- 1Department of Internal and Family Medicine, Lesya Ukrainka Volyn National University, Lutsk, Ukraine
- 2Department of Therapy and Rehabilitation, Ivan Boberskij Ivan Bobersky Lviv State University of Physical Culture, Lviv, Ukraine
Introduction: Our goal was to determine the differences in changes in cardiovascular and cardiorespiratory interaction indicators during a respiratory maneuver with a change in breathing rate in athletes with different types of heart rate regulation.
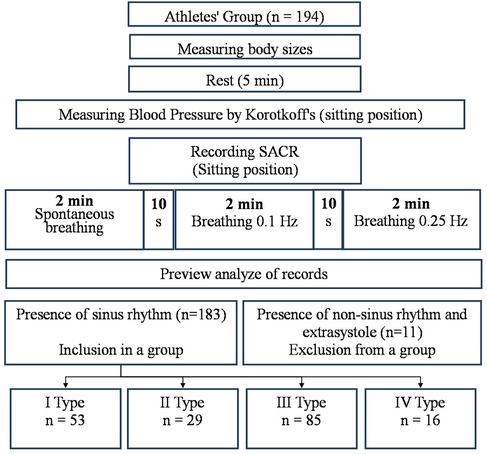
Methods: The results of a study of 183 healthy men aged 21.2 ± 2.3 years, who were systematically involved in various sports, were analyzed. According to the results of the analysis of the HRV study during spontaneous breathing, the athletes were divided into 4 groups taking into account the type of heart rate regulation (HRR). Group 1 (with type I) consisted of 53 people, group 2 (with type II)—29 people, group 3 (with type III)—85 people, group 4 (with type IV)—16 people. The methodology for studying the cardiorespiratory system included combined measurements of the respiratory and cardiovascular system activity indicators in a sitting position using a spiroarteriocardiorhythmograph. The duration of the study was 6 min.
Results: According to changes in cardiorespiratory and cardiovascular interaction indicators during controlled breathing with a frequency of 6 and 15 per minute (CR6 and CR15), it is shown that with a pronounced predominance of parasympathetic influences (type IV) in conditions of excessive cardiorespiratory control and moderate hyperventilation, differences in changes in arterial baroreflex sensitivity (δBRLF and δBRHF) are noted in comparison with other HRR. Athletes with type IV at CR6 in δBRLF significantly differ from athletes with type III (p = 0.026) and do not differ from athletes with type II (p = 0.141). In δBRHF significantly (p = 0.038 and p = 0.043)—from athletes with types I and II. It is shown that with the predominance of sympathetic influences (types I and II), the reactivity of BRS (δBRLF and δBRHF) in response to moderate hyperventilation (CR15) is significantly lower. Changes in the Hildebrandt index and the volume synchronization index additionally differentiate HRR associated with a moderate and pronounced predominance of sympathetic and parasympathetic influences.
Conclusion: The use of a respiratory maneuver in a combined study of the cardiorespiratory system in the conditions of current control of athletes showed informativeness in the differentiation of HRR types and states of functional overstrain.
1 Introduction
The problem of rapid evaluation of the functional state of the athletes' body is extremely relevant in the conditions of training and competitions (1, 2). First of all, this is due to the need to objectify changes in the body as soon as possible (3, 4). Most often, for this purpose, a number of biochemical and instrumental studies are used, which allow assessing the impact of physical exertion, the body's reaction and the course of recovery processes (5–11). Among the instrumental research methods are the analysis of electrical activity of the heart, heart rate variability (HRV), sensorimotor function, and others (12–18).
The heart, as an indicator of the adaptive reactions of the entire organism, reacts to a wide variety of internal and external influences, which is reflected in the indicators of HRV. HRV indicators provide important information about the state of the autonomic nervous system (ANS) and other levels of neurohumoral regulation, therefore research and analysis of HRV is considered a methodology for studying the mechanisms of regulation of physiological functions in the human body (19–26). Taking into account the results of the HRV study allows to increase the effectiveness of diagnosing the condition of athletes, to preventing fatigue, adjusting the training load and assessing the athlete's ability to self-regulate (27–30). The main advantage of HRV is that it is non-invasive, cheap, time-efficient and can be used regularly and simultaneously in a large number of athletes (20, 31–33). According to the two-circuit model of heart rhythm control, which is based on the regulatory activity of the sympathetic and parasympathetic divisions of the ANS Shlyk et al. (34) proposed a .classification of heart rhythm regulation (HRR) types, which provides for the selection of moderate and pronounced variants of the predominance of central (types I and II) and autonomous (types III and IV) regulation circuits. This classification takes into account the values of indicators of total HRV power—TР (ms2), variability in a very low range—VLF (ms2) and stress index—SI (с.u.). The feasibility of using these indicators to assess the current functional state of regulatory systems in healthy people and athletes has been shown in a number of other studies (5, 24, 29, 35, 36). Each of the types of HRR is characterized by the presence of possible states at rest. At the same time, a number of studies showed the individual typological features of such a classification, when a certain type of regulation in an individual person in a state of rest is preserved for a long time, and its changes are caused by the modification of external and internal influencing factors (37). The technique is widely used during monitoring in the educational and training process of athletes. It was even mentioned in the instructions for monitoring in national teams of different countries. Its results make it possible to predict the athlete's functional readiness and the possibility of achieving a sports result (37, 38).
The search for informative criteria for assessing the functional state of athletes' bodies during express screening examinations within the educational and training process arouses interest in the analysis of combined intersystem changes in the cardiorespiratory system (39–44). Such an opportunity is available with the simultaneous registration of indicators of cardiovascular and respiratory systems, because it allows direct assessment of the mechanisms of intersystem interaction (21, 45–53). This is possible under the condition of simultaneous registration of cardio intervals, pulse wave of blood pressure and respiratory flows, which is achieved with the help of Finapres (54) and SAСR (55) devices.
Studying indicators of intersystem interaction, especially taking into account the frequency and depth of breathing, both spontaneous and controlled, is important in understanding HRV changes in the regulation of the autonomic nervous system (49, 50, 56–61). In earlier studies, it was shown that in the range from 6 to 10 spontaneous breaths per minute, there is a clear relationship between the frequency of breathing and indicators of HRV (26, 62). Previous studies have shown that the alternate execution of tests with controlled breathing with a frequency of 0.1 Hz and 0.25 Hz allows determining the peculiarities of the ANS response, which relate to multidirectional changes in most indicators of HRV, blood pressure variability, partially hemodynamics and indicators of the cardiorespiratory system interaction. An assumption was made about the probable prognostic value of these changes in determining the functional state of the athletes' body and patients with different diseases (63–69). A number of authors have proposed assessing the redundancy of cardiorespiratory control and the synergy of this connection during breathing at a rate lower than spontaneous (70).
Among the known indicators of cardiorespiratory interaction—the indicators of the arterial baroreflex sensitivity (BRS) in the low-frequency (BRLF, ms × mmHg−1) and high-frequency (BRHF, ms × mmHg−1) ranges, which characterize the interaction of the cardiac and vascular components of hemodynamic support (71), and also the indicators of frequency (Hildebrandt index, c.u.) and volumetric (VSI, dm3 × L−1) synchronization of the heart and breathing, which are more related with the oxygenation capabilities of the body (72, 73). Their changes during controlled breathing have not been studied enough. In our opinion, their research and analysis can in the future significantly supplement the possibilities of current monitoring of the functional state of athletes.
In this paper, our goal was to determine the differences in the changes in indicators of cardiovascular and cardiorespiratory interaction during a breathing maneuver with a change in breathing frequency in athletes with different types of heart rhythm regulation.
2 Materials and methods
2.1 Study subjects
This study was conducted in the limits scientific programs of departments of Exercises Medicine and Sports Medicine of South Ukrainian National Pedagogical University (September 2012–July 2016) and Sports Medicine of Lviv State University of Physical Culture (January 2021), on different sports bases of Odesa, Lviv and of team Ukraine. We analyzed the results of the study of 183 healthy men aged 21 ± 2 years who regularly engaged in various sports, did not complain of any problems in the state of the body, did not have acute diseases and were allowed to participate in sports according to the results of the last medical examination. The length of time in sports ranged from 3 to 15 years, the level of sportsmanship ranged from a candidate for master of sports to champions of Ukraine, Europe, the World, and the Olympic Games. All examinations were carried out in the morning, 2–3 h after a light breakfast. On the eve of the study, all participants were instructed by trainers to avoid consumption of stimulant beverages (coffee, green tea, energy drinks) before the examination. Taking into account that the examination was carried out in different periods of the annual training cycle, the main condition for admission to the study was the absence of intense and prolonged physical load the day before. Among the athletes who were examined were representatives of athletics (long and middle distance runners), rowers on kayaks and canoes, table tennis players, representatives of single combat sports (boxing, karate, freestyle wrestling, judo, Greco-Roman wrestling), representatives of games (volleyball, water polo, handball, soccer), as well as gymnasts, acrobats and shooters.
2.2 Procedure of study
The procedure for studying the cardiorespiratory system included conducting combined measurements of indicators of activity of the respiratory and cardiovascular systems in a sitting position using a Spiroarteriocardiorhythmograph (SACR) device (RRID:SCR_025431). The duration of the study was 6 min and involved the sequential registration of three measurements (2 min each) with a change in breathing rate. During the first 2 min, SACR indicators were recorded during normal spontaneous breathing (SR), during the second 2 min—during controlled breathing 6 times per minute (5 s inhalation, 5 s exhalation) (CR6), during the third 2 min—during controlled breathing 15 once per minute (2 s inhalation, 2 s exhalation) (CR15).
