- 1Carnegie School of Sport, Leeds Beckett University, Leeds, United Kingdom
- 2Department of Physical Education & Sport Sciences, University of Limerick, Limerick, Ireland
Editorial on the Research Topic
Preventing sarcopenia and promoting musculoskeletal health in middle-aged adults: the role of exercise and nutrition
Sarcopenia, once considered an inevitable consequence of ageing, is now recognised as a complex syndrome influenced by lifestyle, disease, and acute physiological stress. As global life expectancy rises, its prevalence is increasing, placing a burden on healthcare systems (1, 2) due to its association with disability, frailty, and comorbidities (3). Prevalence estimates range from 0.2% to 86.5% depending on diagnostic criteria (4). While typically studied in older adults, evidence suggests an earlier onset, with rates between 8% and 36% in those younger than 60 years and between 10% and 27% in those aged 60 years and older (4). This variability partly reflects differences in classification, with the European Working Group on Sarcopenia in Older People (EWGSOP2) (5) defining primary sarcopenia (driven by ageing) and secondary sarcopenia (driven by disease, inactivity, or malnutrition), each of which poses distinct diagnostic challenges.
Acute sarcopenia, which refers to rapid muscle loss following illness or hospitalisation, has gained attention but remains inconsistently defined. Before Welch et al. (6) introduced the term “acute sarcopenia”, this phenomenon was typically defined as disease-related muscle wasting. EWGSOP2 (2019) (5) incorporated acute sarcopenia in its diagnostic framework, but its clinical application remains limited. A recent systematic review by Aldrich et al. (7) found that acute sarcopenia develops in 18% of hospitalised patients, reaching 59% in intensive care settings; however, current diagnostic criteria may underestimate muscle deterioration, impacting recovery, quality of life, and long-term health outcomes.
Despite ongoing efforts by multiple working groups (8), variability in definitions and diagnostic criteria hinders clinical recognition and intervention. Strengthening collaboration between organisations such as EWGSOP2 and the Global Leadership Initiative on Sarcopenia remains critical to advancing standardised diagnostic frameworks.
Early intervention: a critical window for prevention
While diagnostic precision is important, prevention must not be delayed, particularly in middle-aged adults, where early intervention can prevent muscle loss. Midlife is a key period for musculoskeletal health, with declines in neuromuscular efficiency (9), physical activity (10), hormonal alterations (e.g., oestrogen) (11), and poor dietary habits accelerating muscle loss (12, 13). Gender-specific barriers also influence the risk of sarcopenia, particularly in women during the perimenopausal phase (14, 15). Developing inclusive, accessible resistance training (RT) programmes tailored to women's needs – addressing social, cultural, and practical barriers – could improve long-term engagement.
A summary of the key intervention strategies and modifying factors for the prevention of sarcopenia in midlife is presented in Figure 1. This Research Topic explored key lifestyle approaches, including nutrition, exercise, and gut-muscle interactions, offering solutions for sustaining musculoskeletal health through early, targeted intervention.
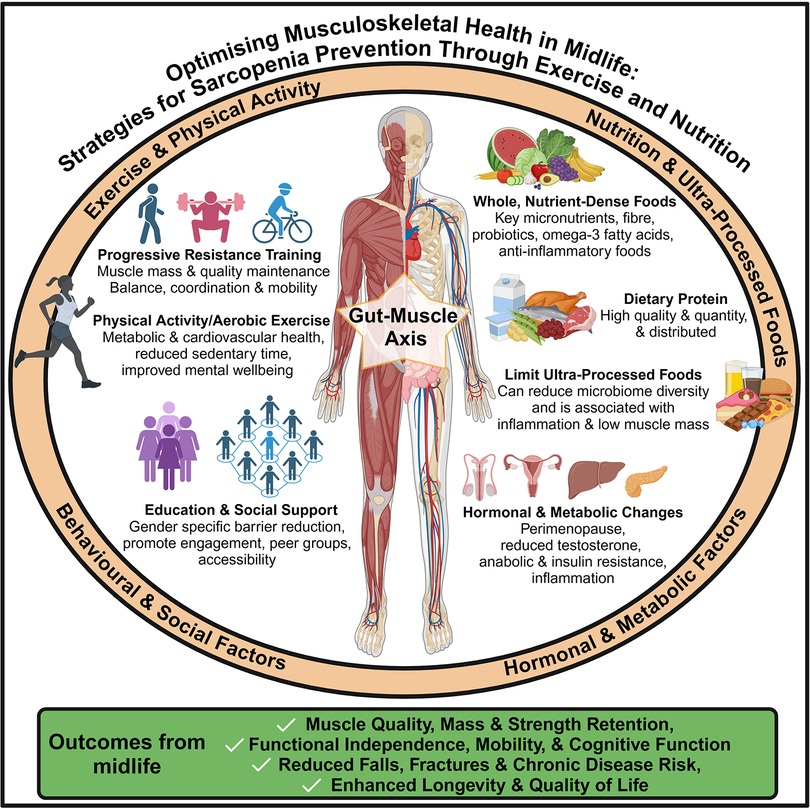
Figure 1. Lifestyle and behavioural strategies—including exercise, nutrition, and social factors—to support musculoskeletal health and prevent sarcopenia in midlife.
