- 1College of General Education, Tongmyong University, Busan, Republic of Korea
- 2Department of Physiology, Dong-A University College of Medicine, Busan, Republic of Korea
- 3College of Arts and Sports, Dong-A University, Busan, Republic of Korea
- 4Waseda Institute for Sport Sciences, Waseda University, Saitama, Japan
Background: Sedentary lifestyles in older individuals are associated with reduced physical function and an increased risk of metabolic diseases such as type 2 diabetes. Physical exercise can enhance muscle mass, insulin sensitivity, and metabolic health. Taekwondo, a martial art that integrates both aerobic and resistance components, may improve strength, balance, and metabolic health in older individuals. This study investigated the effect of long-term Taekwondo training on thigh muscle cross-sectional area, health related physical fitness, and metabolic indicators in sedentary older women.
Methods: Seventeen participants (aged 65 years and older, sedentary time 8 h and more per day) were randomly assigned to a Taekwondo group (n = 9) and a control group (n = 8). Outcomes, including thigh muscle cross-sectional area, health-related physical fitness, Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), and Glucagon-like peptide-1 (GLP-1) were measured before and after the Taekwondo program. The Taekwondo group underwent 60 min of training, three times per week for 12 weeks. Variable changes over time and between groups were analyzed using two-way repeated measures ANOVA performed for significant interactions.
Results: The Taekwondo group exhibited a significant reduction in body weight, body mass index, body fat, and mean arterial blood pressure (p < 0.05), as well as increased thigh muscle cross-sectional area, lean body mass and lower limb muscle mass (p < 0.05). Improvements in balance and gait speed, stride were observed (p < 0.05), indicating reduced fall risk and enhanced mobility. Laboratory analyses revealed reduced triglyceride and free fatty acids and elevated HDL-cholesterol and GLP-1 levels (p < 0.05). Increased thigh muscle cross-sectional area was inversely correlated with fasting glucose, insulin, and HOMA-IR, suggesting improved insulin sensitivity and glucose regulation.
Conclusion: Long-term Taekwondo training improved thigh muscle cross-sectional area, health-related physical fitness and insulin resistance markers in sedentary older women, providing evidence for its use as an effective intervention to promote metabolic health in this population.
1 Introduction
The global average life expectancy was 73.6 years in 2022 and is projected to increase to 78.1 years by 2050 (1). However, as life expectancy increases, the health of the older individual population has emerged as a significant social issue and is recognized as a primary factor contributing to a decline in quality of life (2).
A sedentary lifestyle has a deleterious impact on the general health of older individuals (3, 4). Recent studies have also demonstrated that sedentary behavior negatively impacts thigh muscle cross-sectional area (CSA), leading to accelerated muscle atrophy and reduced functional capacity (5, 6). This is also, associated with an increased risk of metabolism-related conditions such as insulin resistance, metabolic syndrome, and type 2 diabetes, musculoskeletal conditions such as knee pain, sarcopenia and osteoporosis (7–9). In contrast, individuals who engage in reduced sedentary behavior, light physical activities, or simple muscle-strengthening exercises have been reported to exhibit superior health outcomes (10–13). More importantly, exercise can optimize these effects, which is crucial in reducing insulin resistance and preventing metabolic syndrome in older individuals (14, 15). Addressing these issues through targeted exercise interventions is essential for maintaining independence and overall well-being in aging populations.
Aerobic and anaerobic exercise have been independently demonstrated to enhance insulin sensitivity and facilitate glucose homeostasis, contributing to improved glycated hemoglobin (HbA1c) levels (16–18). Furthermore, exercise-induced increases in glucagon-like peptide-1 (GLP-1) levels play a significant role in improving insulin resistance (19). These changes have beneficial effects on muscle mass and insulin resistance, which contribute to overall metabolic health.
Thigh muscle mass is a crucial indicator of physical function and health status in older individuals, as it is directly associated with overall health (20). Thigh muscles comprise approximately 50%–80% of total body muscle mass and are essential for maintaining overall health, balance, and gait stability (21). In older adults, a reduction in thigh muscle mass is associated with an elevated risk of falls, diminished strength, increased prevalence of chronic disease, insulin resistance, and diminished quality of life (8, 22). Exercise that increases thigh muscle mass is effective in addressing these issues, thereby enhancing the overall physical function of older individuals (23).
Taekwondo, a widely accessible martial art in Korea, confers advantageous effects on physical function, and body composition in older individuals (24–27). As a combined aerobic and anaerobic exercise, Taekwondo, can contribute to improvements not only in strength and balance, but also cardiorespiratory endurance (24, 25, 28). The dynamic movements, including kicks, postural shifts, and coordinated footwork, provide significant muscle engagement that stimulates strength gains, particularly in the lower body (29).
Consequently, this can help prevent and improve the risk factors for sarcopenia (30), thus promoting the overall health of older individuals. The present study aims to assess the effects of regular and long-term Taekwondo training on thigh muscle CSA, health-related physical fitness, HbA1c, and GLP-1 levels in sedentary older women, providing valuable insights into the potential of martial arts as a multifaceted approach to healthy aging.
2 Materials and methods
2.1 Study participants
The participants were female residents aged 65 years or older from Busan city, Republic of Korea, who had no limitations in physical activity by a physician. A total of 26 participants were recruited and randomly assigned to either the Taekwondo training group (n = 13) or a control group (n = 13) using simple randomization via a computer-generated random sequence (https://www.ranmomizer.org).
Sedentary time was assessed using a wrist-worn PA monitor (Polar Active, Polar Electro, Finland), which participants were instructed to wear continuously for one week prior to the intervention. To classify sedentary behavior, an energy expenditure threshold of <1.5 METs was applied, following established guidelines for sedentary behavior assessment (31).
