- 1REVAL Rehabilitation Research Center, Faculty of Rehabilitation Sciences, Hasselt University, Hasselt, Belgium
- 2Human Movement Research Laboratory (MOVI-LAB), Department of Physical Education, School of Sciences, Bauru, São Paulo State University (Unesp), Brazil
Background: Due to improvement in movement performance, post-activation performance enhancement (PAPE) may open new possibilities to improve gait performance. However, no study has attempted to translate this phenomenon into walking. Therefore, the study aimed to test whether acute stretching followed by a conditioning exercise can improve subsequent gait performance in healthy adults.
Research question: Can an exercise protocol subsequently improve gait performance?
Methods: Sixteen individuals walked four 10-m trials (in each period) before and after 7 min of an exercise protocol composed of stretching (focusing on the lower limb) and a conditioning exercise (standing calf-raise wearing a vest of 20 kg). Gait spatialtemporal parameters and muscle activity of tibialis anterior and gastrocnemius medialis and lateralis muscles were obtained by a 3D-motion system and wireless electromyography, respectively. Before and after the exercise protocol, kinematic and muscle activity parameters were compared by a one-way ANOVA and the Wilcoxon signed-rank test, respectively.
Results: After the exercise protocol, the participants walked with a faster step velocity (p < 0.018) and with a lower step duration (p < 0.025). Also, higher peak muscle activity (p < 0.008) and low-frequency (p < 0.034) activation of the anterior tibial muscle after the exercise protocol were observed.
Significance: In conclusion, the protocol improves the stability and the muscles’ efficiency during gait, contributing to a new approach to enhancing gait rehabilitation programs
Introduction
Post-activation performance enhancement (PAPE), defined as an acute increment of voluntary actions performance following a voluntary conditioning muscle contraction (1, 2), is a phenomenon well-investigated in the sports context. Previous studies have demonstrated improved performance (e.g., increased squat jumps or soccer kicking velocity) after voluntary conditioning contraction or stretching strategies (1, 3). A wide variety of physical approaches, such as dynamic/ballistic (3) or static (4) stretching and conditioning exercises (5), have been used to induce PAPE, suggesting an optimal effect approximately 7 min after (2, 6). The possible mechanisms underlying the PAPE effect are i) increases in muscle temperature, thus improving the functionality of cross-bridge cycling rates; ii) fluid shifts into the working muscles, enhancing muscle function in a fibre type-specific manner (6). However, the studies investigating PAPE on maximizing performance focused mainly on sports context without exploring the impacts on daily activities, such as walking.
Walking is a fundamental mode of human locomotion requiring different neuromuscular, kinematic, and kinetic demands to move forward and maintain stability. For example, gastrocnemius muscles have a primary role during the forward propulsion phase (7). The tibialis anterior (TA) contributes to vertical support stabilising the ankle joint to facilitate forward propulsion (8) and, with the soleus, breaking the forward velocity to heel strike (9). Enhancing the efficiency of lower limb muscles responsible for forwarding propulsion and stability may improve gait performance, as reported in running after a warm-up with a weighted vest (10) and walking after hip muscles static stretching (11). Nevertheless, Radacki et al., (11) only analysed the effects on kinematic gait parameters without investigating muscle activity. Thus, understanding the mechanisms and effects of an acute exercise protocol involving stretching and conditioning exercises on kinematic and muscle activity gait parameters may open new possibilities to improve and apply gait performance in clinical/rehabilitation programs.
In this proof-of-concept study, we aimed to test whether acute stretching followed by a conditioning exercise can improve subsequent gait performance (kinematic and muscle activity) in healthy young adults. We also hypothesize that the exercise protocol would improve gait performance—by enhancing step velocity and muscle activity.
Methods
Participants
Sixteen physically active participants (7M/9F, 22.8 ± 0.7 years, 1.68 ± 0.21 m, and 73.2 ± 17.8 kg), according to the American College of Sports Medicine (amount of physical activity: 358.82 ± 63.63 min/week), were recruited. A priori sample size calculation indicated a minimum of 15 participants to detect a significant effect between pre vs. post-conditioning exercise [power of 90% and α = 0.05, gait speed variable- Radacki et al., (2009)]. Participants under 18 or older than 30 years with any neurological, orthopedic, or cardiac disease were not eligible to participate. The participants were requested to avoid vigorous physical activity for 48 h and refrain from caffeine consumption three hours before the testing. The participants provided written informed consent before participating (approved by the University Ethics Committee board- # 53311021.1.0000.5398).
