
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Sports Act. Living, 21 July 2023
Sec. Sport and Exercise Nutrition
Volume 5 - 2023 | https://doi.org/10.3389/fspor.2023.1257740
This article is part of the Research TopicPre-workout NutritionView all 8 articles
Editorial on the Research Topic
Pre-workout nutrition
For decades, athletes have manipulated what foods they have consumed to maximize their performance and the adaptations their bodies make to the physical training they complete. The roots of nutrient timing date back to the original Scandinavian research of Bergstrom that popularized percutaneous collection of skeletal muscle tissue and made some of the first inferences to the connection between glycogen levels and carbohydrate intake (1). This work initially led athletes to focus on consuming nutrients before and during workouts (2, 3), which led to the first nutrient timing strategy commonly known as glycogen supercompensation or “carbohydrate loading”. Research in the early 1990s then provided some of the first experimental evidence which demonstrated that when nutrients are consumed may impact recovery potential and subsequent performance (4, 5). The popularity of nutrient timing exploded when research by the late Kevin Tipton first suggested that pre-exercise feeding of essential amino acids with carbohydrate may promote higher rates of muscle protein synthesis than when those nutrients were ingested after exercise (6). The flames of this excitement were further stoked by the initial work by Cribb et al. (7) who reported that consuming nutrients in close proximity to a workout was responsible for greater increases in strength and accretion of fat-free tissues. Since that time, research in this area has been consistent with multiple review articles that have summarized the literature base (8–11). While the majority of the work has focused on outcomes surrounding macronutrient administration, recent investigations have started to explore the impact of various micronutrients (8), caffeine (12), and amino acids such as creatine (13) and, most recently, glutamine (14). Figure 1 has been developed to highlight the different ingredients that have been investigated or purported for their ability to impact physical performance or adaptations to physical training when ingested prior to competition or a training session.
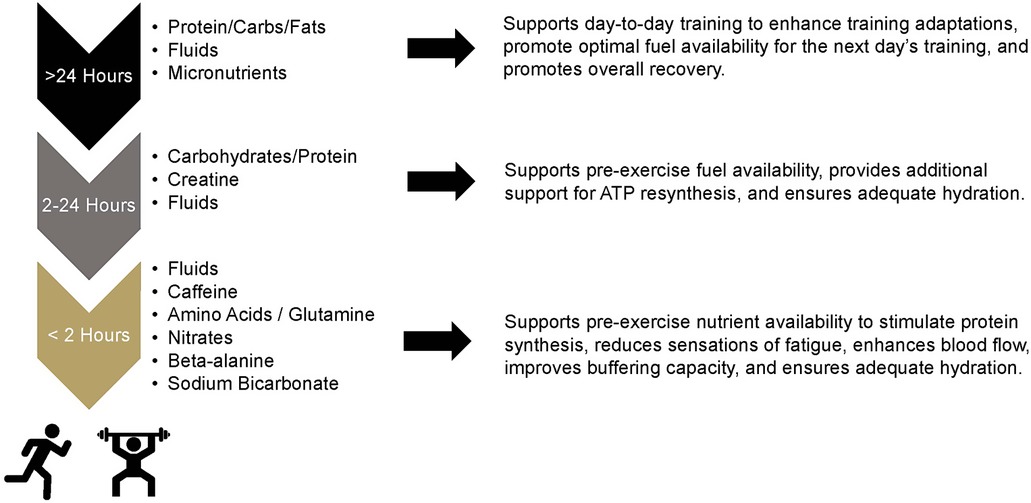
Figure 1. Nutritional ingredients purported to impact acute exercise responses or exercise training adaptations.
The aim of this Research Topic was to provide a direct publication avenue for articles that highlighted relevant topics on nutritional support for athletes including diet composition, supplements, and meal timing surrounding training and sport. As the literature base of nutrient timing has evolved, more research is needed to help scientists, athletes, and practitioners understand how and when nutrient timing should be considered. This Research Topic includes seven original articles that all explored the impact of ingesting different ingredients prior to some form of exercise. Supplementation with ATP has previously been shown to positively impact health and exercise performance, but the optimal dose is unknown. de Moura et al. demonstrated that a 400-mg dose of ATP prior to resistance training exercise was needed to improve performance while lower doses positively impact perceived exertion. Benjamin et al. evaluated the safety of consuming a dose of Citrus Aurantium prior to submaximal aerobic exercise and concluded that a 600 mg dose of a 30% extract of p-synephrine was well tolerated and exhibited no unfavorable cardiac and hemodynamic outcomes. An animal study by Che et al. investigated the impact of oral administration of pyruvate on mitigating the onset and accumulation of metabolic acidosis after high-intensity interval exercise. They concluded that a seven-day regimen of oral pyruvate (616 mg/kg/day, HED: 99.4 mg/kg) prior to exercise attenuates the metabolic acidosis induced by high-intensity interval exercise. Three studies in our Research Topic explored the impact of creatine prior to exercise. Negro et al. supplemented 18 adult males with either a placebo, creatine citrate, or a multi-ingredient combination of nutrients containing creatine and found that differences in the time to perform an established task and various EMG parameters were exhibited between the combination of nutrients and placebo. No differences, however, were found between creatine citrate and the other supplementation conditions. Dinan et al. reported that the timing of creatine ingestion (pre- vs. post-workout consumption) in conjunction with a daily dose of protein and carbohydrate was not responsible for any further changes in strength or body composition after 8 weeks of supplementation in 34 healthy resistance-trained male and female collegiate athletes. Candow