In the final analysis, 17 participants were included, while eight were excluded due to sedentary time non-eligibility (n = 2), relocation (n = 1), medical conditions (n = 1), attendance below 95% of the scheduled sessions (n = 4), and loss to follow-up (n = 1). Consequently, the analysis was conducted using data from 17 participants who fully adhered to the intervention and assessments, with 9 in the Taekwondo group and 8 in the control group. Participants who remained in the study attended at least 95% of the scheduled sessions. No serious injuries were reported during the intervention; however, some participants experienced minor discomfort, such as muscle soreness. Participants taking medication were instructed to maintain their usual lifestyle, including diet and physical activity habits, to ensure consistency. Baseline characteristics of the study participants are presented in Table 1.
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Review Board of D University (IRB No. 2-1040709-AB-N-01-201904-HR-022-04). Written informed consent was obtained from all participants.
2.2 Measurement of body composition
Body composition was evaluated using a body composition analyzer (VENUS 5.5, Jawon Medical Co., Korea) to ascertain height, weight, lean body mass (LBM), and percent body fat. Waist and hip circumferences were measured with a Martin-type measuring tape, and the waist-hip ratio (WHR) was calculated using the following formula: waist circumference divided by hip circumference. Blood pressure was determined using a mercury-free sphygmomanometer (CK-E301, Chin Kow Medical Instrument Co., Ltd., Taiwan), and mean arterial pressure (MAP) was calculated according to the formula MAP = diastolic blood pressure + [(systolic blood pressure − diastolic blood pressure)/3], as previously reported (32).
2.3 Thigh muscle cross-sectional area
The thigh muscle CSA was evaluated via computed tomography (CT) using a CT Max II scanner (General Electric Co., USA). The scan was conducted in a transverse plane at the umbilicus level, specifically crossing the midpoint between the upper border of the patella and the greater trochanter. The mass of the thigh muscles was defined by regions with Hounsfield numbers ranging from −49 to +100, as described by Choi et al. (33).
2.4 Health-related physical fitness
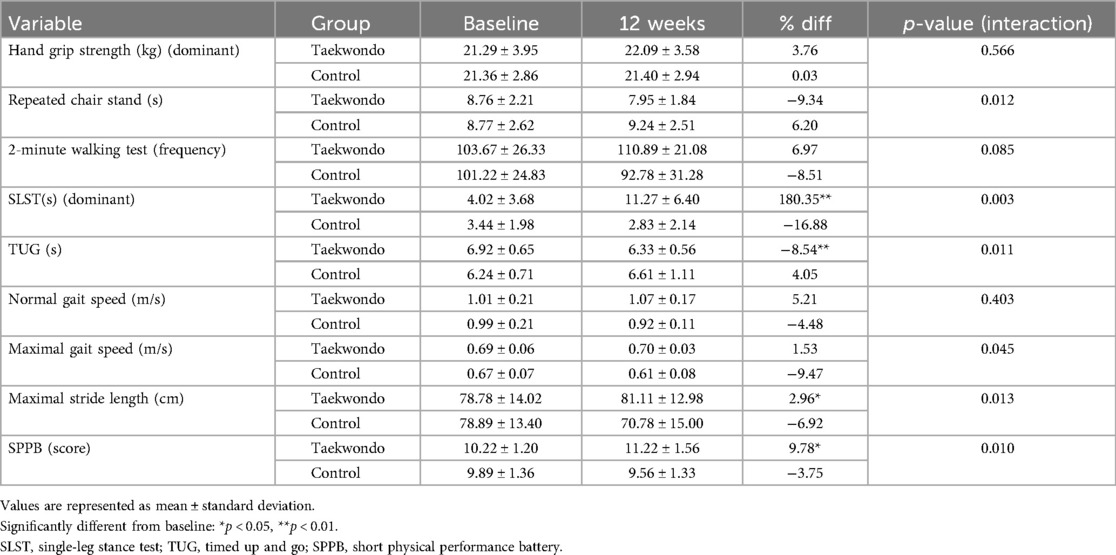
HRPF was assessed using a set of physical fitness tests, including hand grip strength (34), the 2-minute walk test (35), the open-eyed single-leg stance test (SLST) (36), the timed up-and-go (TUG) test (37), the 4-m gait speed test (38), maximal stride length (39), and the short physical performance battery (SPPB) (40).
2.5 Blood collection and laboratory assays
Blood samples were collected and analyzed under standardized procedures. To minimize any circadian rhythm variations, all blood samples were collected in the morning hours (between 08:00 and 11:00 h). Participants were instructed to fast for at least 10–12 h before sample collection. Upon arrival at the laboratory, 15 ml of venous blood was collected from the antecubital vein of each participant.
For the analysis of total cholesterol, triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and fasting blood glucose (FBG) levels, blood was collected into tubes containing clotting activators for serum isolation. The samples were allowed to clot for 45 min at room temperature and centrifuged at 3,000 rpm for 10 min at 4 °C. After centrifugation, serum was dispensed into plain microtubes and stored at −80 °C until analysis. These biomarkers were measured using enzymatic methods (Cobas c702, Roche Diagnostics, Basel, Switzerland).
Insulin levels were quantified using an electrochemiluminescence immunoassay (Elecsys Insulin Kit, Roche Diagnostics, Basel, Switzerland). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated according to the formula [fasting insulin [μU/ml] × fasting plasma glucose [mg/dl]/405], as proposed by Matthews et al. (41). HbA1c levels were determined using an automated glycohemoglobin analyzer (HLC-723G8, Tosoh Corporation, Tokyo, Japan), and GLP-1 levels were assessed using enzyme-linked immunosorbent assays (ELISA Kit, R&D Systems, Minneapolis, MN, USA).
