pre-treatment DBP and unfavourable outcome: WMD = 0.36 mmHg (95%CI −0.76 to 1.49, Z = −0.63), p = 0.53 (Figure 5). No study reported a significant difference in DBP between outcome groups (Supplementary Table S8) and this is reflected in the forest plot. Most studies demonstrated higher DBP in unfavourable outcome groups; however, three studies reported effects in the opposite direction (Anadani et al., 2019; Wu et al., 2019; Yu et al., 2022), two of which had also reported this for SBP (Wu et al., 2019; Yu et al., 2022). The study with largest mean difference, in favour of higher DBP in the unfavourable outcome group, had a low weight due to its small sample (Jiang et al., 2015; N = 84), whereas the highest weighted study was in the opposite direction (Anadani et al., 2019; N = 1149). Variation in the direction of effect was evenly distributed in terms of study weights. Findings were consistent with those reported for other outcomes, including no significant group differences for mortality or final infarct volume (Supplementary Table S8).
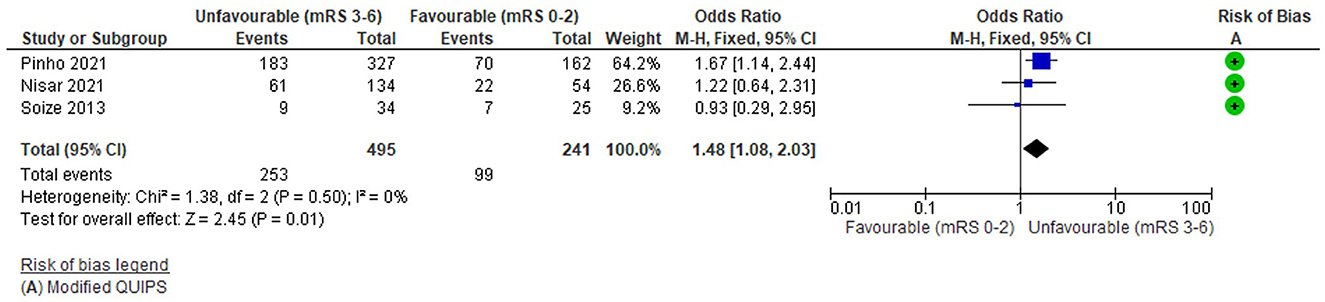
Electrocardiogram (ECG) detected atrial fibrillation
Three studies (736 total patients) considered the relationship between AF detected on a pre-treatment ECG (present vs. absent) and 3-month functional outcome (mRS 0-2 vs 3-6). The average number of patients per study was 245 (489 for one study with significant findings and 124 for 2 studies with non-significant findings). The risk of bias (Modified QUIPS) for AF was low for all studies (mean 12.7/14) and similar for studies with both significant (mean 13.5/14) and non-significant (mean 12.3/14) differences. Further detail on AF studies, as well as alternative outcomes, is reported in Supplementary Table S9.
The funnel plot (Supplementary Figure S5) was fairly symmetrical, although potentially biassed by few studies, there was no significant heterogeneity (Tau2 = 0, I2 = 0%, Chi2 = 1.38 (df = 2), p = 0.5) and there were fewer than five studies so a fixed effects model was applied.
Overall, there was a significant association between pre-treatment AF and unfavourable functional outcome: OR = 1.48 (95%CI 1.08 to 2.03, Z = 2.45), p = 0.01 (Figure 6). The analysis was dominated by one study with 489 patients (Pinho et al., 2021) which reported a significant difference in outcome (Supplementary Table S9); the remaining studies were non-significant and this is reflected in the forest plot. One study reported a non-significant effect in the opposite direction (Soize et al., 2013) but this was weighted substantially lower (N = 59). Other outcomes reported by these studies reflected their small size, with no significant group differences for mortality (Supplementary Table S9).
Level of consciousness
Two studies (99 total patients) assessed the relationship between pre-treatment conscious level, using Glasgow Coma Scale (GCS), and 3-month functional outcome (mRS 0-2 vs. 3-6). The risk of bias for the GCS studies was low overall (mean 10.8/14) but one (Phuong et al., 2022) had a moderate risk. Further details are reported in Supplementary Table S10.
The funnel plot (Supplementary Figure S6) was symmetrical, although the lack of studies suggests possible bias. There was no significant heterogeneity (Tau2 = 0, I2 = 0%, Chi2 = 0.04 (df = 1), p = 0.53) and there were N < 5 studies so a fixed effects model was applied.
Overall, there was a significant association between lower pre-treatment conscious level recorded by GCS (lower score = lower conscious level) and unfavourable outcome: WMD = −2.72 points (95%CI −4.01 to −1.44, Z = 4.16), p < 0.0001 (Figure 7). Confidence intervals did not intersect 0 on the forest plot but studies had similarly small sample sizes and neither reported significant differences in outcome (Supplementary Table S10). The highest weighted study (Phuong et al., 2022) had a moderate risk of bias. There were no other outcome measures for the GCS studies (Supplementary Table S10).
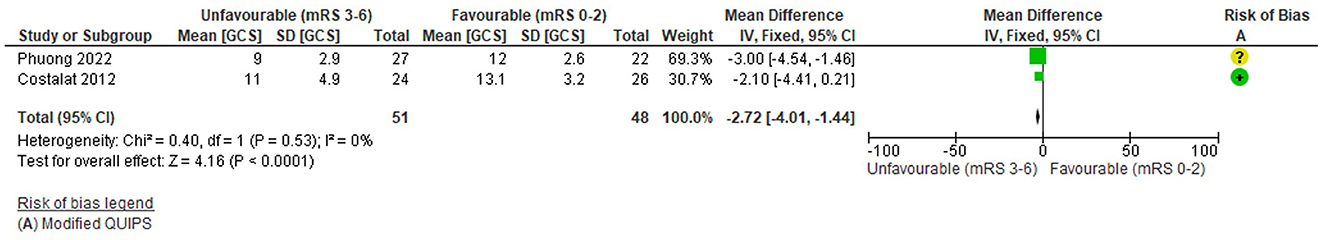
Figure 7. Effect of Pre-treatment Level of Consciousness (GCS; continuous) on outcome. *Note that the x-axis is reversed to accommodate the direction of the GCS.