Ultra-processed foods (UPFs) and muscle deterioration
Diet is fundamental to muscle health, but modern dietary patterns are increasingly dominated by UPFs, which may accelerate muscle loss. Kong et al. investigated the association between ultra-processed food (UPF) consumption and muscle health, revealing that higher UPF intake was significantly associated with low muscle mass in young to middle-aged adults. These findings reinforce the need to prioritise whole, nutrient-dense foods to reduce early muscle deterioration.
However, not all UPFs are inherently harmful. High-protein products, such as protein shakes and bars, fall into the UPF category but can support protein intake when consumed as part of a balanced diet. Alternatives, such as high-protein yoghurts containing probiotics, may also promote gut health. Future research should assess diet quality holistically, considering both benefits and risks when evaluating UPFs in muscle health strategies.
The gut-muscle axis: a novel target for sarcopenia prevention
Emerging research highlights the gut-muscle axis as a key regulator of muscle health, linking gut microbiota to metabolism, inflammation, and function. Microbiome-targeted interventions may complement exercise and nutrition, offering therapeutic potential beyond sarcopenia prevention (16).
Li et al. examined exercise-induced microbiome shifts and suggested that they might enhance muscle protein synthesis and metabolic resilience. Furthermore, diet critically influences gut health, with UPFs [associated with low muscle mass (Li et al.)] shown to reduce microbial α-diversity and increase proinflammatory bacteria (17, 18). By linking gut health to muscle function, new holistic health strategies may help mitigate muscle loss, metabolic disease, autoimmune disease, and chronic inflammation. While promising, causal mechanisms remain unclear, and future research must determine whether microbiome modulation – through diet, probiotics, or exercise – can offer viable therapeutic approaches for sarcopenia and broader health outcomes.
The protein paradox: why diet alone is insufficient
While protein remains crucial, diet alone is insufficient without exercise. Research shows that muscle protein synthesis declines with age, necessitating higher protein intake thresholds to stimulate an adequate anabolic response. Reviews highlight protein quality, timing, and distribution as key factors in maximising muscle preservation (19, 20).
Schalla et al. found that high-protein diets had minimal impact on muscle mass and strength in physically active middle-aged adults, reinforcing the need for an integrated approach combining diet and RT. This highlights a gap in current nutritional recommendations, where protein supplementation is often prioritised over exercise (i.e., RT), limiting its efficacy in the prevention of sarcopenia.
Exercise as a primary prevention strategy
Of all interventions, exercise (i.e., RT) remains the most effective strategy for preventing muscle loss and function. Li et al. conducted a network meta-analysis that identified RT as the most effective approach to counteracting sarcopenia, particularly in clinical populations.
Despite strong evidence supporting RT, participation remains low, with global participation rates of 18%–35% in men and 14%–26% in women (14). Common barriers, especially among midlife women, include perceived time constraints, lack of knowledge and education, exercise modality preferences, and social factors (15). Addressing these barriers through education, community-based programmes, and gender-inclusive training environments is crucial to improving adherence and maximising public health benefits.
Conclusion
Despite the growing recognition of sarcopenia as a midlife health concern, prevention efforts remain fragmented. This collection highlights the urgent need for integrated, proactive strategies targeting modifiable lifestyle factors – diet, resistance exercise, and gut health – before significant muscle loss occurs.
Public health initiatives must prioritise early intervention with a coordinated effort to educate healthcare professionals, policymakers, and the public on the importance of exercise and nutrition for muscle health. While resistance exercise remains the most effective intervention, complementary nutritional strategies should focus on reducing UPF consumption, promoting gut health, and ensuring adequate, high-quality protein intake to maintain muscle.
Policymakers and healthcare systems must invest in accessible, evidence-based interventions tailored to midlife adults, embedding sarcopenia prevention within public health frameworks. Future research should enhance the accessibility, adherence, and long-term sustainability of interventions, making sarcopenia prevention a midlife priority rather than an afterthought.
