
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Stroke, 15 June 2023
Sec. Genetics and Omics of Stroke
Volume 2 - 2023 | https://doi.org/10.3389/fstro.2023.1192465
This article is part of the Research TopicDiversity in Stroke Omic(s) and Epidemiology Research: Opportunities and ChallengesView all 6 articles
 A. Parker Ruhl1,2*
A. Parker Ruhl1,2* Neal Jeffries3
Neal Jeffries3 Yu Yang4
Yu Yang4 Steven D. Brooks1
Steven D. Brooks1 Rakhi P. Naik5
Rakhi P. Naik5 Lydia H. Pecker5
Lydia H. Pecker5 Bryan T. Mott6
Bryan T. Mott6 Cheryl A. Winkler7
Cheryl A. Winkler7 Nicole D. Armstrong8
Nicole D. Armstrong8 Neil A. Zakai9,10
Neil A. Zakai9,10 Orlando M. Gutierrez8,11
Orlando M. Gutierrez8,11 Suzanne E. Judd8
Suzanne E. Judd8 Virginia J. Howard8,11
Virginia J. Howard8,11 George Howard8,11
George Howard8,11 Marguerite R. Irvin8
Marguerite R. Irvin8 Mary Cushman9,10
Mary Cushman9,10 Hans C. Ackerman1
Hans C. Ackerman1Introduction: People with African ancestry have greater stroke risk and greater heritability of stroke risk than people of other ancestries. Given the importance of nitric oxide (NO) in stroke, and recent evidence that alpha globin restricts nitric oxide release from vascular endothelial cells, we hypothesized that alpha globin gene (HBA) deletion would be associated with reduced risk of incident ischemic stroke.
Methods: We evaluated 8,947 participants self-reporting African ancestry in the national, prospective Reasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Incident ischemic stroke was defined as non-hemorrhagic stroke with focal neurological deficit lasting ≥24 h confirmed by the medical record or focal or non-focal neurological deficit with positive imaging confirmed with medical records. Genomic DNA was analyzed using droplet digital PCR to determine HBA copy number. Multivariable Cox proportional hazards regression was used to estimate the hazard ratio (HR) of HBA copy number on time to first ischemic stroke.
Results: Four-hundred seventy-nine (5.3%) participants had an incident ischemic stroke over a median (IQR) of 11.0 (5.7, 14.0) years' follow-up. HBA copy number ranged from 2 to 6: 368 (4%) -α/-α, 2,480 (28%) -α/αα, 6,014 (67%) αα/αα, 83 (1%) ααα/αα, and 2 (<1%) ααα/ααα. The adjusted HR of ischemic stroke with HBA copy number was 1.04; 95%CI 0.89, 1.21; p = 0.66.
Conclusions: Although a reduction in HBA copy number is expected to increase endothelial nitric oxide signaling in the human vascular endothelium, HBA copy number was not associated with incident ischemic stroke in this large cohort of Black Americans.
People with African ancestry have greater stroke risk and greater heritability of stroke risk than people of other ancestries (Howard et al., 2011; Prapiadou et al., 2021). While some risk is explained by genetic variants shared between European and African populations, less is known about African-specific variants (Traylor et al., 2017). Alpha globin (HBA), which regulates endothelial nitric oxide (NO) signaling in resistance arteries, varies in gene copy number among people of African and Asian descent (Dozy et al., 1979; Straub et al., 2012). Genetic deletion of HBA is associated with protection from kidney disease, possibly through increased vascular NO signaling (Ruhl et al., 2022b).
NO plays an important role as a signaling molecule which regulates cerebrovascular blood flow both at rest and during injury (Garry et al., 2015). The regulation of cerebrovascular blood flow via NO occurs via two main mechanisms: (1) autoregulation to keep a consistent blood flow and (2) neurovascular coupling in which increased neuronal activity is influenced by the local regulation of cerebral blood flow. When there is reduced cerebral blood flow, for example during either an ischemic stroke or a hemorrhagic stroke, depletion of NO may exacerbate neuronal injury. However, the role of NO in the cerebral vasculature is complex and an overabundance of NO leading to significant vasodilation and lower cerebral perfusion pressure could potentially contribute to cerebral injury during stroke event. In addition, NO may impact the risk of developing vasospasm in the setting of cerebral hemorrhage (Siuta et al., 2013). Given the importance of NO in stroke (Garry et al., 2015; Woodhouse et al., 2015), and evidence that the alpha subunit of hemoglobin restricts nitric oxide release from vascular endothelial cells, we examined the association between HBA copy number and incident ischemic stroke in the Reasons for Geographic And Racial Differences in Stroke (REGARDS) cohort (Howard et al., 2005). We hypothesized that lower HBA copy number would be associated with lower risk of incident ischemic stroke.