Discussion
This meta-analysis has demonstrated significant associations between basic clinical characteristics routinely recorded pre-thrombectomy and unfavourable outcome including higher blood/serum glucose, higher SBP, presence of AF and lower conscious level. It is important to recognise that the findings do not imply causal effects or that modification of the characteristics could change treatment effectiveness or prognosis but that their relationship with outcome could be useful for patient selection to avoid futile transfers and treatments.
Although our review only includes patients treated with thrombectomy, there have been previous reports in many different settings describing associations between baseline physiological characteristics and short or long term outcomes following acute stroke, which has led to their inclusion in prognostic models and scores (Saposnik et al., 2011; Fahey et al., 2018; Alaka et al., 2020). Although it might be expected that these characteristics are also relevant to the thrombectomy population, it is equally important to specifically consider whether their impact is the same as for the wider acute stroke population because relationships might be changed by selection for treatment (e.g., higher symptom burden) and/or the effects of thrombectomy plus associated interventions (e.g., blood pressure lowering post-treatment).
Prognostic scores have previously been constructed to predict outcomes after thrombectomy using different combinations of clinical information. A comprehensive overview and external validation of scores (Kremers et al., 2021) has shown that some have a good combination of discrimination and calibration for prediction of functional outcome for patients receiving thrombectomy treatment within 6.5 h of onset. For example, when applied retrospectively to a validation cohort of 3,156 thrombectomy registry patients including 1,193 (40.5%) with a 3-month mRS score of 0–2, the THRIVE-c and MR PREDICTS scores predicted outcome with an area under the curve of 0.74 (95%CI 0.72 to 0.76) and 0.80 (95%CI 0.78 to 0.81) respectively. However, these scores also incorporate additional clinical assessments which are not standard for generalists performing initial patient review (e.g., National Institutes of Health Stroke Scale) and/or detailed radiological information, so could not be used for very early triage. Concerns have also been raised about clinician reliance upon predictive scores for making high impact and complex treatment decisions such as thrombectomy, particularly because these cannot reflect all possible combinations of clinical information and they have usually been developed from specific patient populations with uncertain wider generalisability (Pan et al., 2018; Hamann et al., 2021; Kremers et al., 2021). Therefore, it is still valuable to understand the strength and direction of associations between individual clinical characteristics and outcomes, which are reflective of data pooled from similar studies across multiple real-world settings.
The review factors with most data were glucose and SBP. Elevated glucose had the strongest relationship with unfavourable outcomes, with similar results previously described following meta-analysis of data from key RCTs evaluating thrombectomy (Chamorro et al., 2019). The mechanism of this association is not yet clearly established and may be due to biochemical injury of the vascular endothelium and/or arterial smooth muscle resulting in reduced blood flow despite reperfusion, or a neurotoxic effect increasing infarct volume when restoration of oxygenated blood flow boosts the formation of free radicals because of the greater availability of glucose (Martini and Ta, 2007; Natarajan et al., 2011). However, intensive lowering of elevated blood glucose during acute ischaemic stroke does not appear to improve outcome (Johnston et al., 2019) and more investigation is required to determine whether glucose is a modifiable risk factor for unfavourable outcome in the thrombectomy population.
It is perhaps surprising that blood pressure did not have a stronger relationship with post-thrombectomy outcome when it has consistently been shown to be a prognostic factor during acute stroke and higher levels are associated with an increased risk of haemorrhage after reperfusion (Willmot and Leonardi-Bee, 2004; Appleton et al., 2016). There have also been reports of a potential U-shaped association between low and high SBP and unfavourable outcome (Leonardi-Bee et al., 2002). However, patients undergoing thrombectomy - and hence included in this review—have already had a degree of clinical screening to exclude individuals who may not benefit from treatment, including those with severe hypertension (Mathews, 2021). Other explanations for disagreement between studies in our analysis include the timing of reported measures (e.g., on admission or pre-thrombectomy when sedation may have been administered) and the effect of additional treatments. For instance, it is unknown whether some readings were performed after intravenous blood pressure lowering treatment or bridging thrombolysis which could affect pre-treatment blood pressure measures or enhance reperfusion and confound the association with post-thrombectomy outcome. Evidence on potentially adverse effects of blood pressure lowering suggest that it may only be useful for patient selection rather than as a modifiable factor (Yang et al., 2022). Indeed, a recent trial has shown that intensive lowering post-thrombectomy is associated with a poorer outcome (Nam et al., 2023). There are ongoing trials of blood pressure modification before and during thrombectomy which could be included in a future meta-analysis (Bath and Havard, 2021; Li and Zhao, 2022), including in the pre-hospital setting (Song, 2020).
Atrial fibrillation is a well-recognised marker associated with a poorer prognosis, an increased rate of medical complications and a higher in-hospital mortality (Steger et al., 2004). Within the thrombectomy treated population, patients with AF experienced worse outcomes despite similar rates of reperfusion, which is likely to be attributed to their greater age and greater number of comorbidities (Kobeissi et al., 2023). However, it has also been speculated that occlusions associated with AF are technically more difficult to remove during intra-arterial treatment (Staessens et al., 2021).