The only condition for exclusion from the analysis of the examination results was the presence of heart rhythm disturbances in the form of extrasystoles and non-sinus rhythm, which was establishing during the preliminary analysis of the examination records. A total of 11 such cases were registered. From 11 mentioned cases there were extrasystoles in three cases and non-sinus rhythm in two cases in rest. In six cases the extrasystoles appeared in breathing 6 times per minute. They are not included in the analysis group (Figure 1). There were no other complaints or violations during and after the breathing maneuver.
This study was approved by the Ethics Committee of the South Ukrainian National Pedagogical University (No. 121), by the Ethics Committee of the Lviv State University of Physical Culture (No. 33–16). All athletes were informed about the study and signed an informed consent form before the trial.
2.3 Method
The study was conducted using the SAСR device. ECG recording in 1 lead allowed to determine the indicators of HRV according to the spectral analysis of the sequence of RR intervals is total power (ТР, ms2), power in the very low frequency range (VLF, ms2), power in the low frequency (LF, ms2) and power in the high frequency range (HF, ms2) and their derivatives (LFn, n.u., HFn, n.u., LF/HF); according to the math analysis of the sequence of RR intervals were defined RMSSD (square root of the sum of squares of the differences in the values of consecutive pairs of normal intervals, ms), pNN50 (the percentage of NN50 from the total number of consecutive pairs of intervals that differ by more than 50 milliseconds, obtained over the entire time recording,%) (74); according to cardiointervalometry were defined the heart rate (HR, min−1), averages durations and intervals of PQRST-complex and indicator of systemic hemodynamics (75)—cardiac output (CO, dm3); according to the pulse wave recording with the help of a photoplethysmographic sensor on the finger by the Penaz method (54, 76), blood pressure variability in ranges similarly to HRV were determined a total power of SBP variability and DBP variability (ТРSBP, mmHg2 and ТРDBP, mmHg2), power in the very low-frequency range (VLFSBP, mmHg2 and VLFDBP, mmHg2), power in the low-frequency range (LFSBP, mmHg2 and LFDBP, mmHg2) and power in the high-frequency range (HFSBP, mmHg2 and HFDBP, mmHg2) (77–79). By using the spectral method we determined the index of arterial baroreflex sensitivity (BRS, ms × mmHg−1)—α-coefficient, that was calculated in high (BRHF) and low (BRLF) frequencies ranges (80–82).
The ultrasonic sensor of the SACR device allows to measure flows of air on inspiration and expiration and to define the average parameters of a respiration: duration of inspiratory (TI, s), duration of expiratory (TE, s), respiratory rate (RR, min−1) tidal volume (VT, L), as well as the volume of minute respiration (VE, L × min−1) (83, 84).
Indicators of frequency and volume synchronization of the cardio-respiratory system were also calculated—Hildebrandt index (HI) and VSI (72, 85).
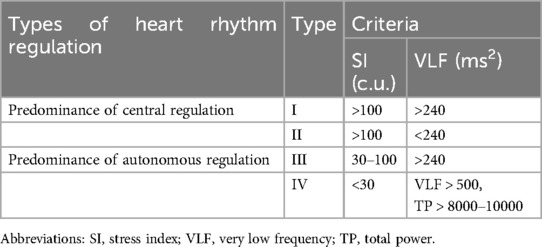
The type of HRR was studied in all athletes and was estimated in accordance with the method proposed by Shlyk et al. (34) (Table 1). Type III is considered the most optimal, which characterizes a moderate predominance of parasympathetic effects, which is characteristic of most people. Types I and II testify to the predominance of the central link activity of regulation and the development of moderate and severe sympathicotonia, but also reflect the activation of stress-realizing systems in the body. As for type IV, when there is a high variability of the heart rate, such a predominance of parasympathetic influences, on the one hand, may indicate the imperfection of central regulation and the development of autonomic dysfunctions, and on the other hand, the peculiarities of autonomic regulation during systematic training loads, especially during the development of the supercompensation condition in athletes (37, 38). In general, II and IV types of HRR are considered unstable and may characterize autonomic dysregulation.
The principles of classification of HRR types, taking into account the above criteria, are presented in Table 1.
According to the analysis of the HRV study results during spontaneous breathing, the athletes were divided into 4 groups, taking into account the type of HRR. Group 1 (with type I) consisted of 53 people, group 2 (with type II)—29 people, group 3 (with type III)—85 people, group 4 (with type IV)—16 people.
2.4 Statistical analysis
The results were processed using the STATISTICA program for Windows (version 10.0), Microsoft Excel 2012. The obtained data are presented as medians with 25%–75% (Q1; Q3) percentiles. Differences between initial and subsequent measurements were obtained using the Wilcoxon matched pairs test. At the next Two Way ANOVA test was performed.
3 Results
3.1 Morphofunctional data result
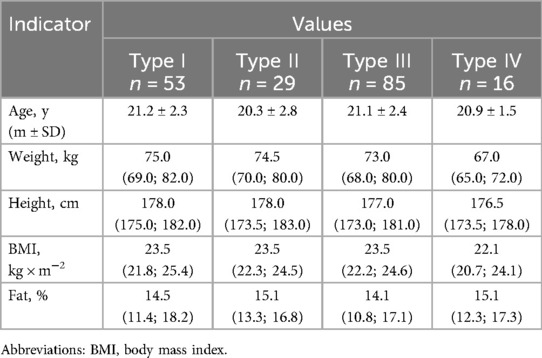
In the Table 2 presents the differences in the main morphometric indicators of athletes of the studied groups.
Analyzing the morphometric indicators, it should be noted that probable differences were obtained for the body weight of athletes with the IV type of HRR. Their weight was significantly lower compared to athletes of other groups (types I–III) (р = 0.010; р = 0.044; р = 0.027, respectively). The height of athletes of types I and IV also differed, p = 0.024, which indicated its lower values in athletes with an excessive predominance of parasympathetic regulation.
3.2 Intergroup differences of the main indicators used for the analysis of changes during a breathing maneuver at rest
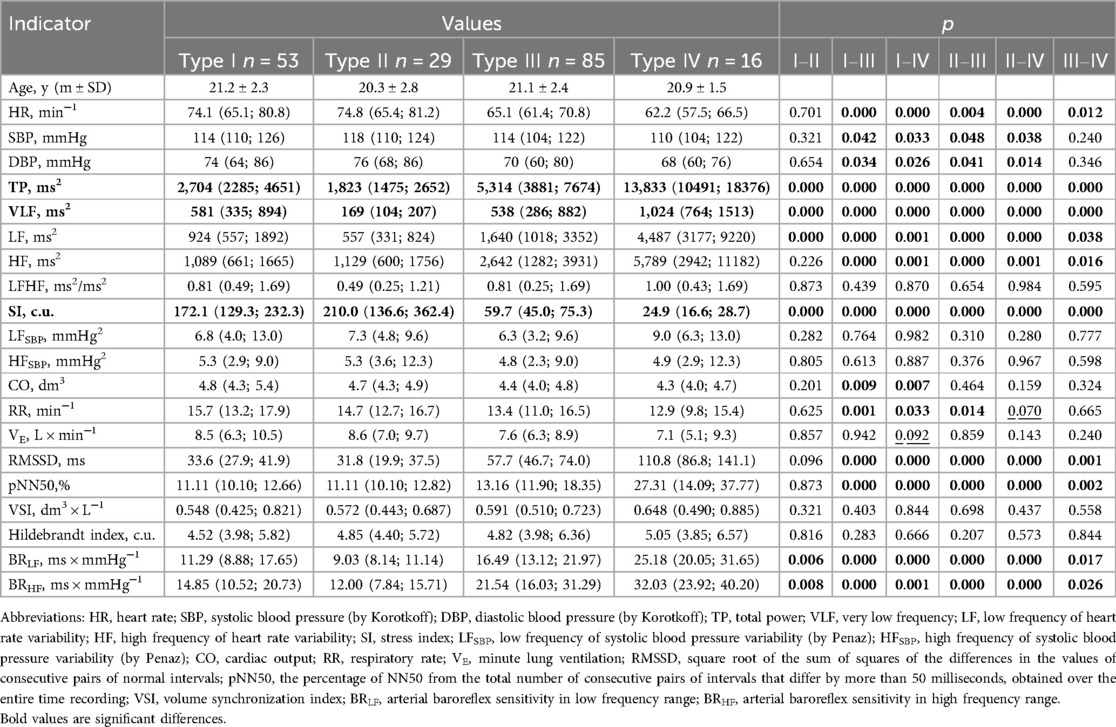
According to the proposed classification (34, 37), groups of athletes were quite clearly differentiated by HRV indicators—TP (ms2), VLF (ms2), SI (c.u.), which was quite expected (Table 3). As for other indicators of HRV, it should be noted that differentiation by indicators characterizing the activity of the parasympathetic link of the ANS—HF (ms2), RMSSD (ms) and pNN50 (%), for groups with a predominance of sympathetic influences (I and II types) not noted, p = 0.226; p = 0.096 and p = 0.873, respectively. Also, HR (min−1) did not differ between athletes with type I and type II. Most notable is the fact that none of the groups differed in LF/HF (ms2/ms2), which is most often used to characterize sympathy-vagal balance. Informative was the absence of differences between indicators of SBP variability in the low-frequency (SBPLF, mmHg2) and high-frequency (SBPHF, mmHg2) ranges, which were later used to calculate BRLF (ms × mmHg−1) and BRHF (ms × mmHg−1). That is, differences in BRS indicators (BRLF and BRHF) at rest were determined by differences in heart rate power in LF and HF ranges (LF, ms2 and HF, ms2). This allows us to state that the proposed approach to assessing HRR types is clearly combined with the differences in BRS in the low-frequency and high-frequency ranges. The highest BRS values are for type IV, slightly lower for type III, even lower for type I, and the lowest for type II of HRR. Accordingly, a significant predominance of parasympathetic influences is characterized by the largest BRS, and an excessive predominance of sympathetic ones is characterized by the smallest.