Gait protocol
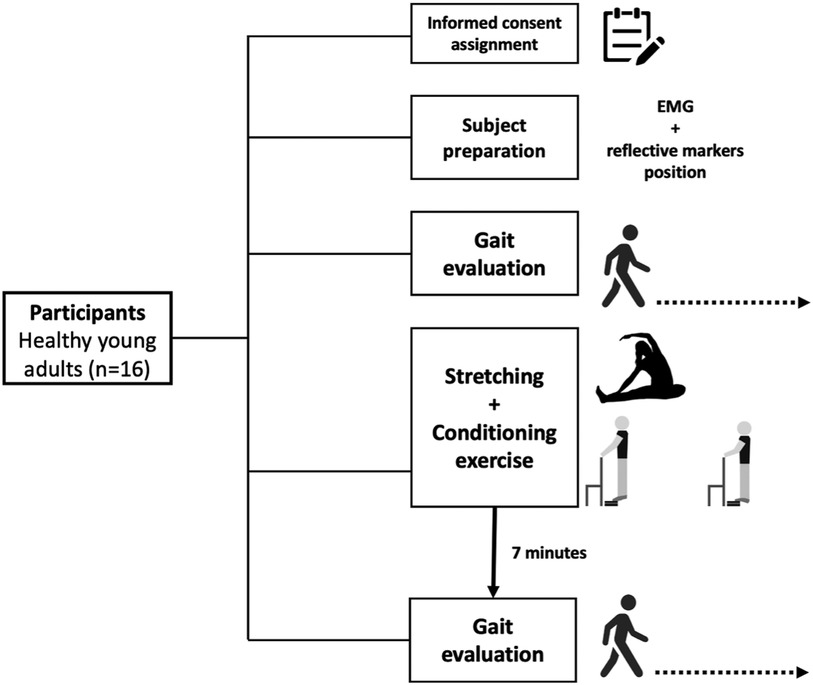
The experimental protocol is presented in Figure 1. Gait was evaluated before and subsequently, after 7 min of recovery, considered the optimal time for PAPE (6, 12) from the exercise protocol. During the recovery, the participants were sitting on a chair. Participants walked in a walkway for four trials each period on their self-selected velocity. A three-dimensional motion capture system (Vicon®, 100 Hz) with ten infrared cameras was used to capture the kinematic parameters. Markers on the 2nd metatarsal and heel, from the left and right feet, were used to calculate kinematic parameters. Synchronized with kinematic data, the EMG activity from both legs of the tibial anterior (TA), gastrocnemius medial (GM), and lateral (GL) muscles were recorded by a Wave Wireless EMG (MiniWave, Cometa System, Italy, 2,000 Hz). The position of the surface electrode followed the recommendation of Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles (13).
Acute exercise protocol: stretching and conditioning exercise
Participants performed a static stretching sequence—two sets of 30s for each focusing on the hamstrings, GL, GM, and TA muscles—followed by a conditioning exercise—four sets of 8 repetitions of a standing calf raise exercise. The standing calf raise exercise was performed while wearing a weighted vest of 20 kg, and the movements were controlled by a metronome (0.5 Hz). At the end of the protocol, the participants were asked how they felt about the effort. For full details, see the Supplementary Material.
Data analysis
Kinematic data were filtered with a low-pass Butterworth filter of 5th order (zero-lag), with a cut-off of 6 Hz. The average of the five central steps was used to calculate the following parameters: step length, width, duration, and velocity, and double support time (percentage of step duration).
Muscle activity data were filtered using a 4th order band-pass, Butterworth filter (20–500 Hz), and rectified afterward. The muscle activity parameters were individually normalized for each participant according to the maximum value for each variable acquired from each muscle in the gait trials before the acute exercise protocol. To determine heel strike and toe-off in muscle activity signals, we interpolated the kinematic data (20×). Then, in every five steps, we identified two 200 ms windows (1) from 100 ms before to 100 ms after the heel strike (TA activity), and (2) from 200 ms before toe-off (GM and GL activity). These periods depict the propulsion phase (GM and GL) or stability and initial loading absorption (heel strike) stage (TA) during the gait cycle. We calculated the peak value (maximum) of muscle activity and root-mean-square (RMS). We also calculated the low frequency—muscle activity between 25 and 82 Hz, which represents the contractions of type I fibers -, and high-frequency—muscle activity between 142 and 300 Hz, meaning the contractions of type II fibers –, by applying Fast Fourier Transformation (14).