In line with expectations, there was an association between lower conscious level and unfavourable outcome. Most studies did not report this feature separately from the NIHSS, which was judged to be a specialist assessment, and so were not included. The GCS is widely used during initial emergency review, but as the speech and movement components can also be affected by stroke, it is likely to reflect symptom severity as well as consciousness (Weir and Bradford, 2003). There is a recent report of NIHSS being used reliably by trained ambulance personnel (Larsen et al., 2022), which may provide more valuable information for early triage of suspected stroke; however this may be difficult to sustainably implement into general paramedic practice in other settings.
It is important to acknowledge limitations in the review process and included studies. Nearly all data were obtained from registries and convenience cohorts, including some that pre-dated landmark RCTs which led to formal regulatory approval of thrombectomy (Goyal et al., 2016). However, despite little attempt at blinding and obvious heterogeneity causing concerns about generalisability, most had a low risk of bias and it is important that the data reflect real-world practice rather than clinical trials with strict inclusion criteria in high performing centres. Although an mRS dichotomisation of 0–2 vs. 3–6 is the most common approach for reporting thrombectomy treatment effects in clinical studies and enabled pooled analysis, it is a binary outcome and so does not allow examination of more complex associations e.g., it has been reported that there is a J shaped association between glucose and outcome (Rinkel et al., 2020; Genceviciute et al., 2022), with more favourable outcomes between 3.7 and 7.3 mmol/L (Ntaios et al., 2010). Also, it does not necessarily reflect treatment effectiveness if patients already had a high level of baseline dependency (mRS 3–6), although most studies only included patients who were independent pre-stroke (mRS 0–2). Robust analyses could not be undertaken for AF and GCS due to insufficient eligible studies and no studies reported any combinations of predictive factors to allow further explanation of relationships between them, e.g., SBP in the group with AF. Studies did not consistently report some variables which could also relate to outcome, such as the thrombolytic agent used and the time of administration and it was not possible to perform any sensitivity analyses based on these. It is also important to note that some studies did not separately report data for anterior and posterior circulation stroke and we took the decision to pool data across all vascular territories for the meta-analysis. Finally, as there are many other dominant factors which should be considered during thrombectomy treatment decisions, the factors described in this review should not be used in isolation to triage suspected stroke patients as they only had small effects on outcome.
The basic clinical factors included in our analysis are usually available during initial non-specialist assessment, and possibly could be used to refine selection of patients suitable for thrombectomy, such as during ambulance triage towards Comprehensive Stroke Centres. Reports of symptom-based pre-hospital redirection pathways confirm that not all patients with LVO arriving at CSC receive thrombectomy, and it is possible that some of these had unfavourable non-stroke characteristics (e.g., high systolic blood pressure) which might especially deter interventionists from treating patients with uncertain benefit (e.g., later in the time window and/or with a larger ischaemic core). For instance, in the Direct Transfer to Endovascular Center of Acute Stroke Patients with Suspected Large Vessel Occlusion in the Catalan Territory (RACECAT) trial (Pérez de la Ossa et al., 2022), amongst 482 intervention patients who were taken directly to a thrombectomy capable centre, 333 had recent onset LVO confirmed but only 235 (70%) received treatment, and it is possible that further consideration of additional prognostic information before transportation could reduce unnecessary patient displacement during implementation of this pathway model. There is little data so far which directly addresses this question, and only one study included in our meta-analysis reported data from the prehospital setting (Shriki et al., 2020). Further evaluation is needed of emergency care pathways which collect and use these characteristics. In England, the Specialist Pre-hospital Redirection for Ischaemic Stroke Thrombectomy (SPEEDY) trial (Shaw, 2022) is currently examining the clinical and cost-effectiveness of pre-hospital patient redirection to thrombectomy providers using a two-stage pre-hospital triage process during which ambulance personnel collect and communicate key information to specialist centres for a direct admission decision. The standard information checklist includes pre-hospital heart rate, blood pressure, oxygen saturations, blood glucose, temperature and conscious level, so the trial is expected to provide additional evidence about the value of these early basic measures for predicting thrombectomy outcome when LVO is present.
Future work in pre-hospital and hospital settings should also consider combining physiological factors with other non-stroke characteristics which may have a bearing on thrombectomy outcome such as frailty, pre-stroke dependency and comorbidities (Adamou et al., 2022; Tan et al., 2022; Barow et al., 2023). Although some have been included in previously published thrombectomy outcome scores (Kremers et al., 2021), especially pre-stroke dependency, it has not yet been demonstrated that they are accurate when used by non-specialist practitioners making early triage decisions towards thrombectomy providers. As it is likely that some factors will interact (e.g., patients with diabetes as a co-morbidity are likely to have high blood glucose), relationships between them may be complex and could also vary according to symptom severity and time since onset. Machine learning models are useful in this scenario and could help to create more accurate prognostic tools which are then embedded in software on portable devices to assist non-specialists when making early triage decisions and interventionists deciding whether to offer thrombectomy to patients with complex medical presentations (Thomas et al., 2021; Lin et al., 2022a). In future such models could potentially incorporate novel biomarker tests and/or technologies which are challenging for clinicians to interpret for individual patients, such as surface EEG (Montellano et al., 2021; Sutcliffe et al., 2022). The outputs would also facilitate discussion with patients and their families about the probability of a good outcome according to individual health and physiological profiles.
Finally, it is important that interventionists and stroke services undertake regular audit of their practice, including descriptions of baseline factors which may influence outcomes. As associations have been demonstrated between basic physiological characteristics and post-thrombectomy dependency, details of these variables should be included in audit reports and registries to illustrate any population variations between settings. Adjustment for the effects of these characteristics could also help to understand differences in outcomes between centres and so facilitate comparisons of care delivery in real world settings (Nie et al., 2023; Quandt et al., 2023).