The significant results of the comparison, in our opinion, should also include the lack of differentiation in the state of rest during spontaneous breathing in the indicators of frequently and volume cardiorespiratory interaction—the HI (c.u.) and VSI (dm3 × L−1), which with different types of HRR did not differ, which proved their sufficient stability in athletes.
3.3 Differences in indicators of cardiorespiratory interaction during the breathing maneuver
At the next stage, the main task of the study was to analyze group differences in indicators of cardiorespiratory interaction during tests with controlled breathing (CR). We studied the increments of the specified indicators separately for CR6 and CR15. As it was mentioned earlier, indicators indicating cardiorespiratory interaction include indicators of frequently (HI) and volume (VSI) synchronization, indicators indicating cardiovascular interaction include indicators of BRS (BRLF and BRHF).
3.3.1 Differences in indicators of hildebrandt index during the breathing maneuver
According to the δ HI (c.u.) at CR6 and δ HI (c.u.) at CR15, central (types I, II) and autonomous (types III, IV) HRV are clearly differentiated (Table 4). That is, the undifferentiated values of the HI during spontaneous breathing in athletes with different types of HRR during controlled breathing CR6 and CR15 compared to SR clearly determine the central and autonomous variants of HRR. They are respectively characterized by a more significant increase in the HI at CR6 and CR15 with a predominance of sympathetic influences, as well as a less significant increase in the HI at CR6 and unchanged with a tendency to decrease at CR15 with a predominance of parasympathetic influences.
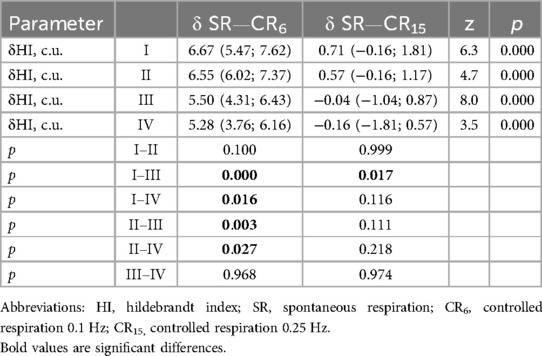
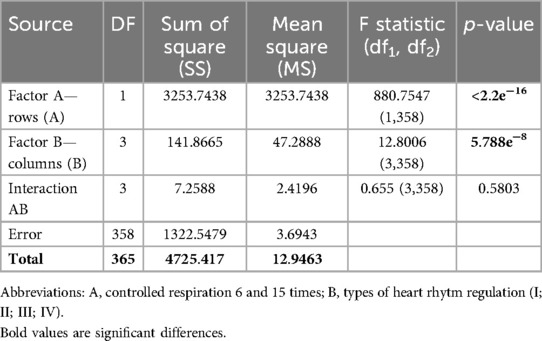
Table 4. Increments of indicators hildebrandt index (HI) in the examined athletes with type I-IV heart rhythm regulation during the breathing maneuver at CR6 and CR15 compared to SR, Med (Q1; Q3).
The results of the Two Way ANOVA test of the δHI (c.u.) indicator taking into account the type of HRR (Factor B) and controlled breathing (Factor A) are presented in Table 5.
3.3.2 Differences in indicators of volumes synchronization index during the breathing maneuver
There were no significant differences in the δVSI-CR6 index (dm3 × L−1) among athletes with different types of HRR. In one case of comparison, there was a tendency for a difference between the δVSI-CR15 (dm3 × L−1) indices in athletes with types I and II of HRR (Table 6), which indicates a lower reactivity of hemodynamic support of lung ventilation during the development of sympathetic overstrain (type II), which may be an additional criterion for the deterioration of the athletes' condition.
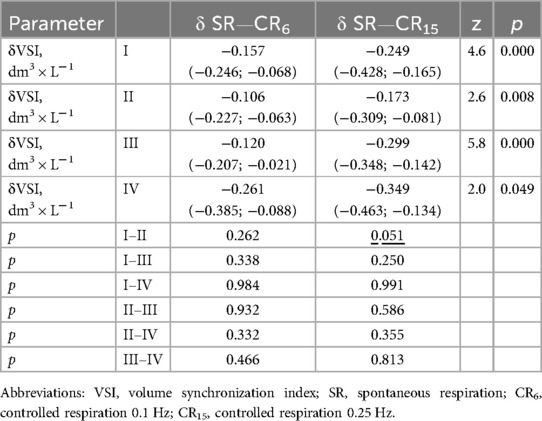
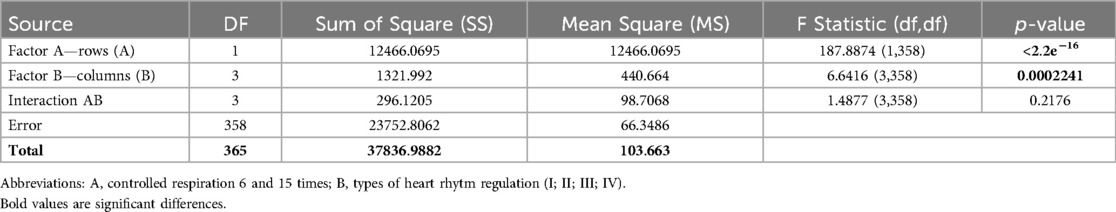
Table 6. Increments of indicators volumes synchronization index in the examined athletes with type I–IV heart rhythm regulation during the breathing maneuver at CR6 and CR15 compared to SR, Med (Q1; Q3).
The results of the Two Way ANOVA test of the δVSI (dm3 × L−1) indicator taking into account the type of HRR (Factor B) and controlled breathing (Factor A) are presented in Table 7.
3.3.3 Differences in indicators of sensitivity of the arterial baroreflex during the breathing maneuver
A number of significant differences during the respiratory maneuver were also obtained among the BRS increments, which indicate interaction in the cardiovascular system.
During spontaneous breathing, the values of BRLF (ms × mmHg−1) and BRHF (ms × mmHg−1) are quite clearly related to the type of HRR (Table 3). At the same time, the highest BRS values indicate a predominance of parasympathetic influences, and the lowest ones indicate a significant predominance of sympathetic influences. These data confirm the well-known facts regarding the influence of sympathetic and parasympathetic activity on BRS (57).
Considering the data presented in Tables 8, 9, it should be noted that δBRLF and δBRHF at CR6 are not differentiated in athletes with types I–III. At the same time, athletes with type IV at CR6 differ from other groups in various ways. For δBRLF significantly (p = 0.026)—from athletes with type III, with a tendency (p = 0.057)—from athletes with type I and do not differ at all from athletes with type II (p = 0.141). For δBRHF significantly (p = 0.038 and p = 0.043)—from athletes with types I and II, respectively, and with a tendency (p = 0.071)—from athletes with type III. That is, deep slow breathing, probably causing excessive vagotonic cardiorespiratory control, causes dysregulation in the low-frequency range, which may reflect similar cardiovascular relationships against the background of different initial states during the development of overstrain according to sympathetic (type II) and parasympathetic (type IV) variants. This distinguishes the latter (athletes with type IV) from athletes with moderate parasympathicotonia (type III). On the other hand, changes in baroreflex sensitivity in the high-frequency range (δBRHF) at CR6 in athletes with type IV still more attest to the features of redundancy characteristic of vagotonia, which is reflected in differences with sympathicotonic variants of HRR, although there is also a certain tendency to differences with moderate parasympathicotonia. On the other hand, hyperventilatory stimulation (CR15), which activates the sympathetic link of the ANS and a more pronounced decrease in BRS in the low-frequency and high-frequency ranges (δBRLF and δBRHF) in athletes with type IV compared to athletes with types I and II, and with a more pronounced tendency compared to type III. The reduced BRS reactivity (δBRLF and δBRHF) in response to a sympathetic stimulus (CR15) in athletes with a predominance of sympathetic influences (types I and II) seems to be quite informative.
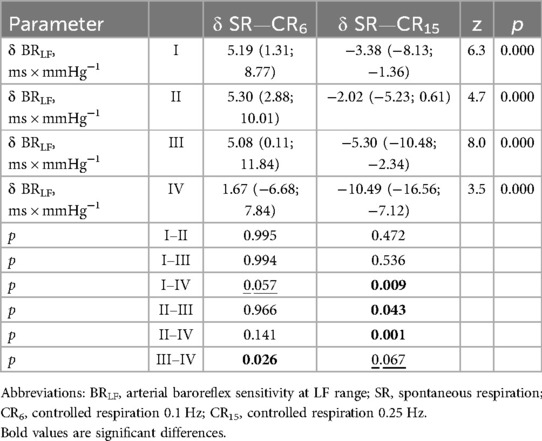
Table 8. Increments of indicators baroreflex sensitivity in low frequency range in the examined athletes with type I-IV heart rhythm regulation during the breathing maneuver at CR6 and CR15 compared to SR, Med (Q1; Q3).
