- 1Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy
- 2Department of Quality of Life Studies, University of Bologna, Rimini, Italy
- 3Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy
Pulsed Electromagnetic Field (PEMF) therapy is a non-invasive treatment that utilizes electromagnetic fields to stimulate and promote natural healing processes within the body. PEMF therapy works by emitting low-frequency electromagnetic pulses, which penetrate deep into tissues and cells, enhancing cellular function and health. PEMF applications are vast, ranging from enhancing recovery in athletes to supporting overall well-being in everyday individuals. PEMF therapy is increasingly recognized in the realm of sports and physical activity for its profound benefits in enhancing performance, accelerating recovery, and preventing injuries. By improving circulation, enhancing tissue oxygenation, and promoting the body's natural healing processes, PEMF therapy has become an invaluable tool in sports medicine, contributing to optimized physical health and prolonged athletic careers. In this review, we explore the effects of PEMF on exercise and the underlying physiological mechanisms.
1 Introduction
Pulsed electromagnetic fields (PEMF) are a non-invasive medical therapy utilized in clinical treatments. The FDA granted approval for the application of PEMF in the repair of non-union fractures in humans in 1979 (1). PEMF therapy is currently employed for treating bone conditions like osteoporosis (2) and fractures (3). PEMF stimulation can be also used to improve tissue oxygenation, microcirculation and angiogenesis (4, 5). Kwan et al. (6) applied PEMF therapy on diabetic subjects, reporting an increase in microcirculation through the enhancement of capillary blood flow. Some authors have proposed PEMF stimulation as an adjunct to exercise (7, 8) but, to the best of our knowledge, there are no comprehensive articles examining the impact of PEMF on physical activity and sport. The aim of this review is to provide up-to-date summary of the current literature on PEMF and physical exercise, elucidating discrepancies, and identifying areas necessitating further research.
2 Biological rationale for PEMF stimulation as an adjunct to exercise
The application of external electromagnetic energy to an injured area triggers modifications in the cellular environment, promoting the restoration of tissue integrity and function (9–11). PEMFs have the potential to enhance tissue oxygenation, microcirculation, and angiogenesis in rats, human erythrocytes, and cell-free assays (4, 12, 13, 14). PEMF application has also showed modulatory effect on microvasculature and can result in its remodeling (15). Moreover, PEMF has the potential to resolve chronic inflammation via inducing changes in gene expression related to heme catabolism, removal of reactive oxygen species, and lipid mediator biosynthesis (16).
From the above-mentioned premises, we can state that the biological effects of PEMF stimulation may be useful to speed up recovery of specific muscles after exercises or to enhance the effects of exercise on specific body parts. Here we speculate about such practical applications.
2.1 Effects of PEMF on bones
The positive effects of PEMF stimulation on bone repair may be beneficial in the prevention and treatment of stress fractures in athletes and soldiers. Stress fractures occur primarily in the lower limbs and result from the repetitive mechanical overload associated with high-volume of endurance training. Standard treatment includes rest, ice and painkillers. Some painkillers may actually impair bone healing (17). PEMF stimulation may be used as an adjunct to exercise training in people at high risk of stress fractures to prevent their development. In athletes and soldiers recovering from stress fractures, PEMF stimulation may accelerate the return to sport or duty by enhancing the healing of the damaged bone tissue (18). Furthermore, PEMF stimulation may relieve pain in people with stress fractures due to its analgesic effects.
2.2 Analgesic effects of PEMF
Because pain is a significant barrier to exercise, the analgesic effects of PEMF stimulation may help people with osteoarthritis perform regular training to improve their physical function (19). A similar strategy may be applied in other situations in which musculoskeletal pain may limit the ability or willingness of people to perform exercise.