Conclusion
Basic physiological observations available during assessment by generalist emergency practitioners are associated with outcome after thrombectomy and might assist with early triage decisions, however these should not yet be considered as independent outcome predictors and associations do not imply that modification of factor(s) can change outcome. There were low numbers of cases for some factors and thrombectomy providers should continue to share data for pooled analysis and assist with evaluation of emergency care pathways which include basic characteristics during early selection of patients for treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
HL: Data curation, Formal analysis, Investigation, Methodology, Resources, Visualisation, Writing—original draft, Writing—review and editing. LS: Conceptualisation, Funding acquisition, Methodology, Supervision, Writing—review and editing. JM: Data curation, Writing—review and editing. AA: Data curation, Formal analysis, Writing—review and editing. PW: Writing—review and editing. GF: Writing—review and editing. MJ: Writing—review and editing. CP: Conceptualisation, Formal analysis, Funding acquisition, Methodology, Resources, Software, Supervision, Writing—original draft, Writing—review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was funded by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration (ARC) North East and North Cumbria (NIHR200173) and by the National Institute for Health and Care Research (NIHR) under its Programme Grants for Applied Research (PGfAR) (Grant Reference Number NIHR202361). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Acknowledgments
Alex Inskip (Information Scientist) assisted with the search strategy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fstro.2023.1283312/full#supplementary-material
References
Adamou, A., Gkana, A., Mavrovounis, G., Beltsios, E. T., Kastrup, A., and P (2022). Outcome of endovascular thrombectomy in pre-stroke dependent patients with acute ischemic stroke: a systematic review and meta-analysis. Front. Neurol. 13, 880046. doi: 10.3389/fneur.2022.880046
Alaka, S. A., Menon, B. K., Brobbey, A., Williamson, T., Goyal, M., Demchuk, A. M., et al. (2020). Functional outcome prediction in ischemic stroke: a comparison of machine learning algorithms and regression models. Front. Neurol. 11, 889. doi: 10.3389/fneur.2020.00889
Anadani, M., Orabi, M.Y., Alawieh, A., Goyal, N., Alexandrov, A.V., Petersen, N., et al. (2019). Blood pressure and outcome after mechanical thrombectomy with successful revascularization. Stroke 50, 2448–2454. doi: 10.1161/STROKEAHA.118.024687
Appleton, J. P., Sprigg, N., and PM B. (2016). Blood pressure management in acute stroke. Stroke Vasc. Neurol. 1, 20. doi: 10.1136/svn-2016-000020
Barow, E., Probst, A. C., Pinnschmidt, H., Heinze, M., Jensen, M., Rimmele, D. L., et al. (2023). Effect of comorbidity burden and polypharmacy on poor functional outcome in acute ischemic stroke. Clin. Neuroradiol. 33, 147–154. doi: 10.1007/s00062-022-01193-8
Bath, P., and Havard, D. (2021) Efficacy of Nitric Oxide in Stroke-2. Available online at: https://www.isrctn.com/ISRCTN17654248 (accessed: 26/09/2023).
Bouslama, M., Barreira, C. M., Haussen, D. C., Grossberg, J. A., Belagaje, S. R., Bianchi, N. A., et al. (2018). Admission hyperglycemia and acute large vessel occlusion stroke outcomes after endovascular therapy. Stroke 49(Supplement 1), 1. doi: 10.1161/str.49.suppl_1.TP21
Broocks, G., Kemmling, A., Aberle, J., Kniep, H., Bechstein, M., Flottmann, F., et al. (2020). Elevated blood glucose is associated with aggravated brain edema in acute stroke. J. Neurol. 267, 440–448. doi: 10.1007/s00415-019-09601-9
Cao, J., Mo, Y., Chen, R., Shao, H., Xuan, J., Peng, Y., et al. (2021). Predictors of functional outcome and mortality in endovascular treatment for acute basilar artery occlusion: a single-centre experience. Front Neurol. 12, 731300. doi: 10.3389/fneur.2021.731300
Cappellari, M., Mangiafico, S., Saia, V., Pracucci, G., Nappini, S., Nencini, P. S., et al. (2020). IER-START nomogram for prediction of three-month unfavorable outcome after thrombectomy for stroke. Int. J. Stroke 15, 412–20. doi: 10.1177/1747493019837756
Chamorro, Á., Brown, S., Amaro, S., Hill, M.D., Muir, K.W., Dippel, D.W.J., et al. (2019). Glucose modifies the effect of endovascular thrombectomy in patients with acute stroke. Stroke 50, 690–696. doi: 10.1161/STROKEAHA.118.023769
Chen, Z., Su, M., Li, Z., Du, H., Zhang, S., Pu, M., and Zhang, Y. et al. (2020). Metabolic syndrome predicts poor outcome in acute ischemic stroke patients after endovascular thrombectomy. Neuropsychiatr Dis. Treat. 16, 2045–2052. doi: 10.2147/NDT.S264300
Cho, B. H., Kim, J. T., Lee, J. S., Park, M. S., Kang, K. W., Choi, K. H., et al. (2019). Associations of various blood pressure parameters with functional outcomes after endovascular thrombectomy in acute ischaemic stroke. Eur. J. Neurol. 26, 1019–1027. doi: 10.1111/ene.13951
Costalat, V., Lobotesis, K., Machi, P., Mourand, I., Maldonado, I., Heroum, C., et al. (2012). Prognostic factors related to clinical outcome following thrombectomy in ischemic stroke (RECOST study). 50 patients prospective study. Eur. J. Radiol. 81, 4075–82. doi: 10.1016/j.ejrad.2012.07.012
Diestro, J. D., Dmytriw, A. A., Broocks, G., Chen, K., Hirsch, J. A., Kemmling, A. A., et al. (2020). Endovascular thrombectomy for low ASPECTS large vessel occlusion ischemic stroke: a systematic review and meta-analysis. Can. J. Neurol. Scie. 47, 612–9. doi: 10.1017/cjn.2020.71