REGARDS is a longitudinal cohort study designed to determine the reasons for racial disparities in stroke and cognitive decline in Black and White Americans aged ≥45 years (Howard et al., 2005). REGARDS enrolled 30,239 participants from the 48 continental United States from 2003 to 2007. All self-reported Black participants consenting to genetic research were included in this study (Figure 1). All participants provided oral and written informed consent. The REGARDS study was approved by the Institutional Review Boards of participating centers. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The analytic plan was pre-specified and approved by the REGARDS Publications Committee. Among 9,999 Black REGARDS participants with HBA and HBB genotyping available, 8,947 participants had no self-reported history of stroke at baseline and complete follow-up data (Figure 1).

Figure 1. Incident ischemic stroke and HBA copy number cohort study flow diagram. REGARDS, Reasons for Geographic and Racial Differences in Stroke (REGARDS) longitudinal cohort study; HBA, alpha globin gene; HBB, beta globin gene.
Incident ischemic stroke was defined as non-hemorrhagic stroke with a focal neurological deficit lasting ≥24 h confirmed by the medical record or a focal or non-focal neurological deficit with positive imaging confirmed with medical records in participants without a prior history of stroke. Genomic DNA was analyzed using droplet digital PCR (Ruhl et al., 2022b) to determine HBA copy number. The covariates age, sex, race, health insurance (yes or no), highest education level obtained (less than high school, high school, some college, college or more), annual income (≤ $20K, $20–34K, $35–74K, ≥$75K), and smoking status (categorized by never, past, or current smoker), history of hyperlipidemia, regular use of lipid lowering medication, and regular use of aspirin were self-reported. Self-reported use of lipid lowering medication was restricted to those also reporting hyperlipidemia. Region was defined as previously described in three geographic areas: stroke belt buckle, stroke belt, and stroke non-belt (Howard and Howard, 2020). Atrial fibrillation was defined by self-report of a physician diagnosis or ECG evidence. The presence of left ventricular hypertrophy (LVH) was defined by 12-lead ECG. Hypertension was defined as systolic blood pressure ≥130, diastolic blood pressure ≥80, or self-reported current medication use to control blood pressure. Chronic kidney disease (CKD) was defined by the 2021 CKD-Epi creatinine-cysteine equation and estimated glomerular filtration rate < 60 mL/min/1.73 m2, including those with end-stage kidney disease, and/or urine albumin to creatinine ratio ≥ 30 mg/g measured on urine collected during the baseline in-home examination. Fasting glucose levels ≥ 126 mg/dL, random glucose ≥ 200 mg/dL, or self-reported use of glucose-lowering medication was used to define diabetes mellitus. Hemoglobin, mean cell volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), and red-cell distribution width-coefficient of variation (RDW-CV) values were measured or calculated from blood collected during the in-home examination. The first 10 principal components of ancestry were calculated from Infinium Expanded Multi-Ethnic Genotyping Array data on 7,032 (79%) participants. HBA regulatory single nucleotide polymorphisms (SNPs) (rs11248850, rs11865131, and rs7203560) (Milton et al., 2013; Raffield et al., 2018) and a NOS3 SNP (rs1549758) (Mishra et al., 2022) were determined from these array data. The HBA regulatory SNPs rs11248850 and rs11865131 were in high linkage disequilibrium and were concordant in all but five participants. Therefore, we evaluated only rs11248850 and not rs11865131 in the models. The Framingham Stroke Risk Score (10-Year probability of stroke risk percentage) (McClure et al., 2014) and Atherosclerosis Risk in Communities Study (ARIC) Stroke Risk Score, 10-year probability of ischemic stroke risk (percentage) were calculated among those who self-reported at baseline never having had a stroke (Chambless et al., 2004).