2.3 Effects of PEMF that may enhance recovery but may also reduce the hypertrophic response to resistance exercise
Exercise can induce significant muscle damage, especially when it includes eccentric muscle contractions (20). PEMF stimulation has many biological effects that may acutely enhance the recovery from such damage. These effects include reduced pain and inflammation, enhanced cellular repair and regeneration, stem cell activation and improved microcirculation, leading to better oxygenation and nutrient delivery to tissues (21). However, muscle damage and related inflammation have been proposed to be important stimuli for chronic muscle adaptations to resistance exercise (22). Therefore, it is important to directly investigate the net effect of PEMF stimulation on the muscle hypertrophy induced by resistance exercise.
2.4 Effects of PEMF stimulation on peripheral blood flow
PEMF stimulation is known to enhance peripheral blood flow. This effect may be particularly helpful as an adjunct to exercise in patients with impaired peripheral blood flow such as diabetic patients and people suffering from peripheral arterial disease. Exercise training is an important component in the management of these conditions (23, 24). PEMF stimulation may further enhance its beneficial effects in body parts particularly affected by vascular and microvascular alterations (25). Steward et al., (25) found that 12 weeks of PEMF stimulation let to improved endothelial vascular function and reduced blood pressure in hypertensive subjects showing that PEMF treatment could be a potential non-pharmacological and non-invasive strategy to manage vascular function and blood pressure in cohorts with peripheral vascular disease as well as hypertension.
3 Effect of PEMF on physical exercise
PEMF therapy impacts physical exercise with both acute and chronic effects. Acutely, PEMF can enhance muscle recovery by increasing blood flow and reducing inflammation, leading to immediate relief from muscle soreness and faster recovery times after workouts. Chronically, regular use of PEMF can improve overall muscular health, endurance, and performance by promoting cellular repair and optimizing metabolic function over time.
3.1 Effects of PEMF stimulation on the acute responses to exercise
Trofè et al. investigated the acute effects PEMF on muscle oxygenation during exercise. The authors found that PEMF enhanced muscle oxygenation and accelerated deoxyhemoglobin on-transition kinetics, indicating improved local oxygen extraction (7). In another study, the authors also found that PEMF stimulation increases the activity and metabolism of muscle fibers during physical exercise, therefore, PEMF has the potential to enhance muscular responses, particularly in low-intensity exercise scenarios (8).
Grote et al. aimed to assess the immediate and short-term effects of PEMF therapy on physiological parameters, particularly heart rate variability, following physical stress tests. Results showed that PEMF exposure influenced heart rate variability components related to sympathetically controlled blood flow rhythms, and accelerated recovery after physical strain. However, the application of PEMFs had no impact on participants overall well-being (26).
Viti et al. (27) investigated the effects of PEMFs stimulation using a PAP ion magnetic induction device, named PAPIMI, on autonomic nervous system activity in patients with chronic musculoskeletal pain. Results showed that PEMFs stimulation induced a significant parasympathetic response, which increased heart rate variability. Musculoskeletal pain conditions have been associated with a reduced heart rate variability [cfr (27)], thus, such acute autonomic change observed following PAPIMI intervention might be interpreted as a health status-related parasympathetic response. This study provided initial evidence on the potential physiological response induced by PEMF stimulation (PAPIMI device).
3.2 Effects of PEMF stimulation on the chronic responses to exercise
In a study by Parhampour et al., (28) PEMFs were applied with a 6-week resistance training program for patients with severe hemophilia A and osteoporosis. The aim was to enhance muscle strength, bone formation, and joint function. The authors enrolled 48 patients who were randomly assigned to one of four groups: resistance training alone, combined resistance training with PEMF, PEMF alone and control. Results showed that the absolute changes in the total score for joint function were significant for knees, ankles, and elbows in the resistance training alone and resistance training with PEMF, compared to the PEMF alone and control groups. The results indicated that combining PEMF stimulation with resistance training could be more effective than PEMF therapy alone.
Kandemir et al. (29) evaluated the effects of 3 months PEMF therapy in the treatment of subacromial impingement syndrome on 80 individuals randomly assigned to experimental (PEMF and exercise) or control (sham PEMF and exercise) groups. Both groups trained 5 days a week for a total of 20 sessions. Data were recorded before treatment (T0), after treatment (T1), and 12 weeks after the end of the intervention (T2). The results showed improvements in both groups compared with baseline. In the comparison between the two groups at T1 and T2, the PEMF group showed more improvements in most parameters.