Diprose, W. K., Liem, B., Wang, M. T. M., Sutcliffe, J. A., Brew, S., Caldwell, J. R., et al. (2020). Impact of body temperature before and after endovascular thrombectomy for large vessel occlusion stroke. Stroke 51, 1218–1225. doi: 10.1161/STROKEAHA.119.028160
Evans, M. R., White, P., Cowley, P., and DJ, W. (2017). Revolution in acute ischaemic stroke care: a practical guide to mechanical thrombectomy. Practical neurology 17, 252–65. doi: 10.1136/practneurol-2017-001685
Fahey, M., Crayton, E., Wolfe, C., and A. D. (2018). Clinical prediction models for mortality and functional outcome following ischemic stroke: a systematic review and meta-analysis. PLoS ONE 13, e0185402. doi: 10.1371/journal.pone.0185402
Genceviciute, K., Göldlin, M. B., Kurmann, C. C., Mujanovic, A., Meinel, T. R., Kaesmacher, J. J., et al. (2022). Association of diabetes mellitus and admission glucose levels with outcome after endovascular therapy in acute ischaemic stroke in anterior circulation. Eur. J. Neurol. 29, 2996–3008. doi: 10.1111/ene.15456
Gordon, W. R., Salamo, R. M., Behera, A., Chibnall, J., Alshekhlee, A., Callison, R. C., et al. (2018). Association of blood glucose and clinical outcome after mechanical thrombectomy for acute ischemic stroke. Interv. Neurol. 7, 182–188. doi: 10.1159/000486456
Goyal, M., Menon, B. K., van Zwam, W. H., Dippel, D. W., Mitchell, P. J., Demchuk, A. M., et al. (2016). Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. The Lancet,387, 1723–31. doi: 10.1016/S0140-6736(16)00163-X
Goyal, N., Tsivgoulis, G., Iftikhar, S., Khorchid, Y., Fawad Ishfaq, M., Doss, V. T., et al. (2017). Admission systolic blood pressure and outcomes in large vessel occlusion strokes treated with endovascular treatment. J. Neurointerv. Surg. 9, 451–454. doi: 10.1136/neurintsurg-2016-012386
Goyal, N., Tsivgoulis, G., Pandhi, A., Dillard, K., Katsanos, A. H., Magoufis, G., et al. (2018). Admission hyperglycemia and outcomes in large vessel occlusion strokes treated with mechanical thrombectomy. J. Neurointerv. Surg. 10, 112–117. doi: 10.1136/neurintsurg-2017-012993
Hamann, J., Herzog, L., Wehrli, C., Dobrocky, T., Bink, A., Piccirelli, M. AR., et al. (2021). Machine-learning-based outcome prediction in stroke patients with middle cerebral artery-M1 occlusions and early thrombectomy. Eur. J. Neurol. 28, 1234–43. doi: 10.1111/ene.14651
Higgins, J. P. (2020). Cochrane Handbook for Systematic Reviews of Interventions Version 6, 1.0 (updated March 2020). Cochrane.
Huo, X., Gao, F., Sun, X., Ma, N., Song, L., Mo, D., et al. (2016). Endovascular mechanical thrombectomy with the solitaire device for the treatment of acute basilar artery occlusion. World Neurosurg. 89, 301–8. doi: 10.1016/j.wneu.2016.02.017
Huo, X., Liu, R., Gao, F., Ma, N., Mo, D., Liao, X., and Miao, Z. et al. (2019). Effect of hyperglycemia at presentation on outcomes in acute large artery occlusion patients treated with solitaire stent thrombectomy. Front. Neurol. 10, 71. doi: 10.3389/fneur.2019.00071
Jiang, S., Fei, A., Peng, Y., Zhang, J., Lu, Y. R., Wang, H. R., et al. (2015). Predictors of outcome and hemorrhage in patients undergoing endovascular therapy with solitaire stent for acute ischemic stroke. PLoS ONE 10, e0144452. doi: 10.1371/journal.pone.0144452
Johnston, K. C., Bruno, A., Pauls, Q., Hall, C. E., Barrett, K. M., Barsan, W. VL., et al. (2019). Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the shine randomized clinical trial. JAMA 322, 326–335. doi: 10.1001/jama.2019.9346
Jonathan, J. D., Julian, P. T., Higgins, G., and Altman, D. G. (2021). Cochrane handbook for systematic reviews of interventions, version 6. Chapter 10: Analysing Data and Undertaking Meta-Analyses'.
Karamchandani, R. R., Satyanarayana, S., Yang, H., Defilipp, G., Strong, D., Rhoten, J. B., et al. (2022). The Charlotte large artery occlusion endovascular therapy outcome score predicts outcome after basilar artery thrombectomy. J. Neuroimag. 32, 860–865. doi: 10.1111/jon.13039
Kim, J. T., Liebeskind, D. S., Jahan, R., Menon, B. K., Goyal, M., Nogueira, R. G., et al. (2018). Impact of hyperglycemia according to the collateral status on outcomes in mechanical thrombectomy. Stroke 49, 2706–2714. doi: 10.1161/STROKEAHA.118.022167
Kobeissi, H., Ghozy, S., Seymour, T., Gupta, R., Bilgin, C., Kadirvel, R. D. F., et al. (2023). Outcomes of patients with atrial fibrillation following thrombectomy for stroke: a systematic review and meta-analysis. JAMA Network Open 6, e2249993–e2249993. doi: 10.1001/jamanetworkopen.2022.49993
Kremers, F., Venema, E., Duvekot, M., Yo, L., and Bokkers, R. (2021). Outcome prediction models for endovascular treatment of ischemic stroke: systematic review and external validation. Stroke 3, 445. doi: 10.1161/STROKEAHA.120.033445
Larsen, K., Jæger, H. S., Hov, M. R., Thorsen, K., Solyga, V., Lund, C. G., et al. (2022). Streamlining acute stroke care by introducing national institutes of health stroke scale in the emergency medical services: a prospective cohort study. Stroke 53, 2050–2057. doi: 10.1161/STROKEAHA.121.036084
Lasek-Bal, A., Zak, A., Binek, L., Student, S., Cieslik, A., Bal, W., et al. (2022). Relevance of admission hyperglycaemia and diabetes mellitus to efficacy and safety of mechanical thrombectomy in stroke patients. Neurol. i neurochirurgia pol. 56, 472–479. doi: 10.5603/PJNNS.a2022.0063
Leonardi-Bee, J., Bath, P. M., and Phillips, S. J. (2002). Blood pressure and clinical outcomes in the international stroke trial. Stroke 33, 1315–20. doi: 10.1161/01.STR.0000014509.11540.66
Li, L. I., and Zhao, H. (2022). Prevention and Treatment of Reperfusion Injury After Mechanical Thrombectomy in Acute Ischemic Stroke. ClinicalTrials.gov.