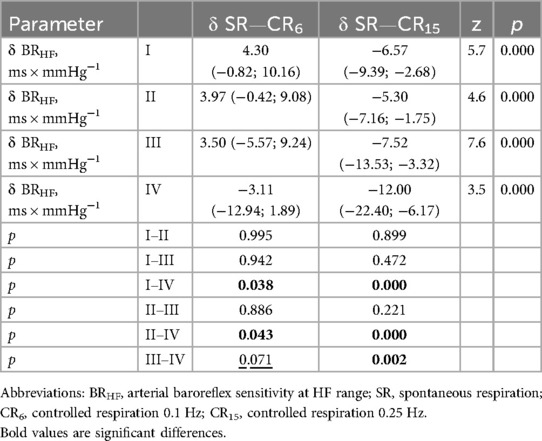
Table 9. Increments of indicators baroreflex sensitivity in high frequency range in the examined athletes with type I-IV heart rhythm regulation during the breathing maneuver at CR6 and CR15 compared to SR, Med (Q1; Q3).
That is, the differences in BRS increases at CR6 and at CR15 provide additional criteria for differentiating excessive parasympathetic overstrain in comparison with sympathetic regulation variants and moderate parasympathetic.
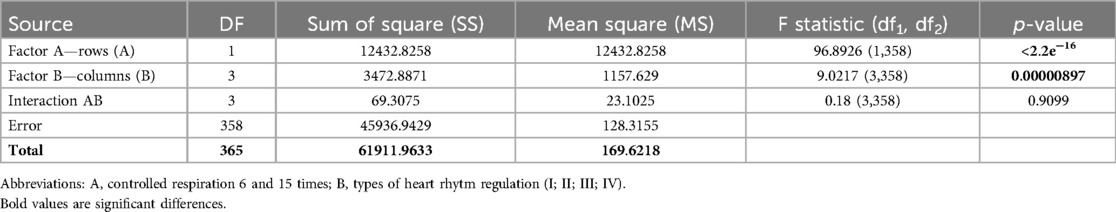
The results of the Two Way ANOVA test of the BRLF (ms × mmHg−1) indicator taking into account the type of HRR (Factor B) and controlled breathing (Factor A) are presented in Table 10.
The results of the Two Way ANOVA test of the BRHF (ms × mmHg−1) indicator taking into account the type of HRR (Factor B) and controlled breathing (Factor A) are presented in Table 11.
Table 12 summarizes the results obtained from the standpoint of the types of HRR differentiation by absolute values during spontaneous breathing and the increments of cardiorespiratory and cardiovascular interaction indicators during the respiratory maneuver, which may contribute to the algorithmization and supplementation of HRR assessment.
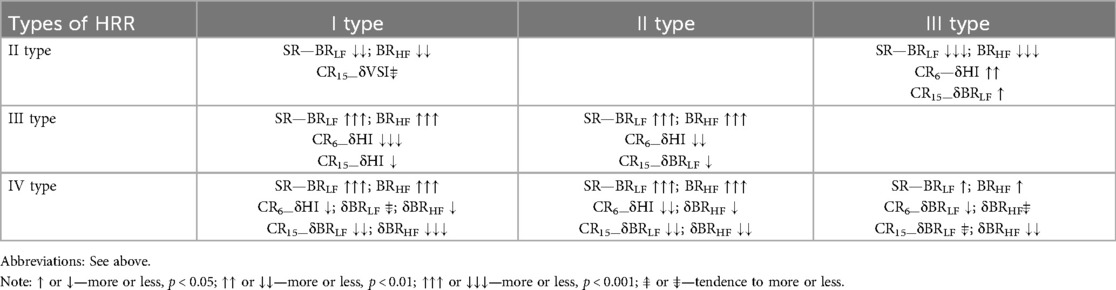
Table 12. Differences in the absolute indicators of intersystem interaction and their increments during a breathing maneuver with different types of heart rate regulation.
Moderate and severe sympathicotonia (types I and II) are poorly differentiated by the indicators of cardiorespiratory interaction during controlled breathing. The only indicator indicating a tendency to differences is the δVSI indicator, which during hyperventilation in athletes with an excessive predominance of sympathetic influences (type II) is lower compared to athletes with moderate sympathicotonia (type I). However, this may indicate dysregulation, which determines the relative deterioration of the heart pumping function during sympathetic activation during CR15.
Moderate and severe parasympathicotonia (types III and IV) are not differentiated at all by the indicators of frequency (HI) and volume (VSI) cardiorespiratory synchronization during controlled breathing. However, significant are the differences in vagotonic stimulation (CR6) by the indicator—δBRLF and sympathicotonic stimulation (CR15) by the indicator δBRHF, the increase of which in parasympathetic overstrain (IV type) indicates a significant decrease in BRS during controlled breathing.
The differences in the increase in the HI indicator during CR6 are noteworthy, which differentiate all variants of sympathicotonic and parasympathicotonic states from each other due to the different increase in HR (min−1), which in different types increases to different extents when performing vagostimulating breathing (CR6). During CR15, the increase in this indicator allows you to differentiate moderate parasympathicotonia (III type) from moderate sympathicotonia (I type).
4 Discussion
In the practice of sports medicine, to assess the functional state of the athlete's body as a whole and the activity of the cardiovascular system during current examinations in the training process is most often used the analysis of HRV indicators, which reflect the degree of load on the autonomous regulation of the cardiovascular system (31, 86–88). However, their joint analysis with the parameters of changes in vascular tone and spontaneous breathing has not been sufficiently studied (40, 89–92). In previous studies of changes in HRV at rest during the training process, its significant dependence on the frequency of spontaneous breathing and tidal volume was shown (62). This prompted us to use controlled breathing tests to standardize and unify the assessment of HRV changes (64, 67, 93). A breathing maneuver procedure has been proposed that allows us to detect the body's reactivity to influences that stimulate, at least, the activation of the sympathetic and parasympathetic branches of the ANS (69). It is important during testing to focus on the choice of frequency and duration of breathing phases. The CR6 with a fixed duration of inhalation and exhalation (5 s each) has been studied in many works and is considered resonant (0.1 Hz) for activating vagal baroreflex effects on HR (94, 95). This allows us to investigate the mechanism of baroreflex activation from the standpoint of its presence and severity in standardized conditions. After all, baroreflex activation also occurs during SR, but depends on its frequency and duration of inhalation and exhalation phases. Regarding the CR15 with a fixed duration of inhalation and exhalation (2 s each), something should be explained. The breathing frequency itself is within the optimal values for a healthy person. However, the duration of the inhalation and exhalation phases is significantly different from the proper ones (1:2). This also causes a change in the wave characteristics of the HRV (68), but with a constant frequency component (0.25 Hz), which is reflected in the HRV indicators, in which there is a more rigid redistribution towards the HF component compared to SR which is a physiological (67). With this breathing option, a certain tension in gas exchange occurs. As a rule, to implement such a breathing rhythm, a person must exhale more actively. As a result, the next breath will also be strengthened, but within the duration (2 s), which in turn will contribute to an increase in the tidal volume. This leads to moderate hyperventilation, which can reach 20 or more (L × min−1) at rest (69). The main thing in such conditions is the ability of the exhalation muscles to releasing the lungs from air. Accordingly, there is an impact on the circulatory system, namely the possibility of implementing the baroreflex mechanism, the sensitivity of which in this case is significantly reduced. That is, breathing with a controlled rhythm (2:2) is not physiological and allows us to characterize the response to moderate hyperventilation.
The main indicators that determined the differences between the types of HRR were the BRS indicators (BRLF and BRHF) during SR, which characterize the functioning of the negative feedback system that buffers short-term fluctuations in blood pressure by modifying cardiovascular parameters—HR (min−1) and SV (ml). There are a sufficient number of publications that analyze changes in BRS under the influence of various factors, including training loads, which indicate the features of intersystem interaction and allow us to characterize the level of stress of the body after performing the load, its recovery, or the tendency to develop overstrain of the cardiovascular system (94, 96, 97). At the same time, the clinical significance of a decrease in BRS during SR is determined by the ability to early diagnose disorders of autonomic function (98–100) due to a decrease in inhibitory activity and an imbalance of physiological sympathovagal outflow to the heart, which leads to chronic adrenergic activation (101). The effects of a decrease in BRS are usually associated with a decrease in the LF component of HRV, which was confirmed in this work. Against the background of an increase in HR (min−1), the LF component of HRV (ms2) decreases and the LF component of BP (mmHg2) increases (102), which additionally affects the decrease in the BRLF (ms × mm Hg−1). The results obtained during the study of highly qualified athletes with signs of the development of sympathetic and parasympathetic overstrain under the influence of intense physical load also showed that BRLF (ms × mmHg−1) and BRHF (ms × mmHg−1) during SR in athletes with both types of overstrain decrease immediately after exercise, and do not recover to their original values the next day (40, 103). A study of athletes at competitions showed differences in the recovery of BRLF (ms × mmHg−1) and BRHF (ms × mm Hg−1). Guzii and Romanchuk (102), which may be an additional criterion for the body's recovery after training and competitions. Subsequently, it was shown that conditions with impaired bronchial patency and hypertension also significantly affect baroreceptor function, which may underlie the mechanisms of formation of hypertensive states (65, 66, 104), and may also be useful for determining and predicting changes in asthma formation due to physical stress and the development of parasympathetic dysregulation. In general, BRS indicators (BRLF and BRHF) are key in determining the mechanisms of maintaining vascular homeostasis and indicators of autonomic control (70, 105, 106). The results obtained in this study indicate that BRS in SR is associated with the features of autonomic HRV and may indicate a significant predominance of central or autonomic mechanisms and evidence the development of dysregulation. The lowest BRS is during the development of fatigue, the formation of sympathetic overstrain (type II), and the highest is during the formation of supercompensation states, or parasympathetic overstrain (type IV). Previous studies have shown that BRS significantly increases during deep slow breathing (CR6) from 14.2 (9.7; 20.1) to 20.4 (14.6; 26.7), p = 0.000 for BRLF (ms × mmHg−1) and from 18.3 (11.9; 27.5) to 20.8 (15.4; 30.4), p = 0.000 for BRHF (ms × mmHg−1). This was confirmed by the results of dynamic observation of endurance athletes, who showed a more pronounced increase in BRLF (ms × mmHg−1) at CR6 (107), which may also indicate the formation of excessive control over cardiorespiratory relationships (45). At the same time, with deeper, more frequent controlled breathing (CR15), BRS significantly decreases, even compared to SR, to 9.4 (6.8; 12.9), p = 0.000 for BRLF (ms × mm Hg−1) and to 10.3 (6.2; 15.6), p = 0.000 for BRHF (ms × mmHg−1) (69), which is due to the activation of sympathetic influences during moderate hyperventilation. In general, the results obtained in this study confirm and complement the known data on the effect of controlled breathing on BRS (95, 108–110). The results of this study demonstrate that δBRLF at CR6 in athletes with type II and IV, which are similar, suggest the formation of dysregulations by sympathetic and parasympathetic types against the background of excessive cardiorespiratory control during vagal-stimulating breathing. At the same time, during CR15, δBRHF in athletes of these groups is significantly different, indicating different reactivity to sympathostimulating influences.