Statistical analysis
The software SPSS® (V.28) was used for analysis (p < 0.05). Shapiro–Wilk, Levene's, and Mauchyly's tests checked data normality, variance, and homogeneity, respectively. An ANOVA for repeated measures with time (before vs. after acute exercise) as the within-subjects factor was used to test for possible differences in gait parameters. For the muscle activity parameters, the variables did not respect assumptions of normality. Therefore, we employed the non-parametric Wilcoxon Signed-Rank test to compare these parameters before and after the acute exercise protocol. The partial eta-square (η2-small >0.01, moderate >0.06, or large >0.14) and r (small-0.1–0.3: moderate-0.3–0.5: large >0.5) for the ANOVA and Wilcoxon test, respectively, were used as effect size (15). We also calculated and reported (Supplementary Material) the intraclass-correlation-coefficients (ICC) and coefficient of variation (CV) (https://www.socscistatistics.com/descriptive/coefficientvariation/default.aspx).
Results
Participants reported no feeling of tiredness after the exercise protocol. Also, they walked 2.9% (+3.8 cm/s) faster (F1,15 = 7.11, p < 0.018, η2 = 0.32) with 1.7% (−0.01 s) shorter step duration (F1,15 = 6.17, p < 0.025, η2 = 0.29) after vs. before (Figure 2).
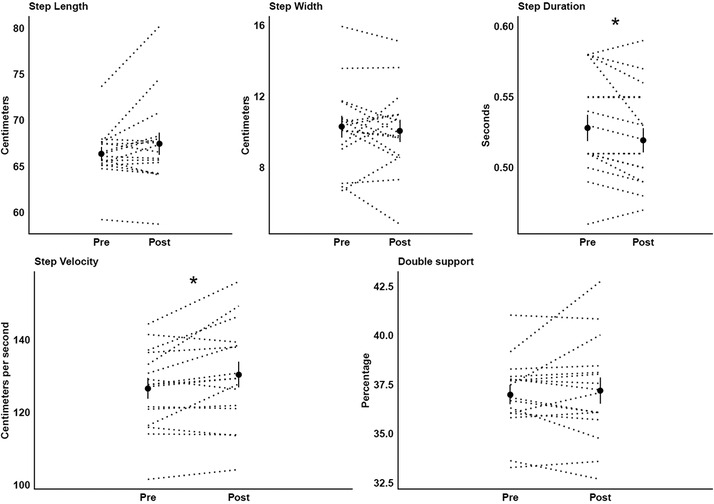
Figure 2. Means (circles) and standard deviations of the kinematic gait parameters before (pre) and after (post) the exercise protocol. Individual data of all participants are represented in dotted lines. * Significant difference between moments.
Three participants were excluded due to noise for muscle activity. The participants increased 13.3% in TA peak muscle activation (Z = 2.668, p < 0.008) and 19.4% recruitments of the TA type I fibre through the low-frequency variable (Z = 2.119, p < 0.034) after vs. before the exercise protocol (Figure 3). For the full values of the statistical analysis, please see Supplementary Table S1 in the supplementary material.
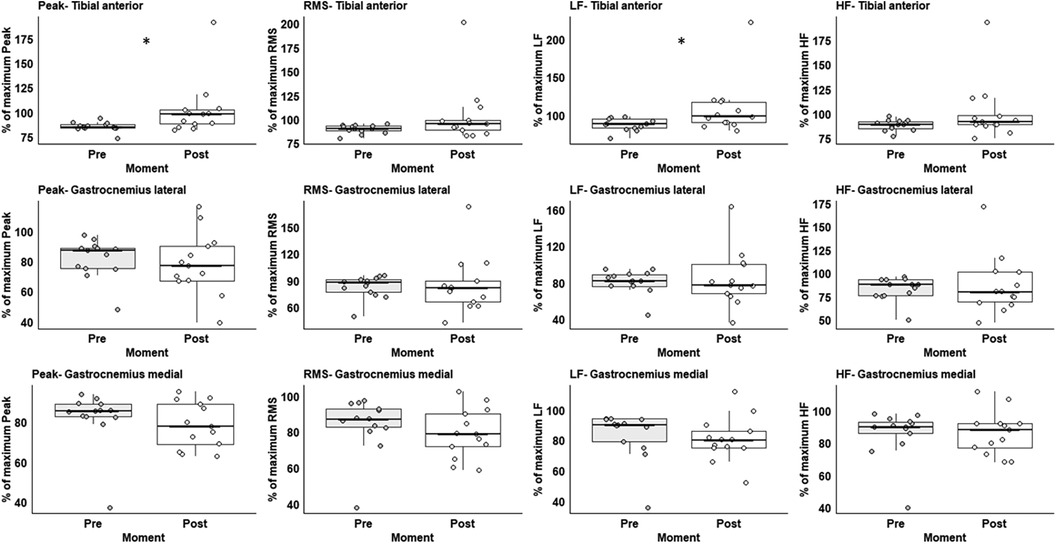
Figure 3. Box plot representation of EMG variables from before (white) and after (grey) preconditioning exercise protocol. Low-frequency (LF), high-frequency (HF), and root-mean-square (RMS). * Significant difference between moments.