2.6 Taekwondo training program
The Taekwondo training program was adapted and modified from the recommendations set forth by Jung et al. (28) and was based on the American College of Sports Medicine's (ACSM) frequency, intensity, type, and time recommendations for older individuals (42–44). Each session consisted of a 10-min warm-up, 40-min main exercise, and 10 min cool-down, conducted three times per week for 12 weeks. The primary exercise consisted of fundamental Taekwondo techniques and kicking maneuvers executed with Thera-Bands for resistance training. In the initial 4-week period, the participants engaged in the practice of fundamental movements. During the subsequent 8-week phase, they performed Taekwondo movements with a yellow Thera-Band. In the final 4-week period, they utilized a red Thera-Band, progressively elevating the intensity of exercises. In accordance with the ACSM (45), the intensity of the exercise was increased at 4-week intervals, and the Rating of Perceived Exertion (RPE) was evaluated according to Borg (46). To ensure participant safety and adherence, adjustments were made when necessary, including modifying the range of motion or reducing the number of repetitions based on individual conditions. All modifications were implemented under the supervision of exercise professionals. The specifics of the Taekwondo training program are outlined in Table 2.
2.7 Statistical analysis
All variables were analyzed using SPSS version 22.0 for Windows (IBM, Chicago, IL, USA). Descriptive statistics, including means and standard deviations, were calculated for all variables.
The homogeneity of baseline characteristics between the Taekwondo and control groups was assessed using either an independent t-test or the Mann–Whitney U test, depending on the data distribution. To evaluate between-group differences over time, a two-way repeated measures ANOVA was conducted. Spearman's rank correlation coefficient was employed to determine inter-variable relationships, and linear regression analysis was conducted to examine scatter plots. Statistical significance was set at p < 0.05.
3 Results
3.1 Thigh muscle cross-sectional area and body composition
As indicated in Table 3, the results of thigh muscle CSA and body composition are as follows. The Taekwondo group exhibited a significant reduction in body mass (p < 0.05), body mass index (BMI) (p < 0.01), percent body fat (p < 0.05), and MAP (p < 0.05) after 12 weeks. Conversely, there was a significant increase in thigh muscle CSA (p < 0.01), appendicular skeletal muscle mass (ASM) (p < 0.05). Furthermore, the interaction between-group and time was significant for thigh muscle CSA (p = 0.013), body mass (p = 0.007), BMI (p < 0.001), LBM (p = 0.013), percent body fat (p = 0.010), WHR (p = 0.040), LLM (p = 0.025), ASM (p = 0.008), and MAP (p = 0.015).
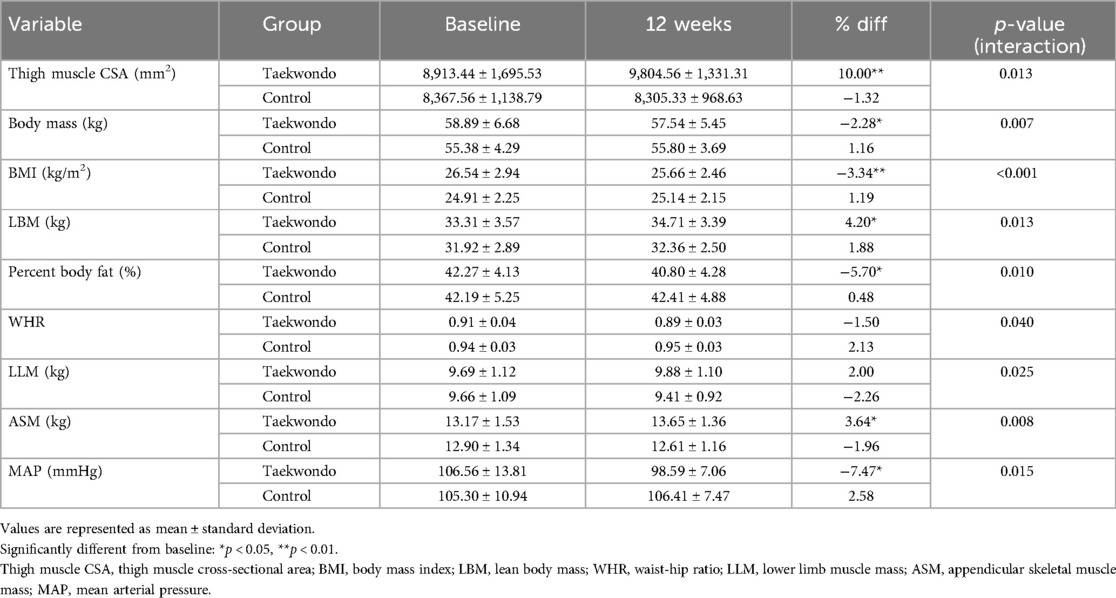
Table 3. Changes in thigh muscle cross-sectional area, body composition between groups at baseline and after 12 weeks.
As shown in Figure 1, the Taekwondo group exhibited a significant reduction in body fat percentage compared to baseline (p < 0.05), whereas the control group showed no significant changes. Additionally, thigh muscle CSA significantly increased in the Taekwondo group (p < 0.01), while the control group exhibited a non-significant decrease. Significant interaction effects were observed for both variables (p < 0.05).
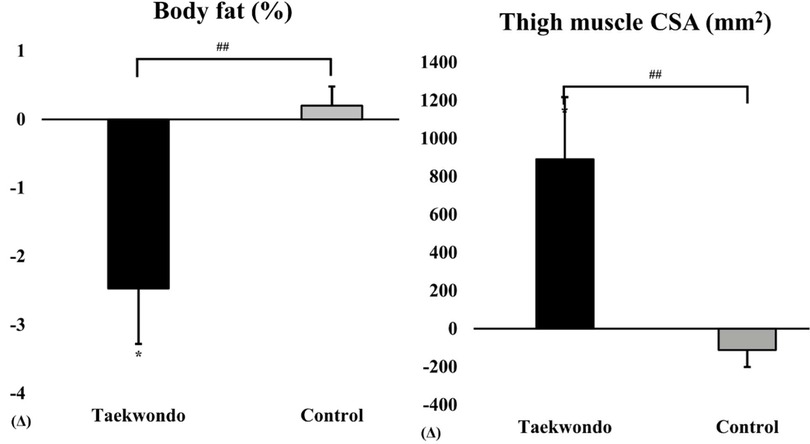
Figure 1. Comparison of body fat (%) and thigh muscle CSA between baseline and after 12 weeks in taekwondo program. #: p-value (interaction) ##p < 0.01. *: p-value (time) *p < 0.05. Δ: Post–Pre. Thigh muscle CSA, thigh muscle cross-sectional area.