3.3 Effects of PEMF on recovery after exercise
Jeon et al. explored the impact of PEMF therapy on recovery from delayed-onset muscle soreness (DOMS) after isometric exercise. A 10-min PEMF on the brachii biceps post-training improved DOMS symptoms in the following days, improving overall recovery quality. Additionally, PEMF treatment increased muscle activation frequency and reduced electromechanical delay, implying a shortened recovery time. However, no significant effect was observed on the peak of isometric force generation, warranting further studies to validate PEMF's positive influence on enhancing and expediting the recovery phase (30).
Galace de Freitas et al. aimed to assess the impact of PEMFs and exercises on pain reduction, function improvement, and muscle strength in patients with shoulder impingement syndrome. They reported that the combination of PEMF and shoulder exercises effectively enhanced function, muscle strength, and reduced pain in SIS patients. However, the results should be interpreted carefully due to the lack of significant differences between the groups (31).
4 Discussion
PEMF can lead to a better performance during exercise via several mechanisms.
The first mechanism by which PEMF can increase oxygenation is via increasing the tissue oxygenation through multiple ways including vessel diameter. Mayrovitz and Larsen (32) investigated the potential effects of a single 45-min pulsed radio frequency field treatment on peri-ulcer microcirculation in 15 subjects with diabetes and chronic foot or toe ulcers. Laser doppler measurements of red blood cell perfusion, volume, and velocity, along with skin temperature, were taken at both the peri-ulcer site and a contralateral nonulcerated limb site before and after treatment. They reported an increase in laser doppler perfusion at the peri-ulcer site, primarily attributed to an elevated volume component. No significant changes were observed at the contralateral control site or in skin temperature at either location. These results were further validated by Smith et al. (14) who showed a noticeable vasodilation in the cremasteric arterioles of PEMF treated rats. It has been suggested that PEMF exerts its vasodilatory effects via increasing the production of nitric oxide (NO). Bragin et al. (12) showed that 30 min of PEMF treatment induced cerebral arteriolar dilation leading to an increase in microvascular blood flow and tissue oxygenation that persisted for at least 3 h. The effects of PEMF were mediated by NO, given that the intravenous injection of the NO synthase inhibitor “L-NAME” prevented PEMF-induced changes in arteriolar diameter, microvascular perfusion, and tissue oxygenation. It has been suggested that the pathway by which PEMF increases NO production is increasing calcium influx. PEMF activates voltage-gated calcium channels which allows an influx of Ca++, consequently leading to the activation of NOS (11).
The second mechanism is via angiogenesis. Roland et al. (5) utilized a microsurgically created arterial loop model in a prospective randomized trial involving 108 rats (n = 12/group). The rats were exposed to pulsed magnetic energies of 0.1 and 2.0 gauss immediately postoperatively and for 4, 8, and 12 weeks. The results revealed a statistically significant increase in neovascularization in the treated animals compared to the control group (5).
The third mechanism regards oxygen consumption. PEMF can ameliorate oxygenation via increasing hemoglobin deoxygenation. Muehsam et al. (4) demonstrates that two clinically used electromagnetic field modalities, pulse-modulated radiofrequency and static magnetic field, independently increased the deoxygenation rate of human hemoglobin in a controlled cell-free assay. Trofè et al. examined the acute effects of PEMFs on muscle oxygenation and pulmonary oxygen kinetics during exercise in male cyclists using the previously mentioned data. Muscle oxygenation was improved by PEMF stimulation, as evidenced by higher primary and steady-state deoxyhemoglobin levels. Furthermore, compared to the non-stimulated condition, PEMF accelerated the kinetics of deoxyhemoglobin on-transition, resulting in a shorter time delay, time constant, and mean reaction time. The pulmonary oxygen kinetics and total oxygenation index did not change significantly during stimulation, despite the increasing lactate concentration. The results imply that without significantly altering overall oxygen kinetics during cycling, local PEMF stimulation can improve muscle oxygen extraction and use (7).