Discussion
Our findings demonstrated that acute static stretching followed by a conditioning exercise (standing calf raise with a fixed load) elicited an enhancement in gait performance and TA muscle activity in young adults. Specifically, we found that after the acute exercise intervention, step velocity and the TA peak muscle activity, and the type I fibers recruitment were increased while step duration was decreased. The PAPE phenomenon could explain the observed enhancement in gait performance and lower limb muscle activity. The mechanism underlying PAPE involves both increases in (i) muscle temperature, which may facilitate the muscle contraction cycle and support an increase in force development (6), and (ii) muscle activity that may increase the efficacy of motoneuron excitability (16), thus improving muscle coordination, and consequently, gait performance. Our study contributes to a new approach to improving gait rehabilitation programs’ efficiency.
Acute static stretching followed by a conditioning exercise improved the stability and the TA muscles’ efficiency during walking. From the perspective of gait stability, a decrease in step duration is associated with a strategy ensuring stability by shortening the unstable phases of the gait cycle (17). In our study, the participants reduced the step duration with a no-significant increase in double leg support time, resulting in a shorter single leg support time. Also, the base of support is smaller in the mediolateral than in the anterior-posterior during walking, which may be a limiting factor in balance control (18). A reduced step duration can facilitate the control of the body's center of mass in the mediolateral (19), mainly when this behavior coincides with an increase in step velocity. Faster walking, which was also observed after a single session of static stretching in older adults (11), decreases mediolateral center-of-mass movements, thus making the gait more stable (20).
From the perspective of muscle efficiency, changes in (higher) TA muscle activity are considered a strategy to keep gait efficiency when gait velocity increases. Specifically, we analyzed the TA muscle activity during early stance, which is a period that TA contracts during ankle plantarflexion to the initial foot contact with the ground (21). Because the amount of leg loading increases with faster walking (22), it is expected an increase in TA muscle activity during this period to control the accelerating and decelerating forces of individual body segments, thus establishing a safe and efficient forward progression (22). Also, increased TA muscle activity is likely critical for interacting with the triceps surae muscles to enhance muscle economy, minimize muscle damage, and contribute to ankle joint stabilization during heel-ground contact (8).
The increased recruitment of TA type I fibers after the exercise protocol is difficult to explain. We can interpret that as TA muscle predominantly presents type I fibers (−69%) (23), it is evident that increased TA muscle activity would cause higher recruitment in this fiber type. Another possible suggestion is related to the characteristics of walking. Despite faster walking velocity requiring higher TA muscle activity, the early stance phase of walking undemand a maximum force production, considering that TA muscle action is isometric during this phase of walking despite muscle-tendon lengthening (21). By acting isometrically, the muscle produces higher forces based on the force-velocity relationship (24), thus storing energy through the elastic properties of the tendon (21). In addition, although the GM/GL has an important role in the propulsion phase of walking, contributing to 34%, the hip joint has a major role in this purpose, contributing to 56% (25). Thus, for not being the major muscle/joint, we were not able to find changes in muscle activation in GM/GL muscles.
These findings presented here are likely to have practical applications. Although acutely, performing stretching in combination with conditioning exercises can improve gait performance, which is essential to prevent falls in many populations. Whether confirmed in populations that suffer from gait impairments, this phenomenon can be an interesting approach and a remarkable intervention strategy to improve gait performance, safety, and stability. However, randomized controlled trials should be done before clinical application.