Multivariable Cox proportional hazards regression modeling was used to estimate the hazard ratio of HBA copy number on time to first ischemic stroke. Covariates included age, sex, region, insurance status, education level, income, hypertension, atrial fibrillation, left ventricular hypertrophy, smoking status, and diabetes mellitus. Pre-specified tests of interaction between HBA copy number and age, sex, and sickle cell trait were performed on fully adjusted models. Pre-specified sensitivity analyses were performed by adding each of the following to the model: hemoglobin, CKD, both hemoglobin and CKD, regular aspirin use, regular statin use, the first 10 principal components of ancestry, and putative HBA regulatory single nucleotide polymorphisms rs11248850 and rs7203560. A post-hoc sensitivity analysis for the association of the rs1549758 NOS3 (nitric oxide synthase 3 or endothelial nitric oxide synthase) SNP with incident ischemic stroke was performed.
For the ischemic stroke time-to-event analysis, time to event was defined as the number of years between the initial in-home interview date and date D where D was the minimum of D1 and D2 which were defined as follows. D1 was the last follow-up date provided by REGARDS as the last time the participant was contacted for status. The outcome associated with this date was either “No Event” or “Death”. D2 was the date when the individual had an ischemic stroke. Individuals with a D2 date prior to their initial in-home interview were excluded from this analysis. Individuals with a D2 date after interview but prior to D1 time were recorded with an ischemic stroke event and D = D2. Individuals with D2 after D1 had stroke onset after their last REGARDS follow-up and they were recorded with “No Event” and censored at date D = D1. This decision to censor stroke events occurring after the end of REGARDS follow-up avoids bias associated with having extended follow-up only for one type of subgroup—those having a stroke event.
Missing data for the primary outcome, secondary outcomes, and explanatory variables were typically rare (< 0.5%) with some exceptions, e.g., hemoglobin values (Table 1). Multiple imputation methods were used in the multivariable analyses. The R package “mice” Version 3.14.0 was used to create and analyze the resulting imputations (Van Buuren and Goorthius-Oudshoom, 2011).
For diagnostic modeling of the model of ischemic stroke incidence using Cox proportional hazard techniques, Schoenfeld residuals were examined over follow-up time to detect violations of proportional hazards assumptions for the covariates in the analysis of ischemic outcomes. Examination of Schoenfeld residuals of the non-imputed and imputed data sets showed no suggestion of violation of proportional hazards for the regression covariates (p = 0.98).
For incident ischemic stroke, we performed an a priori power calculation based upon the Cox proportional-hazards model. We assumed an additive model where each additional HBA copy increased the hazard ratio for prevalent stroke by 25%. Given n = 479 incident ischemic stroke events observed among those without baseline self-reported stroke, and the distribution of alpha globin alleles, the power to reject the hypothesis of no linear trend in HBA copy number on the log of the hazard rate is ~77%. In contrast, there were only 62 incident hemorrhagic stroke events and power to reject a linear trend in HBA copy number on the log of the hazard rate was only 13%; therefore, analysis of hemorrhagic stroke was not performed.
Of the 8,947 REGARDs participants with available data (Figure 1), 479 (5.3%) participants had an incident ischemic stroke over a median (IQR) of 11.0 (5.7, 14.0) years' follow-up. Of these participants with incident ischemic stroke, 393 (82%) were diagnosed with a non-hemorrhagic stroke with a focal neurological deficit lasting ≥24 h and 86 (18%) were found to have a non-focal neurological deficit with positive imaging. There were 62 participants who developed incident hemorrhagic stroke. HBA gene copy number ranged from 2 to 6: 368 (4%) -α/-α, 2,480 (28%) -α/αα, 6,014 (67%) αα/αα, 83 (1%) ααα/αα, and 2 (< 1%) ααα/ααα (Table 1). The HR of ischemic stroke with HBA copy number, fully adjusted for age, sex, region, insurance status, education, income, hypertension, atrial fibrillation, LVH, smoking, and diabetes, was 1.04; 95%CI 0.89, 1.21; p = 0.66; (Table 2). There were no interactions between HBA copy number and age, sex, or sickle cell trait (Table 3). In pre-specified sensitivity analyses, the addition of hemoglobin, chronic kidney disease, aspirin use, statin use, or the first ten principal components of ancestry did not materially change the observed associations (Table 4). In a post-hoc sensitivity analysis we evaluated whether the putative NOS3 SNP was associated with incidence ischemic stroke in this population and no association was found (HR 1.03; 95%CI 0.84, 1.27; p = 0.79).