Li, X., Li, C., Shi, M. Qu., Y., Huo., L., et al. (2022). Which glucose parameter best predicts poor outcome after mechanical thrombectomy for acute large vessel occlusion stroke? Int. Med. J. 52, 1374–1380. doi: 10.1111/imj.15259
Lin, S., Lin, X., Zhang, J., Wan, M., Chen, C., Jie, Q., et al. (2022b). A visualized nomogram to online predict futile recanalization after endovascular thrombectomy in basilar artery occlusion stroke. Front. Neurol. 13, 968037. doi: 10.3389/fneur.2022.968037
Lin, X., Zheng, X., Zhang, J., Cui, X., Zou, D., Zhao, Z. J., et al. (2022a). Machine learning to predict futile recanalization of large vessel occlusion before and after endovascular thrombectomy. Front. Neurol. 13, 909403. doi: 10.3389/fneur.2022.909403
Martini, S. R., and Ta, K. (2007). Hyperglycemia in acute ischemic stroke: a vascular perspective. J. Cereb. Blood Flow Metab. 27, 435–451. doi: 10.1038/sj.jcbfm.9600355
Mokin, M., Ansari, S. A., McTaggart, R. A., Bulsara, K. R., Goyal, M., Chen, M., et al. (2019). Indications for thrombectomy in acute ischemic stroke from emergent large vessel occlusion (ELVO): report of the SNIS Standards and Guidelines Committee. J. Neurointervent. Surg. 11, 215–20. doi: 10.1136/neurintsurg-2018-014640
Mokin, M., Primiani, C. T., and Siddiqui, A. H. (2017). ASPECTS (Alberta Stroke Program Early CT Score) measurement using Hounsfield unit values when selecting patients for stroke thrombectomy. Stroke 48, 1574–9. doi: 10.1161/STROKEAHA.117.016745
Montellano, F. A., Ungethüm, K., Ramiro, L., Nacu, A., Hellwig, S., Fluri, F., et al. (2021). Role of blood-based biomarkers in ischemic stroke prognosis: a systematic review. Stroke 52, 543–51. doi: 10.1161/STROKEAHA.120.029232
Mourad, O., Hossam, H., and Elmagarmid, A. (2016). Rayyan—A web and mobile app for systematic reviews. J. Sys. Rev. 5, 4. doi: 10.1186/s13643-016-0384-4
Nam, H. S., Kim, Y. D., Heo, J., Lee, H., Jung, J. W., Choi, J. K. BM., et al. (2023). Intensive vs conventional blood pressure lowering after endovascular thrombectomy in acute ischemic stroke: The OPTIMAL-BP randomized clinical trial. JAMA 330, 832–42. doi: 10.1001/jama.2023.14590
Natarajan, S. K., Dandona, P., Karmon, Y., Yoo, A. J., Kalia, J. S., Hao, Q., et al. (2011). Prediction of adverse outcomes by blood glucose level after endovascular therapy for acute ischemic stroke. Journal of neurosurgery, 114, 1785–1799. doi: 10.3171/2011.1.JNS10884
National Institute for Health and Care Excellence (2019) Stroke and Transient Ischaemic Attack in over 16s: Diagnosis and Initial Management.