Although the study showed novelty, some inherent weaknesses and methodological considerations are observed: (i) lack of a control condition (i.e., non-exercising condition) or groups with only exercise and/or only stretching in a randomized, cross-over protocol (exercise vs. no-exercise) on different days. However, this is a proof-of-concept study, involving a small number of subjects and providing the first evidence that an acute stretching in combination with a condition exercise can improve subsequent gait performance. Therefore, we suggest that future studies address this issue; (ii) the experimental design was not double-blinding; (iii) experimental procedures confounders such as diet and hydration were not controlled (6); and (iv) all the participants wore a fixed external load (i.e., the 20 kg vest), not being relativized in accord with their weights. In this way, the muscle activation required and intensity performed were different across subjects. However, although the different effort demands could influence performance, the easy clinical application and the report by the participants that they were not tired after the protocol support our expectations. We recommend that future studies amend these issues when designing the experimental protocol.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Comitê de Ética em Pesquisa- UNESP- Faculdade de Ciências. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. AP: Data curation, Formal Analysis, Investigation, Writing – review & editing. VM: Formal Analysis, Investigation, Writing – review & editing. MK: Formal Analysis, Investigation, Writing – review & editing. FB: Conceptualization, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES- Finance Code 001).
Acknowledgments
We would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES- Finance Code 001) and all the participants of the study for collaborating.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fspor.2024.1285247/full#supplementary-material
References
1. Cuenca-Fernández F, Smith IC, Jordan MJ, MacIntosh BR, López-Contreras G, Arellano R, et al. Nonlocalized postactivation performance enhancement (PAPE) effects in trained athletes: a pilot study. Appl Physiol Nutr Metab. (2017) 42:1122–5. doi: 10.1139/apnm-2017-0217
2. Prieske O, Behrens M, Chaabene H, Granacher U, Maffiuletti NA. Time to differentiate postactivation “potentiation” from “performance enhancement” in the strength and conditioning community. Sports Med. (2020) 50:1559–65. doi: 10.1007/s40279-020-01300-0
3. Amiri-Khorasani M, Abu Osman NA, Yusof A. Electromyography assessments of the vastus medialis muscle during soccer instep kicking between dynamic and static stretching. J Hum Kinet. (2010) 24:35–41. doi: 10.2478/v10078-010-0017-2
4. Iglesias-Soler E, Paredes X, Carballeira E, Márquez G, Fernández-Del-Olmo M. Effect of intensity and duration of conditioning protocol on post-activation potentiation and changes in H-reflex. Eur J Sport Sci. (2011) 11:33–8. doi: 10.1080/17461391003770517
5. Seitz LB, Trajano GS, Haff GG. The back squat and the power clean: elicitation of different degrees of potentiation. Int J Sports Physiol Perform. (2014) 9:643–9. doi: 10.1123/ijspp.2013-0358
6. Blazevich AJ, Babault N. Post-activation potentiation versus post-activation performance enhancement in humans: historical perspective, underlying mechanisms, and current issues. Front Physiol. (2019) 10:1359. doi: 10.3389/fphys.2019.01359
7. Gottschall JS, Kram R. Energy cost and muscular activity required for leg swing during walking. J Appl Physiol. (2005) 99:23–30. doi: 10.1152/japplphysiol.01190.2004
8. Li L, Ogden LL. Muscular activity characteristics associated with preparation for gait transition. J Sport Health Sci. (2012) 1:27–35. doi: 10.1016/j.jshs.2012.04.006
9. Francis CA, Lenz AL, Lenhart RL, Thelen DG. The modulation of forward propulsion, vertical support, and center of pressure by the plantarflexors during human walking. Gait Posture. (2013) 38:993–7. doi: 10.1016/j.gaitpost.2013.05.009