3.2 Health-related physical fitness
HRPF and SPPB outcomes are presented in Table 4. In the Taekwondo group, significant post-intervention improvements were observed in the SLST (p < 0.01), maximal stride length (p < 0.05), and SPPB (p < 0.05). Additionally, the TUG test showed a significant reduction (p < 0.01).
Moreover, significant interaction effects between-group and time were identified for repeated chair stand (p = 0.012), SLST (p = 0.003), TUG test (p = 0.011), maximal gait speed (p = 0.045), maximal stride length (p = 0.013), and SPPB (p = 0.010).
As shown in Figure 2, the Taekwondo group exhibited a significant increase in SLST time compared to baseline (p < 0.01), whereas the control group showed no significant changes. Additionally, the SPPB score increased in the Taekwondo group, while the control group exhibited a slight decrease. Significant interaction effects were observed for SLST (p < 0.01) and SPPB (p < 0.05).
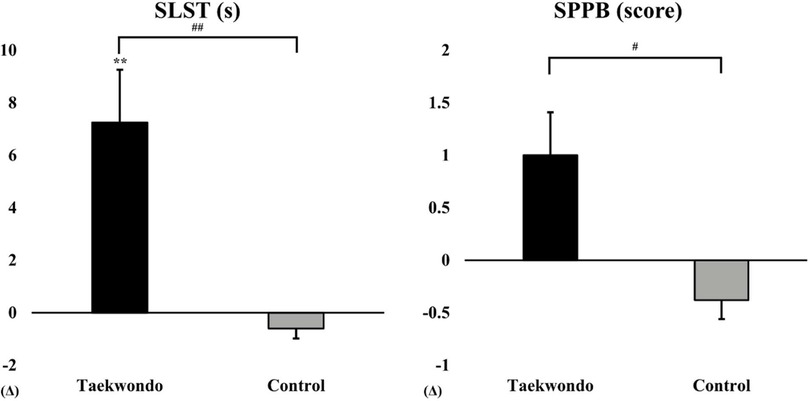
Figure 2. Comparison of SLST and SPPB between baseline and after 12 weeks in taekwondo program. #: p-value (interaction) #p < 0.05, ##p < 0.01. *: p-value (time) **p < 0.01. Δ: Post–Pre. SLST, single-leg stance test; SPPB, short physical performance battery.
3.3 Laboratory markers
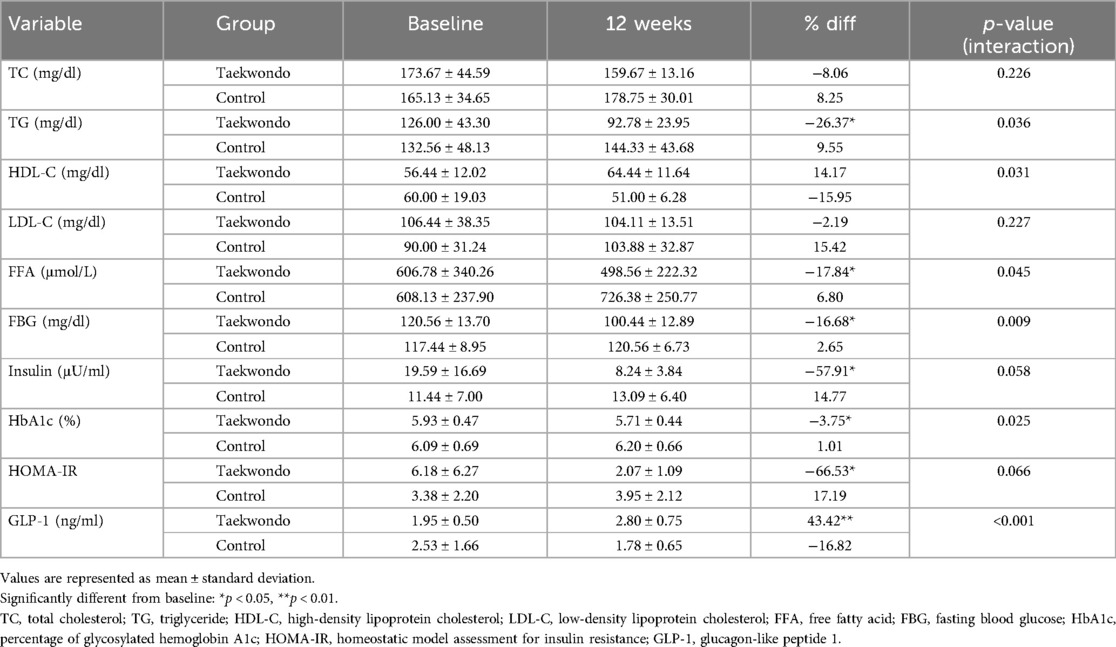
Changes in blood lipid levels and insulin resistance markers are presented in Table 5. In the Taekwondo group, significant reductions were observed in TG, FFA, FBG, insulin, HbA1c, and HOMA-IR whereas GLP-1 levels showed a significant increase. Moreover, significant interaction effects between-group and time were observed for TG (p = 0.036), HDL-C (p = 0.031), FFA (p = 0.045), FBG (p = 0.009), HbA1c (p = 0.025), and GLP-1 (p < 0.001) levels.