It is noteworthy that the impact of PEMF depends on the baseline characteristics of the individual. In the study of Grote et al. (26), 32 healthy male adults underwent standardized physical stress tests, followed by exposure to PEMFs of varying intensity. The research revealed that the influence of electromagnetic fields on the very low-frequency power spectral components of heart rate variability, reflecting sympathetically controlled blood flow rhythms, is contingent upon individual baseline factors. Notably, exposure to a specific magnetic field intensity accelerated recovery after physical strain compared to a placebo, and these effects quickly subsided upon cessation of magnetic field exposure (26).
4.1 Potential practical applications
Interestingly, the positive effects of PEMF therapy have been mostly reported in extreme situations, while outcomes for healthy adults remain yet to be elucidated (33). Athletes are frequently involved in preseason training camps where they undergo intensified training to optimize adaptations for the competition seasons (34). The initiation of such a training regimen after a period of inactivity poses a substantial physical challenge to athletes. Given the existing research and the widespread application of these devices in real-world scenarios, PEMF holds the potential to enhance athletic performance as athletes transition back to regular training seasons. This could be achieved by boosting recovery and reducing fatigue during training camps.
PEMF stimulation should thus gain attention in the athletic and therapeutic exercise communities for its potential benefits in enhancing performance, accelerating recovery, and promoting overall musculoskeletal health. PEMF application helps in reducing muscle soreness and stiffness after intense workouts, enabling athletes to recover faster and perform consistently at high levels (30). PEMF therapy is useful in managing both acute and chronic pain conditions enabling individuals undergoing rehabilitation to continue their therapeutic exercises without discomfort. The regular use of PEMF stimulation can improve muscle performance by enhancing cellular function and energy production leading to improved strength, endurance, and overall athletic performance (7, 8, 18, 29, 31). Athletes may experience better training outcomes and improved results in their competitive activities. Further, PEMF therapy is also effective in reducing inflammation and promoting the healing of joint and ligament injuries being a valuable tool for both injury prevention and recovery (18). Lastly, PEMF stimulation is a non-invasive, drug-free treatment option, which can be particularly important for athletes who wish to avoid the side effects of medications or the risks associated with invasive procedures.
PEMF stimulation offers a range of potential benefits for athletes and those involved in therapeutic exercise. From enhanced recovery and pain reduction to improved performance, PEMF therapy presents a promising adjunctive treatment to support physical health and optimize athletic outcomes.
4.2 Stimulation protocols
Although PEMF stimulation has been used for decades, there are currently no guidelines for categorizing PEMF. The main issue concerns the stimulation protocols, including time of exposure and frequency. However, those parameters are difficult to standardize. A discussion on the electromagnetic characteristics of PEMF stimulation can be found in the review by Flatscher et al. (21). Briefly, electromagnetic fields are composed of magnetic and electric fields that influence each other. PEMF applies intermittent, current pulse-generated magnetic field pulses over a short period of time. Tissues are affected by the applied PEMF field in two ways: firstly, the magnetic field creates a force on tissue-resided molecules which depend on their magnetic reactive properties, and secondly, the induced electrical field, which exerts a force on the ions present in the tissue (21); both result in a forced movement of ions or charged particles, such as proteins (35, 36). Mansourian and Shanei (37) performed a meta-analysis on PEMF in vitro studies showing that the effect of PEMF differs between cell type (stem cells) and origin (human/animal).