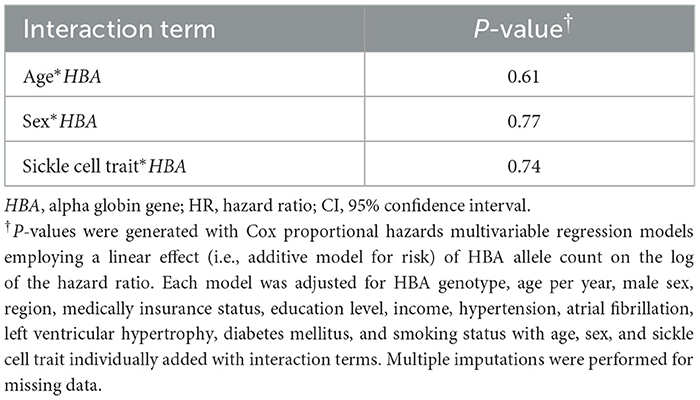
Table 3. Pre-specified tests for interaction between HBA genotype and age, sex, and sickle cell trait on incident ischemic stroke in fully adjusted models.
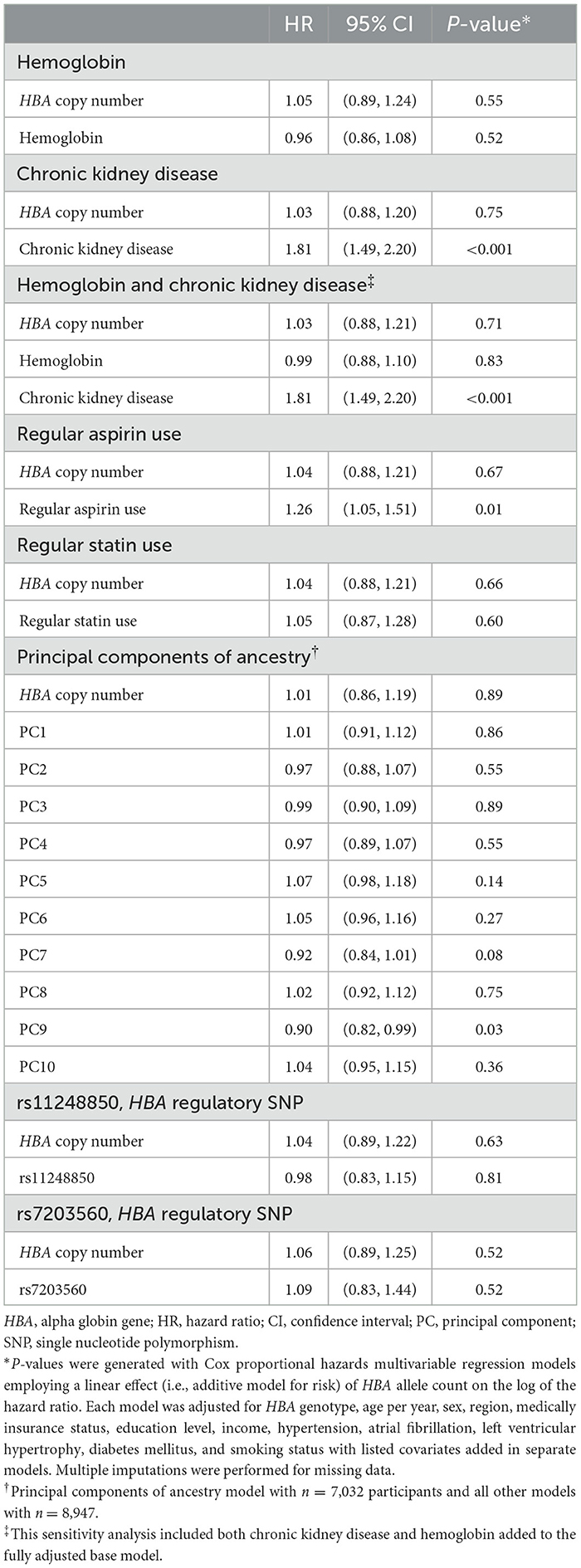
Table 4. Pre-specified sensitivity analyses for the association of HBA genotype with incident ischemic stroke—fully adjusted models with the separate addition of covariates for each model.
Although a reduction in HBA copy number was expected to increase endothelial nitric oxide signaling in the human vascular endothelium, HBA copy number was not associated with incident ischemic stroke in this large longitudinal cohort of Black Americans. This finding is consistent with a prior study demonstrating no association between HBA copy number and hypertension but differs from a study demonstrating an association with chronic and end-stage kidney disease in which HBA gene deletions are protective (Ruhl et al., 2022a,b). Together, these population studies suggest that the physiological roles of alpha globin may differ between the renal and cerebral vascular beds.