Author contributions
TI: Writing – original draft, Investigation, Conceptualization, Supervision, Writing – review & editing. CN: Conceptualization, Writing – review & editing, Investigation. DM: Conceptualization, Investigation, Writing – review & editing, Visualization.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bruyère O, Beaudart C, Ethgen O, Reginster JY, Locquet M. The health economics burden of sarcopenia: a systematic review. Maturitas. (2019) 119:61–9. doi: 10.1016/j.maturitas.2018.11.003
2. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. (2017) 12:e0169548. doi: 10.1371/journal.pone.0169548
3. Laskou F, Fuggle NR, Patel HP, Jameson K, Cooper C, Dennison E. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the hertfordshire cohort study. J Cachexia Sarcopenia Muscle. (2022) 13:220–9. doi: 10.1002/jcsm.12870
4. Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:86–99. doi: 10.1002/jcsm.12783
5. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
6. Welch C, Hassan-Smith ZK, Greig CA, Lord JM, Jackson TA. Acute sarcopenia secondary to hospitalisation - an emerging condition affecting older adults. Aging Dis. (2018) 9:151–64. doi: 10.14336/ad.2017.0315
7. Aldrich L, Ispoglou T, Prokopidis K, Alqallaf J, Wilson O, Stavropoulos-Kalinoglou A. Acute sarcopenia: systematic review and meta-analysis on its incidence and muscle parameter shifts during hospitalisation. J Cachexia Sarcopenia Muscle. (2025) 16:e13662. doi: 10.1002/jcsm.13662
8. Kirk B, Cawthon PM, Arai H, Ávila-Funes JA, Barazzoni R, Bhasin S, et al. The conceptual definition of sarcopenia: Delphi consensus from the global leadership initiative in sarcopenia (GLIS). Age Ageing. (2024) 53:1–10. doi: 10.1093/ageing/afae052
9. Roberts BM, Lavin KM, Many GM, Thalacker-Mercer A, Merritt EK, Bickel CS, et al. Human neuromuscular aging: sex differences revealed at the myocellular level. Exp Gerontol. (2018) 106:116–24. doi: 10.1016/j.exger.2018.02.023
10. Ramsey KA, Rojer AGM, D’Andrea L, Otten RHJ, Heymans MW, Trappenburg MC, et al. The association of objectively measured physical activity and sedentary behavior with skeletal muscle strength and muscle power in older adults: a systematic review and meta-analysis. Ageing Res Rev. (2021) 67:101266. doi: 10.1016/j.arr.2021.101266
11. Collins BC, Laakkonen EK, Lowe DA. Aging of the musculoskeletal system: how the loss of estrogen impacts muscle strength. Bone. (2019) 123:137–44. doi: 10.1016/j.bone.2019.03.033
12. Otsuka R, Nishita Y, Tange C, Tomida M, Kato Y, Imai T, et al. Age-related 12-year changes in dietary diversity and food intakes among community-dwelling Japanese aged 40 to 79 years. J Nutr Health Aging. (2018) 22:594–600. doi: 10.1007/s12603-018-0999-3
13. Rippin HL, Hutchinson J, Jewell J, Breda JJ, Cade JE. Adult nutrient intakes from current national dietary surveys of European populations. Nutrients. (2017) 9:1288. doi: 10.3390/nu9121288
14. Nuzzo JL. Sex difference in participation in muscle-strengthening activities. J Lifestyle Med. (2020) 10:110–5. doi: 10.15280/jlm.2020.10.2.110
15. Stimson AM, Anderson C, Holt A-M, Henderson AJ. Why don't women engage in muscle strength exercise? An integrative review. Health Promot J Austr. (2024) 35:911–23. doi: 10.1002/hpja.857
16. Barry DJ, Wu SSX, Cooke MB. The relationship between gut microbiota, muscle mass and physical function in older individuals: a systematic review. Nutrients. (2025) 17:81. doi: 10.3390/nu17010081
17. Rondinella D, Raoul PC, Valeriani E, Venturini I, Cintoni M, Severino A, et al. The detrimental impact of ultra-processed foods on the human gut microbiome and gut barrier. Nutrients. (2025) 17:859. doi: 10.3390/nu17050859
18. Cuevas-Sierra A, Milagro FI, Aranaz P, Martínez JA, Riezu-Boj JI. Gut microbiota differences according to ultra-processed food consumption in a Spanish population. Nutrients. (2021) 13:1–20. doi: 10.3390/nu13082710
19. Ispoglou T, Wilson O, McCullough D, Aldrich L, Ferentinos P, Lyall G, et al. A narrative review of non-pharmacological strategies for managing sarcopenia in older adults with cardiovascular and metabolic diseases. Biology (Basel). (2023) 12:892. doi: 10.3390/biology12070892
Keywords: sarcopenia, resistance training, ultra-processed foods, gut-muscle axis, middle-aged adults, muscle protein synthesis, anabolic resistance, gender-specific barriers
Citation: Ispoglou T, Norton C and McCullough D (2025) Editorial: Preventing sarcopenia and promoting musculoskeletal health in middle-aged adults: the role of exercise and nutrition. Front. Sports Act. Living 7:1601326. doi: 10.3389/fspor.2025.1601326
Received: 27 March 2025; Accepted: 31 March 2025;
Published: 11 April 2025.
Edited and Reviewed by: David Christopher Nieman, Appalachian State University, United States
Copyright: © 2025 Ispoglou, Norton and McCullough. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Theocharis Ispoglou, dC5pc3BvZ2xvdUBsZWVkc2JlY2tldHQuYWMudWs=
 Theocharis Ispoglou
Theocharis Ispoglou Catherine Norton
Catherine Norton Deaglan McCullough
Deaglan McCullough