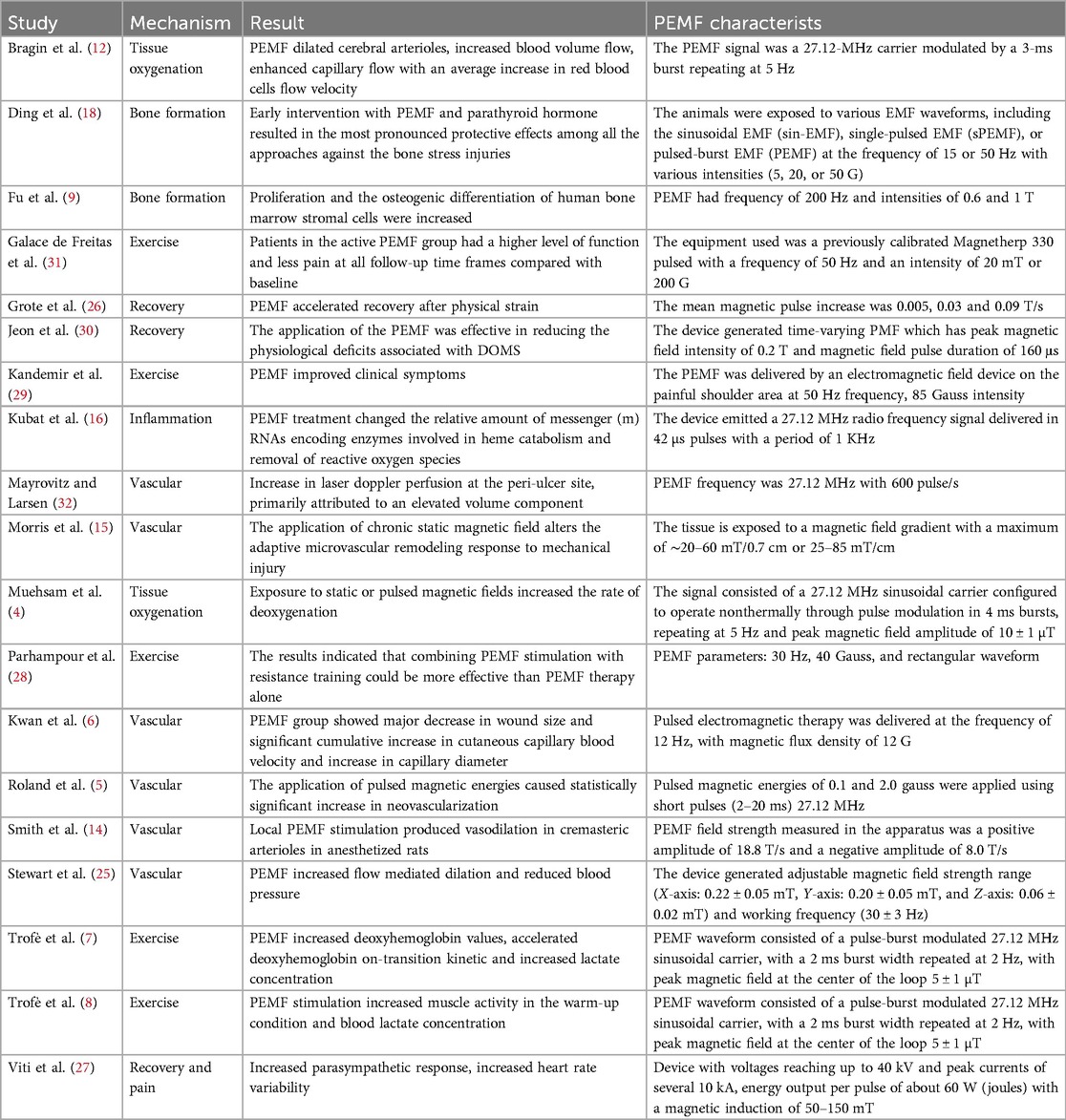
Almost every article discusses the heterogeneity of PEMF parameters used and positive effects are repeatedly reported A summary of results and characteristics of the reviewed research articles is shown in Table 1. Even though reliable research on such parameters has not been performed yet, PEMF is safe, non-invasive, relatively inexpensive and has been shown to positively contribute to many different conditions.
4.3 Conclusions and direction for future research
The data shown in this brief review demonstrate that PEMF application in physical activity and sport is a burgeoning field with considerable potential. Future research should focus on understanding how PEMF influences muscle metabolism, ATP production, and neuromuscular function. Detailed mechanistic studies are needed to elucidate how PEMF can be used to improve strength, endurance, and recovery at systemic level. Further studies should explore optimal frequencies, intensities, durations, and application timings relative to training sessions. Customizing protocols based on sport-specific demands and individual athlete profiles can enhance the practical utility of PEMF.