Nie, X., Leng, X., Miao, Z., and Fisher, M. and L, L. (2023). Clinically ineffective reperfusion after endovascular therapy in acute ischemic stroke. Stroke 54, 873–81. doi: 10.1161/STROKEAHA.122.038466
Nisar, T., Shapouran, S., Abu-Hadid, O., Shaulov, S., Tofade, T., Patel, J., et al. (2021). Association of anemia with functional outcomes in patients with mechanical thrombectomy. Clin. Neurol. Neurosurg. 211, 107028. doi: 10.1016/j.clineuro.2021.107028
Nogueira, R. G., Jadhav, A. P., Haussen, D. C., Bonafe, A., Budzik, R. F., Bhuva, P. CA, S., et al. (2018). Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. New Eng. J. Med. 378, 11–21. doi: 10.1056/NEJMoa1706442
Ntaios, G., Egli, M., and Faouzi, M. and P, M. (2010). J-shaped association between serum glucose and functional outcome in acute ischemic stroke. Stroke 41, 2366–70. doi: 10.1161/STROKEAHA.110.592170
Ozdemir, O., Giray, S., Arlier, Z., Baş, D. F., Inanc, Y., and Colak, E. (2015). Predictors of a good outcome after endovascular stroke treatment with stent retrievers. Sci. WorldJ. 2015, 403726. doi: 10.1155/2015/403726
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Sys. Rev. 10, 89. doi: 10.1186/s13643-021-01626-4
Pan, Y., Peng, Y., Chen, W., Wang, Y., Lin, Y., He, Y., et al. (2018). THRIVE-c score predicts clinical outcomes in Chinese patients after thrombolysis. Brain Behav. 8, e00927. doi: 10.1002/brb3.927
Pérez de la Ossa, N., Abilleira, S., and Jovin, T. G. (2022). Effect of direct transportation to thrombectomy-capable center vs local stroke center on neurological outcomes in patients with suspected large-vessel occlusion stroke in nonurban areas: the RACECAT randomized clinical trial. JAMA 327, 1782–1794. doi: 10.1001/jama.2022.4404
Phuong, N. V., Cong Thanh, N., Keserci, B., Sang, N. V., and Minh Duc, N. (2022). Mechanical thrombectomy treatment of basilar artery occlusion within 24 h of symptom onset: a single-center experience. La Clin. Terap. 173, 400–406. doi: 10.7417/CT.2022.2454
Pinho, J., Küppers, C., Nikoubashman, O., Wiesmann, M., Schulz, J. B., Reich, A., et al. (2021). Frailty is an outcome predictor in patients with acute ischemic stroke receiving endovascular treatment. Age Age. 50, 1785–1791. doi: 10.1093/ageing/afab092
Quandt, F., Meißner, N., Wölfer, T. A., Flottmann, F., Deb-Chatterji, M., Kellert, L. G., et al. (2023). RCT vs. real-world cohorts: differences in patient characteristics drive associations with outcome after EVT. Eur. Stroke J. 8, 231–40. doi: 10.1177/23969873221142642
Quinn, T., Ashton, C., and Mcclelland, G. and S., M. (2022). JRCALC Guidelines - Stroke/Transient Ischaemic Attack'.
Ramos, L. A., Kappelhof, M., Van Os, H. J., Chalos, V., Van Kranendonk, K., Kruyt, N. D., et al. (2020). Predicting poor outcome before endovascular treatment in patients with acute ischemic stroke. Front. Neurol., 11, 580957. doi: 10.3389/fneur.2020.580957
Riley, R. D., Moons, K. G., Snell, K. I., Ensor, J., Hooft, L., Altman, D. G., et al. (2019). A guide to systematic review and meta-analysis of prognostic factor studies. BMJ, 364, 4597. doi: 10.1136/bmj.k4597
Rinkel, L. A., Nguyen, T. T. M., Guglielmi, V., Groot, A. E., Posthuma, L., Roos, Y., et al. (2020). High admission glucose is associated with poor outcome after endovascular treatment for ischemic stroke. Stroke 51, 3215–3223. doi: 10.1161/STROKEAHA.120.029944
Sallustio, F., Toschi, N., Mascolo, A. P., Marrama, F., Morosetti, D., Ros, D. a., et al. (2019). Selection of anterior circulation acute stroke patients for mechanical thrombectomy. J. Neurol. 266, 2620–2628. doi: 10.1007/s00415-019-09454-2
Saposnik, G., Raptis, S., Kapral, M. K., Liu, Y., Tu, J. V., Mamdani, M., et al. (2011). The iScore predicts poor functional outcomes early after hospitalization for an acute ischemic stroke. Stroke 42, 3421–8. doi: 10.1161/STROKEAHA.111.623116
Sarraj, A., Albright, K., Barreto, A. D., Boehme, A. K., Sitton, C. W., Choi, J. O., et al. (2013). Optimizing prediction scores for poor outcome after intra-arterial therapy in anterior circulation acute ischemic stroke. Stroke 44, 3324–30. doi: 10.1161/STROKEAHA.113.001050
Sentinel Stroke National Audit Programme, S. (2023). National Clinical Guideline for Stroke for the United Kingdom and Ireland'.
Shaw, L. (2022). Specialist pre-hospital redirection for ischaemic stroke thrombectomy (SPEEDY). Available online at: https://www.isrctn.com/ISRCTN77453332.
Shi, Z. S., Liebeskind, D. S., Xiang, B., Ge, S. G., Feng, L., Albers, G. W., et al. (2014). Predictors of functional dependence despite successful revascularization in large-vessel occlusion strokes. Stroke 45, 1977–84. doi: 10.1161/STROKEAHA.114.005603
Shriki, J., Johnson, L., Patel, P., McGann, M., Lurie, T., Phipps, M. S., et al. (2020). Transport blood pressures and outcomes in stroke patients requiring thrombectomy. Air Med. J. 39, 166–172. doi: 10.1016/j.amj.2020.03.002
Soize, S., Barbe, C., Kadziolka, K., Estrade, L., Serre, I., and Pierot, L. (2013). Predictive factors of outcome and hemorrhage after acute ischemic stroke treated by mechanical thrombectomy with a stent-retriever. Neuroradiology 55, 977–987. doi: 10.1007/s00234-013-1191-4
Song, L. (2020). Intensive Ambulance-delivered Blood Pressure Reduction in Hyper-Acute Stroke Trial (INTERACT4). ClinicalTrials.gov.