A well-known and widely used indicator reflecting cardiorespiratory interaction is the Hildebrandt index (72). The possibilities of simultaneous measurement of HR (min−1) and RR (min−1) significantly complement the known data, taking into account many components that affect the value of this indicator. Currently, technologies and clothing devices are being developed that will allow for more accurate determination and clear analysis of this parameter in different conditions (111). The technology of simultaneous measurement of indicators of the cardiovascular and respiratory systems makes it possible to develop a number of both frequency and discrete parameters of cardiorespiratory interaction (73, 85, 90). As an example, we can cite the data obtained directly in the procedure of manual correction of the thoracic spine, when this indicator changed due to significant changes in the duration of exhalation (112). In this study, it was shown that none of the groups that differed in the type of HRR during SR differed in HI (cu), despite the differences in HR (min−1). On the other hand, when performing the respiratory maneuver, differences are recorded that clearly distinguish the central and autonomous types of HRR. It was previously shown that the HI (cu) in the group of active men during tests with controlled breathing significantly increases from 4.77 (3.98; 6.20) to 10.92 (9.97; 12.14), p = 0.000 at CR6, but remains unchanged compared to SR 5.00 (4.48; 5.84), p = 0.209 at CR15 (69). Other authors have demonstrated its increase during physical activity, which occurred due to a relative increase in HR (min−1) compared to RR (min−1). The latter, in their opinion, indicates its informativeness regarding the strengthening of sympathetic influences and the “physiological price” of the work performed and predicts the refusal of intensive physical activity (113). Here it is worth recalling the results of the analysis of the breathing pattern, which was carried out in athletes with different types of HRR and showed the development of symptoms of expiratory insufficiency in athletes with type II (84). From these positions, a less pronounced increase in HI (cu) when performing a breathing maneuver with CR6 and CR15 at the end of the training cycle for the development of strength endurance (107) indicates an increase in the reserve capabilities of the cardiorespiratory system.
The possibility of simultaneous registration of ECG and respiratory air flows allowed us to verify the use of a discrete parameter (VSI, dm3 × L−1), which characterizes the ratio between cardiac output and minute lungs ventilation. It takes into account adaptive changes in both the cardiovascular and respiratory systems, which are somehow interconnected, due to the intake and transport of oxygen in the body. When studying VSI (dm3 × L−1), which characterizes volumetric synchronization, it was previously shown that when performing a respiratory maneuver with a change in RR (min−1), it significantly decreases from 0.586 (0.477; 0.745) during SR to 0.441 (0.327; 0.582) at CR6 to 0.360 (0.247; 0.477), p = 0.000 at CR15 (69). Other studies have shown that it increases with load (113). That is, this indicator can be used to characterize the adequacy of the response to physical activity and energy expenditure. When examining 202 highly qualified athletes before, after and the next morning after training, fairly stable indicators were obtained, which indicates the possibility of using this indicator to characterize the individual ability of the body to tolerate the load and recover after it—before 0.566 (0.448; 0.765), after 0.574 (0.432; 0.790), the next morning 0.593 (0.482; 0.891) (114). At the same time, the results obtained during the respiratory maneuver indicate the peculiarities of its changes under the influence of training on the development of strength endurance, which indicates the adaptation of cardiorespiratory relationships under the influence of training, which is characterized by efficiency and economy oxygen supply, which prevents the development of excessive sympathoadrenal activation. Thus, strength endurance training leads to an increase and VSI index (dm3 × L−1) during SR at rest from 0.597 (0.490; 0.832) to 0.725 (0.564; 1.148), p = 0.008, which reflects its increase during CR6 from 0.327 (0.382; 0.529) to 0.532 (0.441; 0.723), p = 0.012, and at CR15 from 0.245 (0.339; 0.455) to 0.481 (0.373; 0.616), p = 0.003. This proved better adaptation of the cardiorespiratory system to the suppression of postganglionic activity at CR6 and the activation of hyperventilation at CR15. From these points of view, the results of this study allowed us to clearly differentiate sympathetic overstrain (type II), which was characterized by the lowest variability of the VSI indicator (dm3 × L−1) during the respiratory maneuver, which can be used as a separate parameter characterizing the development of functional or non-functional overstrain of the athletes' body (52, 115).
That is, determining changes in intersystem relationships during the performance of a respiratory maneuver with a change in RR in athletes with different types of HRR made it possible to supplement information about the conditions for the occurrence of excessive predominance of sympathetic and parasympathetic HRR. The latter characterize the development of sympathetic and parasympathetic overstrain, which correlates with the development of functional and non-functional overstrain and precedes the formation of overtraining in the body of athletes. That's may contribute to the detection of these conditions in the field examinations of athletes during current and operational control.
5 Limitation
The study analyzed the results of testing highly qualified athletes of different sports at different periods of the training process, which does not allow linking the results obtained with the type of loads performed and direct VO2peak. It is also necessary to emphasize that the examined group of athletes was engaged in sports of different orientation and intensity. This somewhat limits the possibility of taking into account indicators of intersystem interaction in determining the functional state of the body in athletes of specific sports. Also, the issues of group differentiation due to their uneven distribution in this study have not been fully resolved. At the same time, the expressness of this study (6 min), taking into account the multifunctionality of such an examination and the possibility of conducting it in field conditions, allows not only to unify and individualize the multiparameter assessment, but also to refine it in the future, which opens up new prospects for current monitoring of the athletes’ condition.