10. Barnes KR, Kilding AE. Strategies to improve running economy. Sports Med. (2015) 45:37–56. doi: 10.1007/s40279-014-0246-y
11. Rodacki ALF, Souza RM, Ugrinowitsch C, Cristopoliski F, Fowler NE. Transient effects of stretching exercises on gait parameters of elderly women. Man Ther. (2009) 14:167–72. doi: 10.1016/j.math.2008.01.006
12. Wilson JMC, Duncan NM, Marin PJ, Brown LE, Loenneke JP, Wilson SMC, et al. (2013). Meta-analysis of postactivation potentiation and power: effects of conditioning activity, volume, gender, rest periods, and training status. J Strength Cond Res 27, 854–9. doi: 10.1519/JSC.0b013e31825c2bdb
13. Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. (2000) 10(5):361–74. doi: 10.1016/s1050-6411(00)00027-4
14. Mündermann A, Wakeling JM, Nigg BM, Humble RN, Stefanyshyn DJ. Foot orthoses affect frequency components of muscle activity in the lower extremity. Gait Posture. (2006) 23:295–302. doi: 10.1016/j.gaitpost.2005.03.004
15. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New York, NY: Routledge (1988). p. 567. doi: 10.4324/9780203771587
16. Nuzzo JL, Barry BK, Gandevia SC, Taylor JL. Acute strength training increases responses to stimulation of corticospinal axons. Med Sci Sports Exerc. (2016) 48:139–50. doi: 10.1249/MSS.0000000000000733
17. Barbieri FA, dos Santos PCR, Simieli L, Orcioli-Silva D, van Dieën JH, Gobbi LTB. Interactions of age and leg muscle fatigue on unobstructed walking and obstacle crossing. Gait Posture. (2014) 39:985–90. doi: 10.1016/j.gaitpost.2013.12.021
18. Dean JC, Alexander NB, Kuo AD. The effect of lateral stabilization on walking in young and old adults. IEEE Trans Biomed Eng. (2007) 54:1919–26. doi: 10.1109/TBME.2007.901031
19. Hof AL, van Bockel RM, Schoppen T, Postema K. Control of lateral balance in walking. Experimental findings in normal subjects and above-knee amputees. Gait Posture. (2007) 25:250–8. doi: 10.1016/j.gaitpost.2006.04.013
20. Orendurff MS, Segal AD, Klute GK, Berge JS, Rohr ES, Kadel NJ. The effect of walking speed on center of mass displacement. J Rehabil Res Dev. (2004) 41:829–34. doi: 10.1682/JRRD.2003.10.0150
21. Maharaj JN, Cresswell AG, Lichtwark GA. Tibialis anterior tendinous tissue plays a key role in energy absorption during human walking. J Exp Biol. (2019) 222(11):jeb191247. doi: 10.1242/jeb.191247
22. Den Otter AR, Geurts ACH, Mulder T, Duysens J. Speed related changes in muscle activity from normal to very slow walking speeds. Gait Posture. (2004) 19:270–8. doi: 10.1016/S0966-6362(03)00071-7
23. Nakagawa Y, Ratkevicius A, Mizuno M, Quistorff B. ATP economy of force maintenance in human tibialis anterior muscle. Med Sci Sports Exerc. (2005) 37:937–43. doi: 10.1249/01.mss.0000166946.94871.9b
24. Usherwood JR, Channon AJ, Myatt JP, Rankin JW, Hubel TY. The human foot and heel-sole-toe walking strategy: a mechanism enabling an inverted pendular gait with low isometric muscle force? J R Soc Interface. (2012) 9:2396–402. doi: 10.1098/rsif.2012.0179
Keywords: locomotion, post-activation performance enhancement (PAPE), muscle activity, conditioning exercise, stability
Citation: Santinelli FB, Prieto Silveira-Ciola A, Moreno VC, Kuroda MH and Barbieri FA (2024) The acute effects of a stretching and conditioning exercise protocol for the lower limbs on gait performance- a proof of concept and single-blind study. Front. Sports Act. Living 6:1285247. doi: 10.3389/fspor.2024.1285247
Received: 29 August 2023; Accepted: 22 January 2024;
Published: 8 February 2024.
Edited by:
Rita Kiss, Budapest University of Technology and Economics, HungaryReviewed by:
Bessem Mkaouer, University of Manouba, TunisiaArnaud Delafontaine, Université libre de Bruxelles, Belgium
© 2024 Santinelli, Prieto Silveira-Ciola, Moreno, Kuroda and Barbieri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabio A. Barbieri ZmFiaW8uYmFyYmllcmlAdW5lc3AuYnI=
 Felipe B. Santinelli
Felipe B. Santinelli Aline Prieto Silveira-Ciola
Aline Prieto Silveira-Ciola Vinicius C. Moreno2
Vinicius C. Moreno2 Fabio A. Barbieri
Fabio A. Barbieri