As shown in Figure 3, HbA1c levels tended to decrease in the Taekwondo group compared to baseline, whereas a slight increase was observed in the control group. A significant interaction effect was found between the two groups (p < 0.05). Additionally, GLP-1 levels significantly increased in the Taekwondo group compared to baseline (p < 0.01), whereas a decreasing trend was observed in the control group. A significant interaction effect was observed between the two groups (p < 0.001).
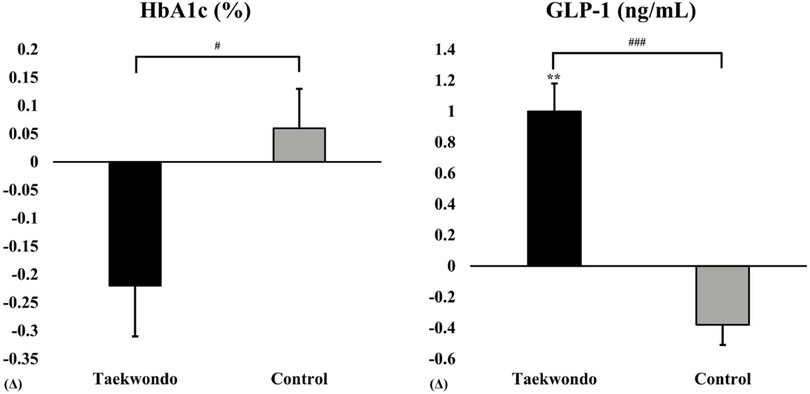
Figure 3. Comparison of HbA1c and GLP-1 between baseline and after 12 weeks in taekwondo program. #: p-value (interaction) #p < 0.05, ###p < 0.001. *: p-value (time) **p < 0.01. Δ: Post–Pre. HbA1c, percentage of glycosylated hemoglobin A1c; GLP-1, glucagon-like peptide 1.
3.4 Correlation between thigh muscle cross-sectional area and other variables
The thigh muscle CSA demonstrated significant negative correlations with TUG (r = −0.658, p < 0.01), TG (r = −0.638, p < 0.01), FFA (r = −0.506, p < 0.05), FBG (r = −0.535, p < 0.05), insulin (r = −0.615, p < 0.01), and HOMA-IR (r = −0.608, p < 0.01) (Table 6). In contrast, significant positive correlations were observed for LBM (r = 0.491, p < 0.05), HDL-C (r = 0.736, p < 0.01), and GLP-1 (r = 0.718, p < 0.01).
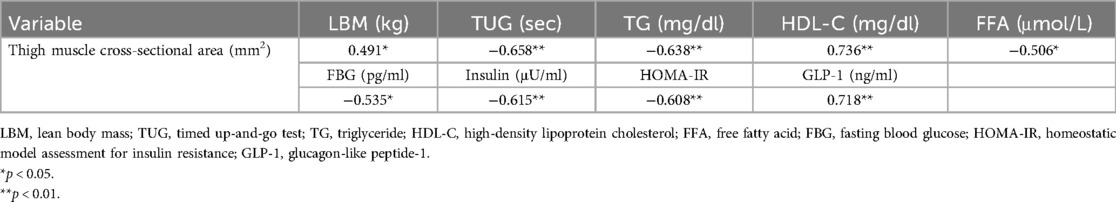
Table 6. Correlation coefficient between changes in thigh muscle cross-sectional area and other variables.
4 Discussion
This study demonstrated that regular, long-term Taekwondo training significantly increased thigh muscle CSA in sedentary older women, an outcome of particular importance given the difficulty in improving muscle mass in this population. Increased thigh muscle CSA is associated with functional mobility, balance, and insulin sensitivity, thereby reducing fall risk and improving metabolic health, especially in older women with a sedentary lifestyle (22).
The significant improvements in thigh muscle CSA observed in this study may be due to the combined aerobic and resistance components of Taekwondo, particularly the dynamic kicking movements and postural changes that stimulate muscle growth (27, 47, 48). These movements effectively engage lower body muscles, promoting hypertrophy and strength in older adults. This is noteworthy as increasing muscle mass is challenging in older women due to age-related declines in muscle synthesis (49–53). However, Taekwondo's unique combination of dynamic resistance and functional movements appears particularly effective for stimulating muscle growth and improving insulin sensitivity in sedentary older women, highlighting its potential as a practical intervention for enhancing muscle mass and metabolic health in this population.
Increased thigh muscle CSA observed a positive association with the HRPF of sedentary older women. More specifically, the significant decline in the TUG test and the significant increase in single-leg stance test, maximum stride length in Taekwondo group indicate that Taekwondo training had a beneficial effects on balance and mobility in older individuals. These improvements are directly linked to the increases in thigh muscle CSA, highlighting the interconnectedness of muscle mass and physical function. The importance of regular exercise in sedentary older women are reducing the risk of falls, improving gait ability, and promoting independence in the older population (25, 54, 55). Our results indicate that the Taekwondo program has the potential to enhance HRPF, particularly balance and gait, thereby reducing fall risk and increasing mobility.
Additionally, significant reductions in HbA1c and increases in GLP-1 levels were observed, suggesting enhanced glucose metabolism. Given the role of GLP-1 in promoting insulin secretion and improving insulin resistance, the observed increase in GLP-1 provides a plausible mechanism for the improvements in HbA1c and insulin sensitivity (56). This highlights the potential of Taekwondo as a multifaceted intervention that not only enhances muscle mass and physical fitness but also positively impacts metabolic health.
These findings underscore the importance of incorporating Taekwondo training as a strategic intervention for enhancing thigh muscle CSA, improving HRPF, and promoting metabolic health in sedentary older women. However, several limitations should be considered. The study's small sample size and the lack of a follow-up limit the generalizability of the results. Future studies should include larger, more diverse populations and consider longitudinal designs to assess the sustainability of the observed benefits. Furthermore, exploring the molecular mechanisms underlying the improvements in muscle mass and metabolic health will provide deeper insights into the efficacy of Taekwondo as an intervention for older adults.