6 Conclusions
The use of a respiratory maneuver in a combined study of the cardiorespiratory system in the conditions of current control of athletes showed informativeness in the differentiation of HRR types and states of functional overstrain.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Lviv State University of Physical Culture (No. 33-16). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
OR: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship. The publication of article was possible with support Frontier.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fspor.2024.1451643/full#supplementary-material
References
1. Pla R, Aubry A, Resseguier N, Merino M, Toussaint J-F, Hellard P. Training organization, physiological profile and heart rate variability changes in an open-water world champion. Int J Sports Med. (2019) 40(08):519–27. doi: 10.1055/a-0877-6981
2. Campos F, Molina Correa JC, Canevari VCM, Branco BHM, Andreato LV, De Paula Ramos S. Monitoring internal training load, stress-recovery responses, and immune-endocrine parameters in Brazilian Jiu-Jitsu training. J Strength Cond Res. (2022) 36(3):723–31. doi: 10.1519/JSC.0000000000003507
3. Greenham G, Buckley JD, Garrett J, Eston R, Norton K. Biomarkers of physiological responses to periods of intensified, non-resistance-based exercise training in well-trained male athletes: a systematic review and meta-analysis. Sports Med. (2018) 48(11):2517–48. doi: 10.1007/s40279-018-0969-2
4. Cadegiani FA, Kater CE. Basal hormones and biochemical markers as predictors of overtraining syndrome in male athletes: the EROS-basal study. J Athl Train. (2019) 54(8):906–14. doi: 10.4085/1062-6050-148-18
5. Blasco-Lafarga C, Martinez-Navarro I, Mateo-March M. Is baseline cardiac autonomic modulation related to performance and physiological responses following a supramaximal judo test? PLoS One. (2013) 8(10):e78584. doi: 10.1371/journal.pone.0078584
6. Porta A, Takahashi ACM, Catai AM. Cardiovascular coupling during graded postural challenge: comparison between linear tools and joint symbolic analysis. Braz J Phys Ther. (2016) 20(5):461–70. doi: 10.1590/bjpt-rbf.2014.0179
7. Ouergui I, Ardigò LP, Selmi O, Levitt DE, Chtourou H, Bouassida A, et al. Changes in perceived exertion, well-being, and recovery during specific judo training: impact of training period and exercise modality. Front Physiol. (2020) 11:931. doi: 10.3389/fphys.2020.00931
8. Mello R, Mello R, Gomes D, Paz GA, Nasser I, Miranda H, et al. Oxidative stress and antioxidant biomarker responses after a moderate-intensity soccer training session. Res Sports Med. (2017) 25(3):322–32. doi: 10.1080/15438627.2017.1345738
9. Horta TAG, BaraFilho MG, Coimbra DR, Miranda R, Werneck FZ. Training load, physical performance, biochemical markers, and psychological stress during a short preparatory period in Brazilian elite male volleyball players. J Strength Cond Res. (2019) 33(12):3392–9. doi: 10.1519/JSC.0000000000002404
10. Parizher G, Emery MS. Exercise stress testing in athletes. Clin Sports Med. (2022) 41(3):441–54. doi: 10.1016/j.csm.2022.02.006
11. Lässing J, Maudrich T, Kenville R, Uyar Z, Bischoff C, Fikenzer S, et al. Intensity-dependent cardiopulmonary response during and after strength training. Sci Rep. (2023) 13(1):6632. doi: 10.1038/s41598-023-33873-x
12. Djaoui L, Haddad M, Chamari K, Dellal A. Monitoring training load and fatigue in soccer players with physiological markers. Physiol Behav. (2017) 181:86–94. doi: 10.1016/j.physbeh.2017.09.004
13. Lee EC, Fragala MS, Kavouras SA, Queen RM, Pryor JL, Casa DJ. Biomarkers in sports and exercise: tracking health, performance, and recovery in athletes. J Strength Cond Res. (2017) 31(10):2920–37. doi: 10.1519/JSC.0000000000002122
14. Flatt AA, Esco MR, Nakamura FY. Individual heart rate variability responses to preseason training in high level female soccer players. J Strength Cond Res. (2017) 31(2):531–8. doi: 10.1519/JSC.0000000000001482
15. Guzii OV, Romanchuk AP, Mahlovanyy AV. Sensorimotor indicators as criteria of the intense physical loads influence on the athlete’s body. Ukrains Kij Zurnal Medicini Biologii ta Sportu. (2020) 5(3):351–8. doi: 10.26693/jmbs05.03.351
16. Romanchuk OP, Guziy OV. The central level of sensorimotor regulation of athletes during the formation of overstrain cardiovascular system. Fizicna Reabilitacia ta Rekreacijno-Ozdorovci Tehnologii. (2020) 5(1):41–51. doi: 10.15391/prrht.2020-5(1).06
17. Korobeynikov G, Mazmanian K, Korobeynikova L, Jagiello W. Diagnostics of psychophysiological states and motivation in elite athletes. Bratisl Med J. (2011) 112(11):637–43. Available online at: http://www.ncbi.nlm.nih.gov/pubmed/22180992
18. Piatysotska S, Podrіgalo L, Romanenko V, Zhernovnikova Y, Dolgopolova N, Yefremenko A. Comparative analysis of motor functional asymmetry indicators in athletes of cyclic sports, martial arts, and esports. Phys Edu Stud. (2023) 27(4):212–20. doi: 10.15561/20755279.2023.0408
19. Bellenger CR, Thomson RL, Davison K, Robertson EY, Buckley JD. The impact of functional overreaching on post-exercise parasympathetic reactivation in runners. Front Physiol. (2021) 11:1–8. doi: 10.3389/fphys.2020.614765
20. Bentley RF, Vecchiarelli E, Banks L, Gonçalves PEO, Thomas SG, Goodman JM. Heart rate variability and recovery following maximal exercise in endurance athletes and physically active individuals. Appl Physiol Nutr Metab. (2020) 45(10):1138–44. doi: 10.1139/apnm-2020-0154
21. Angelova M, Holloway PM, Shelyag S, Rajasegarar S, Rauch HGL. Effect of stress on cardiorespiratory synchronization of ironman athletes. Front Physiol. (2021) 12:1–16. doi: 10.3389/fphys.2021.612245
22. Nuuttila OP, Nikander A, Polomoshnov D, Laukkanen JA, Häkkinen K. Effects of HRV-guided vs predetermined block training on performance, HRV and serum hormones. Int J Sports Med. (2017) 38(12):909–20. doi: 10.1055/s-0043-115122
23. Dong JG. The role of heart rate variability in sports physiology (review). Exp Ther Med. (2016) 11(5):1531–6. doi: 10.3892/etm.2016.3104
24. Tonello L, Rodrigues FB, Souza JWS, Campbell CSG, Leicht AS, Boullosa DA. The role of physical activity and heart rate variability for the control of work related stress. Front Physiol. (2014) 5:67. doi: 10.3389/fphys.2014.00067
25. Censi F, Calcagnini G, Cerutti S. Coupling patterns between spontaneous rhythms and respiration in cardiovascular variability signals. Comput Methods Programs Biomed. (2002) 68(1):37–47. doi: 10.1016/S0169-2607(01)00158-4
26. Saul JP, Berger RD, Chen MH, Cohen RJ. Transfer function analysis of autonomic regulation. II. Respiratory sinus arrhythmia. Am J Physiol. (1989) 256(1):H153–61. doi: 10.1152/ajpheart.1989.256.1.h153
27. Bellenger CR, Karavirta L, Thomson RL, Robertson EY, Davison K, Buckley JD. Contextualizing parasympathetic hyperactivity in functionally overreached athletes with perceptions of training tolerance. Int J Sports Physiol Perform. (2016) 11(7):685–92. doi: 10.1123/ijspp.2015-0495
28. Le Meur Y, Pichon A, Schaal K, Schmitt L, Louis J, Gueneron J, et al. Evidence of parasympathetic hyperactivity in functionally overreached athletes. Med Sci Sports Exercise. (2013) 45(11):2061–71. doi: 10.1249/MSS.0b013e3182980125
29. Leti T, Bricout VA. Interest of analyses of heart rate variability in the prevention of fatigue states in senior runners. Auton Neurosci. (2013) 173(1–2):14–21. doi: 10.1016/j.autneu.2012.10.007
30. Meeusen R, Duclos M, Foster C, Fry A, Gleeson M, Nieman D, et al. Prevention, diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the European college of sport science and the American college of sports medicine. Med Sci Sports Exercise. (2013) 45(1):186–205. doi: 10.1249/MSS.0b013e318279a10a
31. Esco MR, Williford HN, Flatt AA, Freeborn TJ, Nakamura FY. Ultra-shortened time-domain HRV parameters at rest and following exercise in athletes: an alternative to frequency computation of sympathovagal balance. Eur J Appl Physiol. (2018) 118(1):175–84. doi: 10.1007/s00421-017-3759-x
32. Romanchuk OP, Guziy OV. Modern approaches to the objectification of the functional state of the athletes’ body during current examinations. Fiz Reabil ta Rekreac Ozdor Tehnol. (2020) 5(1):8–18. doi: 10.15391/prrht.2020-5(1).02
33. Guzii O, Romanchuk А, Mahlovanyy A. Post-loading dynamics of heart rate variability indices in highly qualified athletes in the formation of overstrains by sympathetic and parasympathetic types. Art Med. (2020) 4(16):28–36. doi: 10.21802/artm.2020.4.16.28
34. Shlyk NI, Sapozhnikova EN, Kirillova TG, Semenov VG. Typoloagical characteristics of the functional state of regulatory systems in schoolchildren and young athletes (according to heart rate variability data). Hum Physiol. (2009) 35(6):730–8. doi: 10.1134/S0362119709060103
35. Alderman BL, Olson RL. The relation of aerobic fitness to cognitive control and heart rate variability: a neurovisceral integration study. Biol Psychol. (2014) 99(1):26–33. doi: 10.1016/j.biopsycho.2014.02.007