The strengths of this study include a robust measurement of HBA copy number with ddPCR with the analysis performed in a well-characterized, large, national cohort of Black Americans with clearly defined cerebrovascular outcomes followed for an extensive period. Moreover, we adjusted for key stroke risk factors including demographic, social, and biomedical variables to address the novel question of whether HBA copy number is associated with stroke risk. We explored the relevance of the NOS3 SNP rs1549758 and found no association between this SNP and incident ischemic stroke in a fully adjusted model. This replicates the finding of no association with stroke among participants with African ancestry in a prior GWAS study (Mishra et al., 2022). Given the absence of a significant main effect for either HBA CNV or the NOS3 SNP on incident ischemic stroke in this cohort, we did not pursue a model with both main effects nor did we test for interaction.
Our study has limitations, including that the population is limited to Black Americans and the associations of HBA copy number and ischemic stroke risk may vary in other populations with HBA copy number variation outside of the U.S. We did not have sufficient power to evaluate other stroke subtypes such as hemorrhagic stroke, though increases in vascular NO signaling could affect bleeding risk through its effects on platelets. The association of HBA copy number with hemorrhagic stroke risk could be evaluated in other populations that have higher rates of hemorrhagic stroke, such as in sub-Saharan Africa (Akinyemi et al., 2021).
In a large longitudinal cohort of Black American adults, we found that HBA copy number was not associated with incident ischemic stroke.
The data analyzed in this study is subject to the following licenses/restrictions: Data are available from the University of Alabama at Birmingham. Requests to access these datasets should be directed to cmVnYXJkc2FkbWluQHVhYi5lZHU=.
The Institutional Review Board of the University of Alabama at Birmingham, the lead study center, approved this study. The REGARDS study was also approved by the Institutional Review Boards of participating centers. The patients/participants provided their written informed consent to participate in this study.
AR, SB, and HA conceived and designed the study and drafted the first version of the manuscript. YY, BM, and CW performed sample analyses. NJ designed and implemented the statistical analyses. All authors contributed to data interpretation and revised and approved the manuscript.
This is an ancillary study supported by Cooperative Agreement U01 NS041588 (MC, VH, and GH) co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. This research was supported in part by the Divisions of Intramural Research, National Institute of Allergy and Infectious Diseases Project AI001150 (AR and HA), National Heart, Lung, and Blood Institute (NHLBI) Project HL006196 (AR, YY, SB, LP, and HA). This work was also funded in part by the National Cancer Institute (NCI) Intramural Research Program Under Contract HHSN26120080001E (CW), and the NHLBI Grants K08HL12510 (RN) and K08HL096841 (NZ).
CW, GH, MI, and HA declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor HH declared a past collaboration with the authors SJ, VH, GH, MI, and MC.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS, NIA, NIAID, NCI, or NHLBI. The content of this publication does not necessarily reflect the view or policy of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the government. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. Government.
Akinyemi, R. O., Ovbiagele, B., Adeniji, O. A., Sarfo, F. S., Abd-Allah, F., Adoukonou, T., et al. (2021). Stroke in Africa: profile, progress, prospects and priorities. Nat. Rev. Neurol. 17, 634–656. doi: 10.1038/s41582-021-00542-4
Chambless, L. E., Heiss, G., Shahar, E., Earp, M. J., and Toole, J. (2004). Prediction of ischemic stroke risk in the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 160, 259–269. doi: 10.1093/aje/kwh189
Dozy, A. M., Kan, Y. W., Embury, S. H., Mentzer, W. C., Wang, W. C., Lubin, B., et al. (1979). alpha-Globin gene organisation in blacks precludes the severe form of alpha-thalassaemia. Nature. 280, 605–607. doi: 10.1038/280605a0