5 Conclusion
Regular, long-term Taekwondo training has been demonstrated to enhance HRPF, total muscle mass, and thigh muscle CSA in sedentary older women, thereby contributing to improved insulin resistance risk factors. These findings suggest that Taekwondo training may be an effective exercise program to promote metabolic health in this population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by This study was conducted in accordance with the guidelines set forth by the institutional review board of D University (IRB No. 2-1040709-AB-N-01-201904-HR-022-04). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JP: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. BK: Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – review & editing. MJ: Data curation, Investigation, Software, Writing – review & editing. H-HJ: Data curation, Investigation, Software, Writing – review & editing. GH: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. SP: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank the participants who volunteered to take part in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was employed to refine sentence structure, enhance clarity, and translate content from Korean to English during the manuscript preparation process. The authors ensured all AI-assisted outputs were carefully reviewed and revised to maintain scientific accuracy and originality.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACSM, American college of sports medicine's; ASM, appendicular skeletal muscle mass; BMI, body mass index; CSA, cross-sectional area; FBG, fasting blood glucose; GLP-1, glucagon-like peptide-1; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment for insulin resistance; HRPF, health-related physical function; LBM, lean body mass; LDL-C, low-density lipoprotein cholesterol; LLM, lower limb muscle mass; MAP, mean arterial pressure; RPE, rating of perceived exertion; SLST, single-leg stance test; SPPB, short physical performance battery; TG, triglyceride; TUG, timed up-and-go; WHR, waist-hip ratio.
References
1. GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022–2050: a forecasting analysis for the Global Burden of Disease Study 2021. Lancet. (2024) 403(10440):2204–56. doi: 10.1016/S0140-6736(24)00685-8
2. OECD Health Statistics. Organization for Economic Cooperation and Development. Paris: OECD (2024). Available online at: https://data-explorer.oecd.org/vis?fs[0]=Topic%2C1%7CHealth%23HEA%23%7CHealth%20atus%23HEA_STA%23&pg=0&bp=true&snb=16&vw=tb&df[ds]=dsDisseminateFinalDMZ&df[id]=DSD_HEALTH_STAT%40DF_HEALTH_STATUS&df[ag]=OECD.ELS.HD&df[vs]=1.0&pd=2015%2C&dq=.A.LFEXP.Y.Y0……&ly[rw]=REF_AREA&ly[cl]=TIME_PERIOD&ly[rs]=SEX&to[TIME_PERIOD]=false (Accessed March, 2024).
3. Cunningham C, O’Sullivan R, Caserotti P, Tully MA. Consequences of physical inactivity in older adults: a systematic review of reviews and meta-analyses. Scand J Med Sci Sports. (2020) 30:816–27. doi: 10.1111/sms.13616
4. Park JH, Moon JH, Kim HJ, Kong MH, Oh YH. Sedentary lifestyle: overview of updated evidence of potential health risks. Korean J Fam Med. (2020) 41:365–73. doi: 10.4082/kjfm.20.0165
5. Wullems JA, Degens H, Verschueren SMP, Morse CI, Grant DM, Onambélé-Pearson GL. Sedentary behaviour (especially accumulation pattern) has an independent negative impact on skeletal muscle size and architecture in community-dwelling older adults. PLoS One. (2024) 19(2):e0294555. doi: 10.1371/journal.pone.0294555
6. Huschtscha Z, Parr A, Porter J, Costa RJS. Sarcopenic characteristics of active older adults: a cross-sectional exploration. Sports Med Open. (2021) 7(1):32. doi: 10.1186/s40798-021-00323-9
7. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. (2017) 15:131. doi: 10.1186/s12916-017-0901-x
8. Vitale JA, Messina C, Albano D, Fascio E, Galbusera F, Corbetta S, et al. Appendicular muscle mass, thigh intermuscular fat infiltration, and risk of fall in postmenopausal osteoporotic elder women. Gerontology. (2021) 67:415–24. doi: 10.1159/000513597
9. Seo M-W, Jung S-W, Kim S-W, Lee J-M, Jung HC, Song J-K. Effects of 16 weeks of resistance training on muscle quality and muscle growth factors in older adult women with sarcopenia: a randomized controlled trial. Int J Environ Res Public Health. (2021) 18:6762. doi: 10.3390/ijerph18136762
10. Lee S-H, Hong G-R, Kim B-J, Kim E-H. Effects of aging process exercise program on walking ability and frailty of elderly women in rural. Korean J Sports Sci. (2018) 27(2):1115–26. doi: 10.35159/kjss.2018.04.27.2.1115
11. Ramsey KA, Rojer AGM, D’Andrea L, Otten RHJ, Heymans MW, Trappenburg MC, et al. The association of objectively measured physical activity and sedentary behavior with skeletal muscle strength and muscle power in older adults: a systematic review and meta-analysis. Ageing Res Rev. (2021) 67:101266. doi: 10.1016/j.arr.2021.101266
12. Saunders TJ, McIsaac T, Douillette K, Gaulton N, Hunter S, Rhodes RE, et al. Sedentary behaviour and health in adults: an overview of systematic reviews. Appl Physiol Nutr Metab. (2020) 45:S197–217. doi: 10.1139/apnm-2020-0272
13. Fragala MS, Cadore EL, Dorgo S, Izquierdo M, Kraemer WJ, Peterson MD, et al. Resistance training for older adults: position statement from the national strength and conditioning association. J Strength Cond Res. (2019) 33:2019–52. doi: 10.1519/JSC.0000000000003230
14. Kim E, Park S, Kwon Y. The effects of combined exercise on functional fitness and risk factors of metabolic syndrome in the older women. Jpn J Phys Fit Sports Med. (2008) 57:207–16.