et al. reviewed the literature on creatine timing and concluded that the current literature is inconclusive on whether or not creatine ingestion or after a workout offers a strategic advantage and put forth a call for researchers to further investigate this topic. Finally, Ratliff et al. used a randomized, crossover design with 15 healthy females to examine the impact of pre-exercise ingestion of carbohydrate or two different sources of protein on energy expenditure and substrate oxidation throughout and after a 60-minute bout of moderate intensity treadmill exercise. The authors concluded that carbohydrate ingestion increased carbohydrate oxidation greater than no nutrient ingestion while no changes were observed for carbohydrate or fat oxidation when either source of protein was consumed prior to each exercise bout. Alternatively, both protein sources triggered increased rates of energy expenditure when compared to carbohydrate ingestion. In summary, the articles submitted to this Research Topic contribute to our understanding of how pre-exercise consumption of different nutrients can impact exercise performance and how we can expect our body to response to regular exercise training.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
CK has received and continues to receive external funding from companies that do business in exercise and nutrition and currently serves as a paid advisor for different companies who do business in areas related to exercise and nutrition.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bergstrom J, Hultman E. Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature. (1966) 210(5033):309–10. doi: 10.1038/210309a0
2. Sherman WM, Costill DL, Fink WJ, Miller JM. Effect of exercise-diet manipulation on muscle glycogen and its subsequent utilization during performance. Int J Sports Med. (1981) 2(2):114–8. doi: 10.1055/s-2008-1034594
3. Sherman WM, Brodowicz G, Wright DA, Allen WK, Simonsen J, Dernbach A. Effects of 4 h preexercise carbohydrate feedings on cycling performance. Med Sci Sports Exerc. (1989) 21(5):598–604. doi: 10.1249/00005768-198910000-00017
4. Ivy JL, Goforth HW Jr., Damon BM, McCauley TR, Parsons EC, Price TB. Early postexercise muscle glycogen recovery is enhanced with a carbohydrate-protein supplement. J Appl Physiol. (2002) 93(4):1337–44. doi: 10.1152/japplphysiol.00394.2002
5. Widrick JJ, Costill DL, Fink WJ, Hickey MS, McConell GK, Tanaka H. Carbohydrate feedings and exercise performance: effect of initial muscle glycogen concentration. J Appl Physiol. (1993) 74(6):2998–3005. doi: 10.1152/jappl.1993.74.6.2998
6. Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall SK, Petrini BE, et al. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab. (2001) 281(2):E197–206. doi: 10.1152/ajpendo.2001.281.2.E197
7. Cribb PJ, Hayes A. Effects of supplement timing and resistance exercise on skeletal muscle hypertrophy. Med Sci Sports Exerc. (2006) 38(11):1918–25. doi: 10.1249/01.mss.0000233790.08788.3e
8. Stecker RA, Harty PS, Jagim AR, Candow DG, Kerksick CM. Timing of ergogenic aids and micronutrients on muscle and exercise performance. J Int Soc Sports Nutr. (2019) 16(1):37. doi: 10.1186/s12970-019-0304-9
9. Kerksick C, Harvey T, Stout J, Campbell B, Wilborn C, Kreider R, et al. International society of sports nutrition position stand: nutrient timing. J Int Soc Sports Nutr. (2008) 5:17. doi: 10.1186/1550-2783-5-17
10. Kerksick CM, Arent S, Schoenfeld BJ, Stout JR, Campbell B, Wilborn CD, et al. International society of sports nutrition position stand: nutrient timing. J Int Soc Sports Nutr. (2017) 14:33. doi: 10.1186/s12970-017-0189-4
11. Aragon AA, Schoenfeld BJ. Nutrient timing revisited: is there a post-exercise anabolic window? J Int Soc Sports Nutr. (2013) 10(1):5. doi: 10.1186/1550-2783-10-5
12. Harty PS, Zabriskie HA, Stecker RA, Currier BS, Tinsley GM, Surowiec K, et al. Caffeine timing improves lower-body muscular performance: a randomized trial. Front Nutr. (2020) 7:585900. doi: 10.3389/fnut.2020.585900
13. Forbes SC, Krentz JR, Candow DG. Timing of creatine supplementation does not influence gains in unilateral muscle hypertrophy or strength from resistance training in young adults: a within-subject design. J Sports Med Phys Fitness. (2021) 61(9):1219–25. doi: 10.23736/S0022-4707.20.11668-2
Keywords: timing, peri-nutrition, post-exercise, pre-exercise, performance, training adaptations
Citation: Kerksick CM and Pugh JN (2023) Editorial: Pre-workout nutrition. Front. Sports Act. Living 5:1257740. doi: 10.3389/fspor.2023.1257740
Received: 12 July 2023; Accepted: 13 July 2023;
Published: 21 July 2023.
Edited and Reviewed by: David Christopher Nieman, Appalachian State University, United States
© 2023 Kerksick and Pugh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chad M. Kerksick Y2tlcmtzaWNrQGxpbmRlbndvb2QuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.