Garry, P. S., Ezra, M., Rowland, M. J., Westbrook, J., and Pattinson, K. T. S. (2015). The role of the nitric oxide pathway in brain injury and its treatment—From bench to bedside. Exp. Neurol. 263, 235–243. doi: 10.1016/j.expneurol.2014.10.017
Howard, G., Cushman, M., Kissela, B. M., Kleindorfer, D. O., McClure, L. A., Safford, M. M., et al. (2011). Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke. 42, 277. doi: 10.1161/STROKEAHA.111.625277
Howard, G., and Howard, V. J. (2020). Twenty years of progress toward understanding the stroke belt. Stroke. 51, 742–750. doi: 10.1161/STROKEAHA.119.024155
Howard, V. J., Cushman, M., Pulley, L., Gomez, C. R., Go, R. C., Prineas, R. J., et al. (2005). The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 25, 678. doi: 10.1159/000086678
McClure, L. A., Kleindorfer, D. O., Kissela, B. M., Cushman, M., Soliman, E. Z., Howard, G., et al. (2014). Assessing the performance of the Framingham stroke risk score in the reasons for geographic and racial differences in stroke cohort. Stroke. 45, 1716–1720. doi: 10.1161/STROKEAHA.114.004915
Milton, J. N., Rooks, H., Drasar, E., McCabe, E. L., Baldwin, C. T., Melista, E., et al. (2013). Genetic determinants of haemolysis in sickle cell anaemia. Br. J. Haematol. 161, 270–278. doi: 10.1111/bjh.12245
Mishra, A., Malik, R., Hachiya, T., Jürgenson, T., Namba, S., Posner, D. C., et al. (2022). Stroke genetics informs drug discovery and risk prediction across ancestries. Nature. 611, 115–123. doi: 10.1038/s41586-022-05165-3
Prapiadou, S., Demel, S. L., and Hyacinth, H. I. (2021). Genetic and genomic epidemiology of stroke in people of African ancestry. Genes. 12, 1825. doi: 10.3390/genes12111825
Raffield, L. M., Ulirsch, J. C., Naik, R. P., Lessard, S., Handsaker, R. E., Jain, D., et al. (2018). Common α-globin variants modify hematologic and other clinical phenotypes in sickle cell trait and disease. PLoS Genet. 14, e1007293. doi: 10.1371/journal.pgen.1007293
Ruhl, A. P., Jeffries, N., Yang, Y., Gutierrez, O. M., Muntner, P., Naik, R. P., et al. (2022a). Alpha globin gene copy number and hypertension risk among Black Americans. PLoS ONE. 17, e0271031. doi: 10.1371/journal.pone.0271031
Ruhl, A. P., Jeffries, N., Yang, Y., Naik, R. P., Patki, A., Pecker, L. H., et al. (2022b). Alpha globin gene copy number is associated with prevalent chronic kidney disease and incident end-stage kidney disease among Black Americans. J. Am. Soc. Nephrol. 33, 213–224. doi: 10.1681/ASN.2021050653
Siuta, M., Zuckerman, S. L., and Mocco, J. (2013). Nitric oxide in cerebral vasospasm: theories, measurement, and treatment. Neurol. Res. Int. 2013, 972417. doi: 10.1155/2013/972417
Straub, A. C., Lohman, A. W., Billaud, M., Johnstone, S. R., Dwyer, S. T., Lee, M. Y., et al. (2012). Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature. 491, 473–477. doi: 10.1038/nature11626
Traylor, M., Rutten-Jacobs, L., Curtis, C., Patel, H., Breen, G., Newhouse, S., et al. (2017). Genetics of stroke in a UK African ancestry case-control study: South London Ethnicity and Stroke Study. Neurol. Genet. 3, e142. doi: 10.1212/NXG.0000000000000142
Van Buuren, S., and Goorthius-Oudshoom, K. (2011). mice: multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67. doi: 10.18637/jss.v045.i03
Keywords: alpha globin, alpha thalassaemia, ischemic stroke, hemoglobin, nitric oxide, endothelial nitric oxide synthase
Citation: Ruhl AP, Jeffries N, Yang Y, Brooks SD, Naik RP, Pecker LH, Mott BT, Winkler CA, Armstrong ND, Zakai NA, Gutierrez OM, Judd SE, Howard VJ, Howard G, Irvin MR, Cushman M and Ackerman HC (2023) Alpha globin gene copy number and incident ischemic stroke risk among Black Americans. Front. Stroke 2:1192465. doi: 10.3389/fstro.2023.1192465
Received: 23 March 2023; Accepted: 26 May 2023;
Published: 15 June 2023.
Edited by:
Hyacinth Idu Hyacinth, University of Cincinnati, United StatesReviewed by:
Fenella Jane Kirkham, University College London, United KingdomCopyright © 2023 Ruhl, Jeffries, Yang, Brooks, Naik, Pecker, Mott, Winkler, Armstrong, Zakai, Gutierrez, Judd, Howard, Howard, Irvin, Cushman and Ackerman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: A. Parker Ruhl, cGFya2VyLnJ1aGxAbmloLmdvdg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.