15. Bir SC, Kelley RE. Carotid atherosclerotic disease: a systematic review of pathogenesis and management. Brain Circ. (2022) 8:127–36. doi: 10.4103/bc.bc_36_22
16. Hamasaki H. Exercise and glucagon-like peptide-1: does exercise potentiate the effect of treatment? World J Diabetes. (2018) 9:138–40. doi: 10.4239/wjd.v9.i8.138
17. Kim E-H, Park S-K, Kim E-Y, Hong G-R. Effects of combined exercise program on glucose, cardiovascular disease risk factors and health-related quality of life in elderly women with type II diabetes. Korean J Sports Sci. (2013) 22(4):1133–45.
18. Jung H-H, Park S-K, Jeong M-K, Kim Y-H, Ryu J-K. The effects of combined exercise on self-reliance health fitness and cardiovascular risk factor in prehypertension elderly women. Korean J Sports Sci. (2019) 28(2):1021–32. doi: 10.35159/kjss.2019.04.28.2.1021
19. Sampath Kumar A, Maiya AG, Shastry BA, Vaishali K, Ravishankar N, Hazari A, et al. Exercise and insulin resistance in type 2 diabetes mellitus: a systematic review and meta-analysis. Ann Phys Rehabil Med. (2019) 62(2):98–103. doi: 10.1016/j.rehab.2018.11.001
20. Wang DXM, Yao J, Zirek Y, Reijnierse EM, Maier AB. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle. (2020) 11(1):3–25. doi: 10.1002/jcsm.12502
21. Bobowik P, Wiszomirska I. Diagnostic dependence of muscle strength measurements and the risk of falls in the elderly. Int J Rehabil Res. (2020) 43:330. doi: 10.1097/MRR.0000000000000430
22. Bani Hassan E, Phu S, Vogrin S, Duque G. Appendicular and mid-thigh lean mass are associated with muscle strength, physical performance, and dynamic balance in older persons at high risk of falls. Gait Posture. (2022) 93:90–5. doi: 10.1016/j.gaitpost.2022.01.022
23. Orwoll ES, Blackwell T, Cummings SR, Cauley JA, Lane NE, Hoffman AR, et al. CT muscle density, D3Cr muscle mass, and body fat associations with physical performance, mobility outcomes, and mortality risk in older men. J Gerontol Ser A. (2022) 77:790–9. doi: 10.1093/gerona/glab266
24. Baek S-H, Hong G-R, Min D-K, Kim E-H, Park S-K. Effects of functional fitness enhancement through taekwondo training on physical characteristics and risk factors of dementia in elderly women with depression. Int J Environ Res Public Health. (2021) 18:7961. doi: 10.3390/ijerph18157961
25. Kim YH, Jeong MK, Park H, Park SK. Effects of regular taekwondo intervention on health-related physical fitness, cardiovascular disease risk factors and epicardial adipose tissue in elderly women with hypertension. Int J Environ Res Public Health. (2021) 18:2935. doi: 10.3390/ijerph18062935
26. Li X, Bae J-H, Lim B, Seo J, Sung Y, Jiang S, et al. Impact of taekwondo training on cognitive and physical function in elderly individuals: a comprehensive review of randomized controlled trials. Complement Ther Clin Pract. (2024) 57:101878. doi: 10.1016/j.ctcp.2024.101878
27. Valdés-Badilla P, Herrera-Valenzuela T, Guzmán-Muñoz E, Branco BHM, Zapata-Bastias J, Lucero B, et al. Effectiveness of adapted taekwondo, multi-component training and walking exercise on health Status in independent older women: study protocol for a randomized controlled trial (TKD & aging project). Biology (Basel). (2022) 11:816. doi: 10.3390/biology11060816
28. Jeong M-K, Ryu J-K, Jung H-H, Kim Y-H, Park S-K. Effects of taekwondo aerobic and combined exercise program on health-related physical fitness and physical activity and depression scale in menopausal obesity women. Korean J Sports Sci. (2018) 27(6):1199–210. doi: 10.35159/kjss.2018.12.27.6.1199
29. Bao W, Sun Y, Zhang T, Zou L, Wu X, Wang D, et al. Exercise programs for muscle mass, muscle strength and physical performance in older adults with sarcopenia: a systematic review and meta-analysis. Aging Dis. (2020) 11:863–73. doi: 10.14336/AD.2019.1012
30. Martínez-Gómez D, Guallar-Castillón P, León-Muñoz LM, López-García E, Rodríguez-Artalejo F. Combined impact of traditional and non-traditional health behaviors on mortality: a national prospective cohort study in Spanish older adults. BMC Med. (2013) 11:47. doi: 10.1186/1741-7015-11-47
31. Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, et al. Sedentary behavior research network (SBRN)—terminology consensus project process and outcome. Int J Behav Nutr Phys Act. (2017) 14(1):75. doi: 10.1186/s12966-017-0525-8
32. Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. (2000) 102:1270–5. doi: 10.1161/01.CIR.102.11.1270
33. Choi S-J, Park S-M, Kwak Y-S. Comparison of the thigh composition and its functional contractility in obese and nonobese elderly patients. J Life Sci. (2014) 24:1125–31. doi: 10.5352/JLS.2014.24.10.1125
34. Reuter SE, Massy-Westropp N, Evans AM. Reliability and validity of indices of hand-grip strength and endurance. Aust Occup Ther J. (2011) 58(2):82–7. doi: 10.1111/j.1440-1630.2010.00888.x
35. Chan WLS, Pin TW. Reliability, validity and minimal detectable change of 2-min walk test and 10-m walk test in frail older adults receiving day care and residential care. Aging Clin Exp Res. (2020) 32(4):597–604. doi: 10.1007/s40520-019-01255-x
36. Ngai SP, Cheung RT, Lam PL, Chiu JK, Fung EY. Validation and reliability of the physical activity scale for the elderly in Chinese population. J Rehabil Med. (2012) 44(5):462–5. doi: 10.2340/16501977-0953