Staessens, S., François, O., Brinjikji, W., Doyle, K. M., Vanacker, P., Andersson, T., et al. (2021). Studying stroke thrombus composition after thrombectomy: what can we learn? Stroke 52, 3718–27. doi: 10.1161/STROKEAHA.121.034289
Steger, C., Pratter, A., Martinek-Bregel, M., Avanzini, M., Valentin, A., Slany, J., et al. (2004). Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian Stroke registry. Eur. Heart J. 25, 1734–40. doi: 10.1016/j.ehj.2004.06.030
Suissa, L., Guigonis, J. M., Graslin, F., Doche, E., Osman, O., Chau, Y., et al. (2020). Metabolome of cerebral thrombi reveals an association between high glycemia at stroke onset and good clinical outcome. Metabolites 10, 483. doi: 10.3390/metabo10120483
Sun, H., Zhou, F., Zhang, G., Hou, J., Liu, Y., Chen, X., et al. (2021). A novel nomogram for predicting prognosis after mechanical thrombectomy in patients with acute ischemic stroke. Curr. Neurovasc. Res. 18, 479–488. doi: 10.2174/1567202618666211210154739
Sutcliffe, L., Lumley, H., Shaw, L., and Francis, R. (2022). Surface electroencephalography (EEG) during the acute phase of stroke to assist with diagnosis and prediction of prognosis: a scoping review. BMC Emerg. Med. 1, 1–30. doi: 10.1186/s12873-022-00585-w
Tan, B. Y., Ho, J. S., Leow, A. S., Chia, M. L., Sia, C. H., Koh, Y. Y., et al. (2022). Effect of frailty on outcomes of endovascular treatment for acute ischaemic stroke in older patients. Age Age. 51, afac096. doi: 10.1093/ageing/afac096
Thomas, S., De la Pena, P., Butler L., Akbilgic, O., Heiferman, D. M., Garg, R., and Gill, R. (2021). Machine learning models improve prediction of large vessel occlusion and mechanical thrombectomy candidacy in acute ischemic stroke. J. Clin. Neurosci. 91, 383–90. doi: 10.1016/j.jocn.2021.07.021
Tufanaru, C., Munn, Z., and Stephenson, M. (2015). Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. JBI Evid. Implement. 13, 196–207. doi: 10.1097/XEB.0000000000000065
Venema, E., Roozenbeek, B., Mulder, M. J., Brown, S., Majoie, C. B., Steyerberg, E. W. S., et al. (2021). Prediction of outcome and endovascular treatment benefit: validation and update of the MR PREDICTS decision tool. Stroke 52, 2764–72. doi: 10.1161/STROKEAHA.120.032935
Wang, Y., and Zhou, Z. (2020). FLAIR vascular hyperintensity-DWI mismatch most likely to benefit from recanalization and good outcome after stroke. Medicine 99, 18665. doi: 10.1097/MD.0000000000018665
Weir, C. J., and Bradford, A. P. (2003). The prognostic value of the components of the Glasgow Coma Scale following acute stroke. Stroke 96, 67–74. doi: 10.1093/qjmed/hcg008
Willmot, M., and Leonardi-Bee, J. (2004). High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension. 43, 18–24. doi: 10.1161/01.HYP.0000105052.65787.35
Wu, X., Liu, G., Zhou, W., Ou, A., Liu, X., Wang, Y., et al. (2019). Outcome prediction for patients with anterior circulation acute ischemic stroke following endovascular treatment: a single-center study. Exp. Ther. Med. 18, 3869–3876. doi: 10.3892/etm.2019.8054
Yang, P., Song, L., Zhang, Y., Zhang, X., Chen, X., Li, Y., et al. (2022). Intensive blood pressure control after endovascular thrombectomy for acute ischaemic stroke (ENCHANTED2/MT): a multicentre, open-label, blinded-endpoint, randomised controlled trial. Lancet Neurol. 400, 1585–96. doi: 10.1016/S0140-6736(22)01882-7
Yu, S., Jiang, Q., Guo, Z., You, S., Huang, Z., Hou, J., Xiao, G., et al. (2022). Development and validation of a dynamic nomogram predicting futile recanalization after thrombectomy in acute ischemic stroke. Chin. J. Neurol. 55, 1118–1127. doi: 10.3760/cma.j.cn113694-20220323-00225
Yuan, Z., Chen, N., Zhou, M., Guo, J., Zhang, Y., Li, Y., et al. (2020). Effects of hypertension in patients receiving mechanical thrombectomy: a meta-analysis. Medicine 99, e19803. doi: 10.1097/MD.0000000000019803
Zeng, W., Li, W., Huang, K., Lin, Z., Dai, H., He, Z., et al. (2022). Predicting futile recanalization, malignant cerebral edema, and cerebral herniation using intelligible ensemble machine learning following mechanical thrombectomy for acute ischemic stroke. Front. Neurol. 13, 982783. doi: 10.3389/fneur.2022.982783
Zhang, X. G., Wang, J. H., Yang, W. H., Zhu, X. Q., Xue, J., Li, Z. Z., et al. (2022). Nomogram to predict 3-month unfavorable outcome after thrombectomy for stroke. BMC Neurology 22, 111. doi: 10.1186/s12883-022-02633-1
Zhou, T., Yi, T., Li, T., Zhu, L., Li, Y., Li, Z., et al. (2022). Predictors of futile recanalization in patients undergoing endovascular treatment in the DIRECT-MT trial. J. NeuroIntervent. Surg. 14, 752–5. doi: 10.1136/neurintsurg-2021-017765
Keywords: thrombectomy, outcome, prognosis, modified Rankin, physiological observations
Citation: Lumley HA, Shaw L, Morris J, Alton A, White P, Ford GA, James M and Price C (2023) Associations between basic physiological observations recorded pre-thrombectomy and functional outcome: a systematic review and meta-analysis. Front. Stroke 2:1283312. doi: 10.3389/fstro.2023.1283312
Received: 25 August 2023; Accepted: 27 September 2023;
Published: 19 October 2023.
Edited by:
Yang Liu, Shanghai Jiao Tong University, ChinaReviewed by:
Jorge Arturo Larco, Henry Ford Hospital, United StatesCem Bilgin, Mayo Clinic, United States
Copyright © 2023 Lumley, Shaw, Morris, Alton, White, Ford, James and Price. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hannah A. Lumley, SGFubmFoLmx1bWxleUBuZXdjYXN0bGUuYWMudWs=
 Hannah A. Lumley
Hannah A. Lumley Lisa Shaw1
Lisa Shaw1 Gary A. Ford
Gary A. Ford