It is important to underline that, to date, the long-term effects of regular PEMF use on athletic performance and injury rates are underexplored. Longitudinal studies should track athletes over extended periods to assess how sustained PEMF use influences performance metrics, injury incidence, and career longevity providing insights into the viability of PEMF as a long-term tool in sports. Further, athletes of different ages and genders may respond differently to PEMF therapy. Future studies should investigate how factors such as age, sex, and hormonal variations influence the efficacy of PEMF enhancing personalized approaches to athlete care.
In conclusion, while the current literature on PEMF in physical activity and sport is promising, extensive research is necessary to fully harness its potential.
Author contributions
SG: Writing – original draft, Writing – review & editing. MP: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. AP: Data curation, Writing – review & editing. SM: Supervision, Writing – review & editing. MR: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Supported by University of Bologna Almaidea 2022 project, funded by European Union—NextGenerationEU.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Heckman JD, Ingram AJ, Loyd RD, Luck JV Jr, Mayer PW. Nonunion treatment with pulsed electromagnetic fields. Clin Orthop Relat Res. (1981) (161):58–66. PMID: 6975692.
2. Zhu S, He H, Zhang C, Wang H, Gao C, Yu X, et al. Effects of pulsed electromagnetic fields on postmenopausal osteoporosis. Bioelectromagnetics. (2017) 38(6):406–24. doi: 10.1002/bem.22065
3. Chalidis B, Sachinis N, Assiotis A, MaccauroG GF. Stimulation of bone formation and fracture healing with pulsed electromagnetic fields: biologic responses and clinical implications. Int J Immunopathol Pharmacol. (2011) 24:17–20. doi: 10.1177/03946320110241S204
4. Muehsam D, Lalezari P, Lekhraj R, Abruzzo PM, Bolotta A, Marini M, et al. Non-thermal radio frequency and static magnetic fields increase rate of hemoglobin deoxygenation in a cell-free preparation. PLoS One. (2013) 8(4):e61752. doi: 10.1371/journal.pone.0061752
5. Roland D, Ferder M, Kothuru R, Faierman T, Strauch B. Effects of pulsed magnetic energy on a microsurgically transferred vessel. Plast Reconstr Surg. (2000) 105(4):1371–4. doi: 10.1097/00006534-200004040-00016
6. Kwan RL, Wong WC, Yip SL, Chan KL, Zheng YP, Cheing GL. Pulsed electromagnetic field therapy promotes healing and microcirculation of chronic diabetic foot ulcers: a pilot study. Adv Skin Wound Care. (2015) 28(5):212–9. doi: 10.1097/01.ASW.0000462012.58911.53
7. Trofè A, Raffi M, Muehsam D, Meoni A, Campa F, Toselli S, et al. Effect of PEMF on muscle oxygenation during cycling: a single-blind controlled pilot study. Appl Sci. (2021) 11(8):3624. doi: 10.3390/app11083624
8. Trofè A, Piras A, Muehsam D, Meoni A, Campa F, Toselli S, et al. Effect of pulsed electromagnetic fields (PEMFs) on muscular activation during cycling: a single-blind controlled pilot study. Healthcare (Basel). (2023) 11(6):922. doi: 10.3390/healthcare11060922
9. Fu YC, Lin CC, Chang JK, Chen CH, Tai IC, Wang GJ, et al. A novel single pulsed electromagnetic field stimulates osteogenesis of bone marrow mesenchymal stem cells and bone repair. PLoS One. (2014) 9(3):e91581. doi: 10.1371/journal.pone.0091581
10. Walleczek J. Electromagnetic field effects on cells of the immune system: the role of calcium signaling 1. FASEB J. (1992) 6:3177–85. doi: 10.1096/fasebj.6.13.1397839