37. Botolfsen P, Helbostad JL, Moe-Nilssen R, Wall JC. Reliability and concurrent validity of the expanded timed up-and-go test in older people with impaired mobility. Physiother Res Int. (2008) 13(2):94–106. doi: 10.1002/pri.394
38. Maggio M, Ceda GP, Ticinesi A, De Vita F, Gelmini G, Costantino C, et al. Instrumental and non-instrumental evaluation of 4-meter walking speed in older individuals. PLoS One. (2016) 11(4):e0153583. doi: 10.1371/journal.pone.0153583
39. Goldberg A, Schepens S, Wallace M. Concurrent validity and reliability of the maximum step length test in older adults. J Geriatr Phys Ther. (2010) 33(3):122–7. doi: 10.1097/JPT.0b013e3181eda302
40. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. (1994) 49:M85–94. doi: 10.1093/geronj/49.2.M85
41. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. (1985) 28:412–9. doi: 10.1007/BF00280883
42. ACSM. American college of sports medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. (2009) 41:687–708. doi: 10.1249/MSS.0b013e3181915670
43. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee I-M, et al. American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0b013e318213fefb
44. Nelson M, Rejeski W, Blair S, Duncan P, Judge J, King A, et al. Physical activity and public health in older adults: recommendation from the American college of sports medicine and the American Heart Association. Circulation. (2007) 116:1094–105. doi: 10.1161/CIRCULATIONAHA.107.185650
45. ACSM AC of SM. ACSM’s Health-Related Physical Fitness Assessment Manual. Lippincott Williams & Wilkins (2013). Available online at: https://books.google.co.kr/books?hl=ko&lr=&id=ZPo96rd3PpAC&oi=fnd&pg=PP2&dq=ACSM%27s+health-related+physical+fitness+assessment+manual.&ots=S_o-MOdTdu&sig=J7UVYGHDaKpCgInWqLD-lH0BCdo (Accessed October 15, 2024).
46. Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human kinetics (1998). Available online at: https://psycnet.apa.org/record/1998-07179-000 (Accessed December 11, 2023).
47. Valdés-Badilla P, Herrera-Valenzuela T, Guzmán-Muñoz E, Hernandez-Martinez J, Cid-Calfucura I, Vásquez-Carrasco E, et al. Adapted taekwondo improves postural balance and health-related quality of life concerning multicomponent training and walking exercise in older females: a randomized controlled trial (TKD and aging project). J Clin Med. (2024) 13(23):7250. doi: 10.3390/jcm13237250
48. Valdés-Badilla P, Guzmán-Muñoz E, Herrera-Valenzuela T, Branco BHM, Hernandez-Martinez J, Nobari H. Impact of adapted taekwondo vs. Multicomponent training on health status in independent older women: a randomized controlled trial. Front Public Health. (2023) 11:1236402. doi: 10.3389/fpubh.2023.1236402
49. Janssen I, Ross R. Linking age-related changes in skeletal muscle mass and composition with metabolism and disease. J Nutr Health Aging. (2005) 9(6):408–19.16395513
50. Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. (2019) 99(1):427–511. doi: 10.1152/physrev.00061.2017
51. Aragon AA, Tipton KD, Schoenfeld BJ. Age-related muscle anabolic resistance: inevitable or preventable? Nutr Rev. (2023) 81(4):441–54. doi: 10.1093/nutrit/nuac062
52. Chen H, Huang X, Dong M, Wen S, Zhou L, Yuan X. The association between sarcopenia and diabetes: from pathophysiology mechanism to therapeutic strategy. Diabetes Metab Syndr Obes. (2023) 16:1541–54. doi: 10.2147/DMSO.S410834
53. Wohlwend M, Laurila PP, Goeminne LJE, Lima T, Daskalaki I, Li X, et al. Inhibition of CERS1 in skeletal muscle exacerbates age-related muscle dysfunction. Elife. (2024) 12:RP90522. doi: 10.7554/eLife.90522. Erratum in: Elife. 2024 May 20;13:e99846. doi: 10.7554/eLife.99846.38506902
54. Shin J-S, Kim H-J. The effect of 12-week resistance exercise on muscle loss and metabolic syndrome-related variables in obese elderly with sarcopenia. J Korean Soc Integr Med. (2022) 10:165–73. doi: 10.15268/ksim.2022.10.4.165
55. Patti A, Zangla D, Sahin FN, Cataldi S, Lavanco G, Palma A, et al. Physical exercise and prevention of falls. Effects of a pilates training method compared with a general physical activity program: a randomized controlled trial. Medicine. (2021) 100:e25289. doi: 10.1097/MD.0000000000025289
Keywords: Taekwondo, thigh muscle cross-sectional area, health-related physical fitness, metabolic syndrome, insulin resistance, older women
Citation: Park J, Kim B, Jeong M, Jung H-H, Hong G and Park SK (2025) Effects of Taekwondo training on thigh muscle cross-sectional area, health-related physical fitness, HbA1c, and GLP-1 in sedentary older women. Front. Sports Act. Living 7:1553202. doi: 10.3389/fspor.2025.1553202
Received: 30 December 2024; Accepted: 17 March 2025;
Published: 3 April 2025.
Edited by:
Bojan Masanovic, University of Montenegro, MontenegroReviewed by:
Ebrahim Norouzi, Farhangian University, IranMila Vukadinović Jurišić, University of Novi Sad, Serbia
Milovan Ljubojevic, University of Montenegro, Montenegro
Artan R. Kryeziu, University of Pristina, Albania
Copyright: © 2025 Park, Kim, Jeong, Jung, Hong and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Garam Hong, Z3JoMjAwNjA5QGdtYWlsLmNvbQ==; Sang Kab Park, c2dwYXJrQGRhdS5hYy5rcg==
†These authors have contributed equally to this work
 Jaehyun Park1,†
Jaehyun Park1,† Bongjo Kim
Bongjo Kim