36. Blake TA, McKay CD, Meeuwisse WH, Emery CA. The impact of concussion on cardiac autonomic function: a systematic review. Brain Inj. (2016) 30(2):132–45. doi: 10.3109/02699052.2015.1093659
37. Shlyk NI. Management of athletic training taking into account individual heart rate variability characteristics. Hum Physiol. (2016) 42(6):655–64. doi: 10.1134/S0362119716060189
38. Shlyk N. Heart rate variability at rest and during an orthostatic challenge at different ranges of MxDMn values in female skiers in the training process. Sci Sport Curr Trends. (2020) 8(1):83–96. doi: 10.36028/2308-8826-2020-8-1-83-96
39. Abreu RM, Cairo B, Porta A. On the significance of estimating cardiorespiratory coupling strength in sports medicine. Front Netw Physiol. (2023) 2:1–7. doi: 10.3389/fnetp.2022.1114733
40. Guzii O, Mahlovanyi A, Romanchuk O. Multifunctional changes in the athletes’ body during the formation of autonomic regulations’ overstrain under the influence of training load. Phys Rehab Recreat Health Technol. (2023) 8(2):91–104. doi: 10.15391/prrht.2023-8(2).03
41. Bringard A, Adami A, Fagoni N, Fontolliet T, Lador F, Moia C, et al. Dynamics of the RR-interval versus blood pressure relationship at exercise onset in humans. Eur J Appl Physiol. (2017) 117(4):619–30. doi: 10.1007/s00421-017-3564-6
42. Sin PYW, Galletly DC, Tzeng YC. Influence of breathing frequency on the pattern of respiratory sinus arrhythmia and blood pressure: old questions revisited. Am J Physiol Heart Circ Physiol. (2010) 298(5):H1588–99. doi: 10.1152/ajpheart.00036.2010
43. Cottin F, Papelier Y, Escourrou P. Effects of exercise load and breathing frequency on heart rate and blood pressure variability during dynamic exercise. Int J Sports Med. (1999) 20(4):232–8. doi: 10.1055/s-2007-971123
44. Cottin F, Medigue C, Papelier Y. Effect of heavy exercise on spectral baroreflex sensitivity, heart rate, and blood pressure variability in well-trained humans. Am J Physiol Heart Circ Physiol. (2008) 295(3):H1150–5. doi: 10.1152/ajpheart.00003.2008
45. Porta A, de Abreu RM, Bari V, Gelpi F, De Maria B, Catai AM, et al. On the validity of the state space correspondence strategy based on k-nearest neighbor cross-predictability in assessing directionality in stochastic systems: application to cardiorespiratory coupling estimation. Chaos. (2024) 34:5. doi: 10.1063/5.0192645
46. Krohn F, Novello M, van der Giessen RS, De Zeeuw CI, Pel JJM, Bosman LWJ. The integrated brain network that controls respiration. eLife. (2023) 12:1–67. doi: 10.7554/eLife.83654
47. Abreu RM de, Porta A, Rehder-Santos P, Cairo B, Sakaguchi CA, da Silva CD, et al. Cardiorespiratory coupling strength in athletes and nonathletes. Respir Physiol Neurobiol. (2022) 305:103943. doi: 10.1016/j.resp.2022.103943
48. Zoccal DB, Machado BH, Moraes DJA. Cardiorespiratory interactions in health and disease. In: Biaggioni I, Browning K, Fink G, Jordan J, Low PA, Paton JFR, editors. Primer on the Autonomic Nervous System. 4 ed. London: Academic Press (2023). p. 165–9. doi: 10.1016/B978-0-323-85492-4.00043-0
49. Pinsky MR. Cardiopulmonary interactions: physiologic basis and clinical applications. Ann Am Thorac Soc. (2018) 15(1):S45–8. doi: 10.1513/AnnalsATS.201704-339FR
50. Russo MA, Santarelli DM, O’Rourke D. The physiological effects of slow breathing in the healthy human. Breathe. (2017) 13(4):298–309. doi: 10.1183/20734735.009817
51. Bilo G, Revera M, Bussotti M, Bonacina D, Styczkiewicz K, Caldara G, et al. Effects of slow deep breathing at high altitude on oxygen saturation, pulmonary and systemic hemodynamics. PLoS One. (2012) 7(11):e49074. doi: 10.1371/journal.pone.0049074
52. Baumert M, Brechtel L, Lock J, Hermsdorf M, Wolff R, Baier V, et al. Heart rate variability, blood pressure variability, and baroreflex sensitivity in overtrained athletes. Clin J Sport Med. (2006) 16(5):412–7. doi: 10.1097/01.jsm.0000244610.34594.07
53. Dempsey JA, Sheel AW, St. Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol. (2002) 130(1):3–20. doi: 10.1016/S0034-5687(01)00327-9
54. Wesseling KH. Finapres, continuous noninvasive finger arterial pressure based on the method of penaz. In: Meyer-Sabellek W, Gotzen R, Anlauf M, Steinfeld L, editors. Blood Pressure Measurements. Heidelberg: Steinkopff (1990). p. 161–72. doi: 10.1007/978-3-642-72423-7_18
55. Pivovarov VV. A spiroarteriocardiorhythmograph. Med Tekh. (2006) (1):38–40. Available online at: http://www.ncbi.nlm.nih.gov/pubmed/16610287
56. Fisher JP, Zera T, Paton JFR. Respiratory-cardiovascular interactions. In: Chen R, Guyenet PG, editors. Handbook of Clinical Neurology. Elsevier B.V (2022). p. 279–308. doi: 10.1016/B978-0-323-91534-2.00006-0
57. Porta A, Guzzetti S, Montano N, Pagani M, Somers V, Malliani A, et al. Information domain analysis of cardiovascular variability signals: evaluation of regularity, synchronisation and co-ordination. Med Biol Eng Comput. (2000) 38(2):180–8. doi: 10.1007/BF02344774
58. Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen PS, Esler M, et al. Clinical neurocardiology defining the value of neuroscience based cardiovascular therapeutics. J Physiol. (2016) 594(14):3911–54. doi: 10.1113/JP271870
59. Dick TE, Hsieh YH, Dhingra RR, Baekey DM, Galán RF, Wehrwein E, et al. Cardiorespiratory coupling: common rhythms in cardiac, sympathetic, and respiratory activities. In Prog Brain Res. (2014) 209:191–205. doi: 10.1016/B978-0-444-63274-6.00010-2
60. Garcia AJ, Koschnitzky JE, Dashevskiy T, Ramirez J-M. Cardiorespiratory coupling in health and disease. Auton Neurosci. (2013) 175(1–2):26–37. doi: 10.1016/j.autneu.2013.02.006
61. Wang YP, Kuo TBJ, Lai CT, Chu JW, Yang CCH. Effects of respiratory time ratio on heart rate variability and spontaneous baroreflex sensitivity. J Appl Physiol. (2013) 115(11):1648–55. doi: 10.1152/japplphysiol.00163.2013
62. Romanchuk O. To a question of an estimation of activity of autonomic nervous system at sportsmen. Med Rehab Balneol Phys. (2005) (4):31–4. doi: 10.5281/zenodo.18109
63. Romanchuk A, Pisaruk V. Change of central hemodynamics of qualified athletes for testing the use of controlled breathing and evaluation. Pedagog Psychol Med Biol Prob Phys Training Sports. (2013) 11:77–84. doi: 10.6084/m9.figshare.817930
64. Guzii OV, Romanchuk AP. Heart rate variability during controlled respiration after endurance training. J Phys Educ Sport. (2017) 17(3):2024–9. doi: 10.7752/jpes.2017.03203
65. Romanchuk A, Shtanko V, Bekalo I. Lizinopril monotherapy and sensitivity of the baroreflex in patients with hypertension. IOSR J Dental Med Sci. (2019) 18(1):74–9. doi: 10.9790/0853-1801127479
66. Romanchuk OP, Velychko VI, Bazhora YI. Reactivity of cardiorespiratory system in bronchial asthma patients according to the tests with respiratory maneuvers performance. Zaporozhye Med J. (2019) 21(4):449–57. doi: 10.14739/2310-1210.2019.4.173191
67. Guzii О, Romanchuk A, Мahlovanyi A, Trach V. Polyfunctional express-evaluation criteria of the sportsman organism state. J Phys Edu Sport. (2019) 2019(4):2352–8. doi: 10.7752/jpes.2019.04356
68. de Maria B, Dalla Vecchia LA, Maestri R, Pinna GD, Parati M, Perego F, et al. Lack of association between heart period variability asymmetry and respiratory sinus arrhythmia in healthy and chronic heart failure individuals. PLoS One. (2021) 16:e0247145. doi: 10.1371/journal.pone.0247145
69. Romanchuk O. Cardiorespiratory dynamics during respiratory maneuver in athletes. Front Netw Physiol. (2023) 3:1–22. doi: 10.3389/fnetp.2023.1276899
70. Porta A, Maestri R, Bari V, De Maria B, Cairo B, Vaini E, et al. Paced breathing increases the redundancy of cardiorespiratory control in healthy individuals and chronic heart failure patients. Entropy. (2018) 20(12):949. doi: 10.3390/e20120949
71. Fadel PJ. Arterial baroreflex control of the peripheral vasculature in humans: rest and exercise. Med Sci Sports Exercise. (2008) 40(12):2055–62. doi: 10.1249/MSS.0b013e318180bc80
72. Hildebrandt G. Uber die rhythmische funktionsordnung von puls und atem. Z Klin Med. (1953) 150(5):444–54. Available online at: http://www.ncbi.nlm.nih.gov/pubmed/1313728813137288
73. Noskin LA, Rubinsky AV, Romanchuk AP. Indications of the level individual cardiovascular and respiratory homeostasis using continuous spiroarteriocardiorhythmography. Biomed J Sci Tech Res. (2018) 6(1):1–2. doi: 10.26717/bjstr.2018.06.001309
74. Malik M, Camm AJ, Bigger JT, Breithardt G, Cerutti S, Cohen RJ, et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. (1996) 17(3):354–81. doi: 10.1093/oxfordjournals.eurheartj.a014868
75. Kim TH, Hur J, Kim SJ, Kim HS, Choi BW, Choe KO, et al. Two-phase reconstruction for the assessment of left ventricular volume and function using retrospective ECG-gated MDCT: comparison with echocardiography. Am J Roentgenol. (2005) 185(2):319–25. doi: 10.2214/ajr.185.2.01850319
76. Penaz J. Photoelectric measurement of blood pressure, volume and flow in the finger. Digest of the 10th International Conference on Medical and Biological Engineering, Dresden, Germany. (1973). p. 104.
77. Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation: a critical appraisal. Hypertension. (1995) 25(6):1276–86. doi: 10.1161/01.HYP.25.6.1276
78. Pinna GD, Maestri R, Mortara A. Estimation of arterial blood pressure variability by spectral analysis: comparison between finapres and invasive measurements. Physiol Meas. (1996) 17(3):147–69. doi: 10.1088/0967-3334/17/3/002
79. Karemaker JM. Heart rate variability: why do spectral analysis? Heart. (1997) 77(2):99–101. doi: 10.1136/hrt.77.2.99
80. Karemaker JM, Wesseling KH. Variability in cardiovascular control: the baroreflex reconsidered. Cardiovasc Eng. (2008) 8(1):23–9. doi: 10.1007/s10558-007-9046-4
81. Papaioannou TG, Fasoulis R, Toumpaniaris P, Tsioufis C, Dilaveris P, Soulis D, et al. Assessment of arterial baroreflex sensitivity by different computational analyses of pressure wave signals alone. Comput Methods Programs Biomed. (2019) 172:25–34. doi: 10.1016/j.cmpb.2019.02.002
82. Rydlewska A, Ponikowska B, Borodulin-Nadzieja L, Banasiak W, Jankowska EA, Ponikowski P. Assessment of the functioning of autonomic nervous system in the context of cardiorespiratory reflex control. Kardiol Pol. (2010) 68(8):951–7. Available online at: http://www.ncbi.nlm.nih.gov/pubmed/2073073420730734
83. Bazhora YI, Romanchuk OP. Variability and respiration pattern of patients with persistent asthma and obesity. Ukrains kij Zurnal Medicini Biologii ta Sportu. (2018) 3(7):74–83. doi: 10.26693/jmbs03.07.074
84. Romanchuk A, Guzii O. Variability and pattern of spontaneous respiration in different types of cardiac rhythm regulation of highly trained athletes. Int J Hum Mov Sports Sci. (2020) 8(6):483–93. doi: 10.13189/saj.2020.080622
85. Noskin LA, Rubinskiy AV, Romanchuk AP, Marchenko VN, Pivovarov VV, Cherepov AB, et al. Study of cardiovascular and respiratory synchronization in different types of breathing. Nauchno Prakticheskii Zhurnal “Patogenez”. (2018) (4):90–6. doi: 10.25557/2310-0435.2018.04.90-96
86. Armstrong LE, Bergeron MF, Lee EC, Mershon JE, Armstrong EM. Overtraining syndrome as a complex systems phenomenon. Front Netw Physiol. (2022) 1:1–20. doi: 10.3389/fnetp.2021.794392
87. Hoffmann B, Flatt AA, Silva LEV, Młyńczak M, Baranowski R, Dziedzic E, et al. A pilot study of the reliability and agreement of heart rate, respiratory rate and short-term heart rate variability in elite modern pentathlon athletes. Diagnostics. (2020) 10(10):833–22. doi: 10.3390/diagnostics10100833
88. Guzii O, Romanchuk A. Differentiation of hemodynamics of top athletes depending on heart rate variability after training. J Adv Med Med Res. (2017) 22(3):1–10. doi: 10.9734/JAMMR/2017/33619
89. Guzii OV, Romanchuk OP. Assessment of individual changes in the activity of the athletes cardiorespiratory system during current examinations. Phys Rehab Recreat Health Technol. (2021) 6(3):5–19. doi: 10.15391/prrht.2021-6(3).01
90. Matić Z, Kalauzi A, Moser M, Platiša MM, Lazarević M, Bojić T. Pulse respiration quotient as a measure sensitive to changes in dynamic behavior of cardiorespiratory coupling such as body posture and breathing regime. Front Physiol. (2022) 13:1–15. doi: 10.3389/fphys.2022.946613
91. Volpes G, Barà C, Busacca A, Stivala S, Javorka M, Faes L, et al. Feasibility of ultra-short-term analysis of heart rate and systolic arterial pressure variability at rest and during stress via time-domain and entropybased measures. Sensors. (2022) 22(23):9149. doi: 10.3390/s22239149
92. Baumert M, Javorka M, Kabir M. Joint symbolic dynamics for the assessment of cardiovascular and cardiorespiratory interactions. Philos Trans A Math Phys Eng Sci. (2015) 373(2034):20140097. doi: 10.1098/rsta.2014.0097
93. Guzii O, Romanchuk A. Determinants of the functional state of sportsmen using heart rate variability measurements in tests with controlled respiration. J Phys Edu Sport. (2018) 2018(2):715–24. doi: 10.7752/jpes.2018.02105
94. Pinna GD, Porta A, Maestri R, De Maria B, Dalla Vecchia LA, La Rovere MT. Different estimation methods of spontaneous baroreflex sensitivity have different predictive value in heart failure patients. J Hypertens. (2017) 35(8):1666–75. doi: 10.1097/HJH.0000000000001377
95. Radaelii A, Raco R, Perfetti P, Viola A, Azzellino A, Signorini MG, et al. Effects of slow, controlled breathing on baroreceptor control of heart rate and blood pressure in healthy men. J Hypertens. (2004) 22(7):1361–70. doi: 10.1097/01.hjh.0000125446.28861.51
96. Bernardi L, Gabutti A, Porta C, Spicuzza L. Slow breathing reduces chemoreflex response to hypoxia and hypercapnia, and increases baroreflex sensitivity. J Hypertens. (2001) 19(12):2221–9. doi: 10.1097/00004872-200112000-00016
97. Norcliffe-Kaufmann L. Stress and the baroreflex. Auton Neurosci. (2022) 238:102946. doi: 10.1016/j.autneu.2022.102946
98. Frattola A, Parati G, Gamba P, Paleari F, Mauri G, Di Rienzo M, et al. Time and frequency domain estimates of spontaneous baroreflex sensitivity provide early detection of autonomic dysfunction in diabetes mellitus. Diabetologia. (1997) 40(12):1470–5. doi: 10.1007/s001250050851
99. Parati G, Mancia G, Di Rienzo M, Castiglioni P, Taylor JA, Studinger P. Point: counterpoint: cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol. (2006) 101(2):676–82. doi: 10.1152/japplphysiol.00446.2006
100. Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. (2014) 114(6):1004–21. doi: 10.1161/CIRCRESAHA.113.302549
101. La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol. (2008) 13(2):191–207. doi: 10.1111/j.1542-474X.2008.00219.x
102. Guzii OV, Romanchuk AP. Sensitivity of arterial baroreflex in the terms of body recovery after training load. Zaporozhye Med J. (2016) (3):24–9. doi: 10.14739/2310-1210.2016.3.76922
103. Guzii O, Romanchuk A, Мahlovanyi A, Trach V. Post-loading dynamics of beat-to-beat blood pressure variability in highly trained athletes during sympathetic and parasympathetic overstrain formation. J Phys Edu Sport. (2021) 21(5):2622–32. doi: 10.7752/jpes.2021.05350
104. Romanchuk O, Bazhora Y. Regulatory peculiar features of uncontrolled bronchial asthma. J Edu Health Sport. (2018) 8(1):330–46. doi: 10.5281/zenodo.1408134
105. Kishi T. Baroreflex failure and beat-to-beat blood pressure variation. Hypertens Res. (2018) 41(8):547–52. doi: 10.1038/s41440-018-0056-y
106. Kawada T, Akiyama T, Shimizu S, Sata Y, Turner MJ, Shirai M, et al. Acute effects of arterial baroreflex on sympathetic nerve activity and plasma norepinephrine concentration. Auton Neurosci. (2014) 186:62–8. doi: 10.1016/j.autneu.2014.10.016
107. Romanchuk O, Guzii O, Mahliovanyi A, Smirnov I. Cardiorespiratory synchronization under the influence of strength endurance training. Phys Rehab Recreat Health Technol. (2024) 9(1):25–35. doi: 10.15391/prrht.2024-9(1).04
108. Incognito AV, Duplea S-G, Lee JB, Sussman J, Shepherd AD, Doherty CJ, et al. Arterial baroreflex regulation of muscle sympathetic nerve activity at rest and during stress. J Physiol. (2019) 597(18):4729–41. doi: 10.1113/JP278376
109. Li C, Chang Q, Zhang J, Chai W. Effects of slow breathing rate on heart rate variability and arterial baroreflex sensitivity in essential hypertension. Medicine (Baltimore). (2018) 97(18):e0639. doi: 10.1097/MD.0000000000010639
110. Lukarski D, Stavrov D, Stankovski T. Variability of cardiorespiratory interactions under different breathing patterns. Biomed Signal Process Control. (2022) 71:103152. doi: 10.1016/j.bspc.2021.103152
111. Harford M, Catherall J, Gerry S, Young J, Watkinson P. Availability and performance of image-based, non-contact methods of monitoring heart rate, blood pressure, respiratory rate, and oxygen saturation: a systematic review. Physiol Meas. (2019) 40(6):06TR01. doi: 10.1088/1361-6579/ab1f1d
112. Romanchuk O. The immediate effects of the manual therapy traction manipulations on parameters of cardiorespiratory system functioning. Int J Hum Mov Sports Sci. (2022) 10(4):832–40. doi: 10.13189/saj.2022.100424
113. Gastinger S, Sorel A, Nicolas G, Gratas-Delamarche A, Prioux J. A comparison between ventilation and heart rate as indicator of oxygen uptake during different intensities of exercise. J Sports Sci Med. (2010) 9(1):110–8. Available online at: http://www.ncbi.nlm.nih.gov/24149394
114. Guzii O, Romanchuk O. Post-loading dynamics of beat-to-beat blood pressure variability in highly qualified athletes. Fiz Reabil ta Rekreac Ozdor Tehnol. (2021) 6(1):5–14. doi: 10.15391/prrht.2021-6(1).01
Keywords: heart rhythm regulation, athletes, respiratory maneuver, arterial baroreflex sensitivity, hildebrandt index, volume synchronization index
Citation: Romanchuk O (2025) Peculiarities of cardio-respiratory relationships in qualified athletes with different types of heart rhythm regulation according to respiratory maneuver data. Front. Sports Act. Living 6:1451643. doi: 10.3389/fspor.2024.1451643
Received: 19 June 2024; Accepted: 18 December 2024;
Published: 13 January 2025.
Edited by:
Raphael Martins de Abreu, Lunex University, LuxembourgReviewed by:
Alberto Porta, University of Milan, ItalyVictor Ribeiro Neves, Universidade de Pernambuco, Brazil
Copyright: © 2025 Romanchuk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oleksandr Romanchuk, ZG9jbGZjQHVhLmZt
 Oleksandr Romanchuk
Oleksandr Romanchuk