11. Pall ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med. (2013) 17:958–65. doi: 10.1111/jcmm.12088
12. Bragin DE, Statom GL, Hagberg S, Nemoto EM. Increases in microvascular perfusion and tissue oxygenation via pulsed electromagnetic fields in the healthy rat brain. J Neurosurg. (2015) 122:1239–47. doi: 10.3171/2014.8.JNS132083
13. McKay JC, Prato FS, Thomas AW. A literature review: the effects of magnetic field exposure on blood flow and blood vessels in the microvasculature. Bioelectromagnetics. (2007) 28(2):81–98. doi: 10.1002/bem.20284
14. Smith TL, Wong-Gibbons D, Maultsby J. Microcirculatory effects of pulsed electromagnetic fields. J Orthop Res. (2004) 22(1):80–4. doi: 10.1016/S0736-0266(03)00157-8
15. Morris CE, Skalak TC. Chronic static magnetic field exposure alters microvessel enlargement resulting from surgical intervention. J Appl Physiol (1985). (2007) 103(2):629–36. doi: 10.1152/japplphysiol.01133.2006
16. Kubat NJ, Moffett J, Fray LM. Effect of pulsed electromagnetic field treatment on programmed resolution of inflammation pathway markers in human cells in culture. J Inflamm Res. (2015) 8:59–69. doi: 10.2147/JIR.S78631
17. Saunier J, Chapurlat R. Stress fracture in athletes. Joint Bone Spine Revue Du Rhumatisme. (2018) 85(3):307–10. doi: 10.1016/j.jbspin.2017.04.013
18. Ding Y, Yang Y, Xu F, Tan Z, Liu X, Shao X, et al. Early protection against bone stress injuries by mobilization of endogenous targeted bone remodeling. iScience. (2023) 26(9):107605. doi: 10.1016/j.isci.2023.107605
19. Kanavaki AM, Rushton A, Efstathiou N, Alrushud A, Klocke R, Abhishek A, et al. Barriers and facilitators of physical activity in knee and hip osteoarthritis: a systematic review of qualitative evidence. BMJ Open. (2017) 7(12):e017042. doi: 10.1136/bmjopen-2017-017042
20. Markus I, Constantini K, Hoffman JR, Bartolomei S, Gepner Y. Exercise-induced muscle damage: mechanism, assessment and nutritional factors to accelerate recovery. Eur J Appl Physiol. (2021) 121(4):969–92. doi: 10.1007/s00421-020-04566-4
21. Flatscher J, Pavez Loriè E, Mittermayr R, Meznik P, Slezak P, Redl H, et al. Pulsed electromagnetic fields (PEMF)-physiological response and its potential in trauma treatment. Int J Mol Sci. (2023) 24(14):11239. doi: 10.3390/ijms241411239
22. Lim C, Nunes EA, Currier BS, McLeod JC, Thomas ACQ, Phillips SM. An evidence-based narrative review of mechanisms of resistance exercise-induced human skeletal muscle hypertrophy. Med Sci Sports Exerc. (2022) 54(9):1546–59. doi: 10.1249/MSS.0000000000002929
23. Bonaca MP, Hamburg NM, Creager MA. Contemporary medical management of peripheral artery disease. Circ Res. (2021) 128(12):1868–84. doi: 10.1161/CIRCRESAHA.121.318258
24. Magkos F, Hjorth M, Astrup A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. (2020) 16(10):545–55. doi: 10.1038/s41574-020-0381-5
25. Stewart GM, Wheatley-Guy CM, Johnson BD, Shen WK, Kim CH. Impact of pulsed electromagnetic field therapy on vascular function and blood pressure in hypertensive individuals. J Clin Hypertens (Greenwich). (2020) 22(6):1083–9. doi: 10.1111/jch.13877
26. Grote V, Lackner H, Kelz C, Trapp M, Aichinger F, Puff H, et al. Short-term effects of pulsed electromagnetic fields after physical exercise are dependent on autonomic tone before exposure. Eur J Appl Physiol. (2007) 101(4):495–502. doi: 10.1007/s00421-007-0520-x
27. Viti A, Panconi G, Guarducci S, Garfagnini S, Mondonico M, Bravi R, et al. Modulation of heart rate variability following PAP Ion magnetic induction intervention in subjects with chronic musculoskeletal pain: a pilot randomized controlled study. Int J Environ Res Public Health. (2023) 20(5):3934. doi: 10.3390/ijerph20053934
28. Parhampour B, Torkaman G, Hoorfar H, Hedayati M, Ravanbod R. Effects of short-term resistance training and pulsed electromagnetic fields on bone metabolism and joint function in severe haemophilia A patients with osteoporosis: a randomized controlled trial. Clin Rehabil. (2014) 28(5):440–50. doi: 10.1177/0269215513505299
29. Kandemir O, Adar S, Dündar Ü, Toktaş H, Yeşil H, Eroğlu S, et al. Effectiveness of pulse electromagnetic field therapy in patients with subacromial impingement syndrome: a double-blind randomized sham controlled study. Arch Phys Med Rehabil. (2024) 105(2):199–207. doi: 10.1016/j.apmr.2023.09.020
30. Jeon HS, Kang SY, Park JH, Lee HS. Effects of pulsed electromagnetic field therapy on delayed-onset muscle soreness in biceps brachii. Phys Ther Sport. (2015) 16(1):34–9. doi: 10.1016/j.ptsp.2014.02.006
31. Galace de Freitas D, Marcondes FB, Monteiro RL, Rosa SG, Maria de Moraes Barros Fucs P, Fukuda TY. Pulsed electromagnetic field and exercises in patients with shoulder impingement syndrome: a randomized, double-blind, placebo-controlled clinical trial. Arch Phys Med Rehabil. (2014) 95(2):345–52. doi: 10.1016/j.apmr.2013.09.022
32. Mayrovitz HN, Larsen PB. A preliminary study to evaluate the effect of pulsed radio frequency field treatment on lower extremity peri-ulcer skin microcirculation of diabetic patients. Wounds. (1995) 7:90–3.
33. Hug K, Röösli M. Therapeutic effects of whole-body devices applying pulsed electromagnetic fields (PEMF): a systematic literature review. Bioelectromagnetics. (2012) 33(2):95–105. doi: 10.1002/bem.20703
34. Buchheit M, Racinais S, Bilsborough JC, Bourdon PC, Voss SC, Hocking J, et al. Monitoring fitness, fatigue and running performance during a pre-season training camp in elite football players. J Sci Med Sport. (2013) 16(6):550–5. doi: 10.1016/j.jsams.2012.12.003
35. Rahbek U, Tritsaris K, Dissing S. Interactions of low-frequency, pulsed electromagnetic fields with living tissue: biochemical responses and clinical results. Oral Biosci Med. (2005) 2:29–40.
36. Wade B. A review of pulsed electromagnetic field (PEMF) mechanisms at a cellular level: a rationale for clinical use. AJHR. (2013) 1:51–5. doi: 10.11648/j.ajhr.20130103.13
Keywords: physical exercise, physical activity, PEMF, performance, recovery, sport, athletes, DOMS
Citation: Ghanbari Ghoshchi S, Petroni ML, Piras A, Marcora SM and Raffi M (2024) Pulsed Electromagnetic Field (PEMF) stimulation as an adjunct to exercise: a brief review. Front. Sports Act. Living 6:1471087. doi: 10.3389/fspor.2024.1471087
Received: 26 July 2024; Accepted: 26 August 2024;
Published: 9 September 2024.
Edited by:
Hanjun Wang, University of Nebraska Medical Center, United StatesReviewed by:
Bruce Rogers, University of Central Florida, United StatesCopyright: © 2024 Ghanbari Ghoshchi, Petroni, Piras, Marcora and Raffi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Milena Raffi, bWlsZW5hLnJhZmZpQHVuaWJvLml0
 Sheyda Ghanbari Ghoshchi
Sheyda Ghanbari Ghoshchi Maria Letizia Petroni1
Maria Letizia Petroni1 Alessandro Piras
Alessandro Piras Samuele Maria Marcora
Samuele Maria Marcora Milena Raffi
Milena Raffi