
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Sports Act. Living , 11 August 2023
Sec. Sport and Exercise Nutrition
Volume 5 - 2023 | https://doi.org/10.3389/fspor.2023.1207013
Introduction: This study examined the effects of Libifem® on exercise performance and body composition in females 25–45 years old.
Methods: Participants were randomized to three equal groups to consume: 600 mg Libifem®/day, 300 mg Libifem®/day or a placebo for 8 weeks. Participants completed a whole-body exercise program three times a week for 8 weeks. At baseline, week 4 and week 8, muscle strength and endurance, functional threshold power, body composition, and sex hormones were measured. At week 8, all three groups increased leg press 1RM compared to baseline.
Results: A significant difference between group treatment effect was seen for leg press at week 8 (p = 0.045), with the 600 mg Libifem® group significantly increasing their leg press 1RM compared to placebo (p = 0.014). The 600 mg Libifem® group significantly reduced their total fat mass (0.96 kg loss) from baseline compared to placebo group (0.09 kg gain). There was no significant difference in fat mass for the 300 mg Libifem® group (0.23 kg loss). The 600 mg Libifem® group had a significant increase in lean mass compared to both the 300 mg and placebo groups (p = 0.011 and 0.009, respectively).
Discussion: Overall, there were significant and dose-related changes in body composition and ergogenic parameters, comparable with previous findings in males.
Clinical Trial Registration: This trial was registered with the Australian and New Zealand Clinical Trials registry [ACTRN12618001538235].
Regular physical activity is associated with improvements in body composition, bone, and cardiovascular health (1), as well as positively affecting mood and mental health (2). A strong interest in performance enhancement via natural and legal (and safe) routes exists, and while much of this research has focused on male performance, a growing demand for analogous research applicable to women arises. Phytochemical research is currently investigating herbal strategies designed to specifically improve training, recovery, and performance among female athletes and competitors (3).
Many of the benefits associated with physical activity are largely influenced by the sex hormones. Physical activity, in particular resistance training, is impacted by the presence of circulating testosterone in both males and females. Resistance training is well known to induce muscle hypertrophy, increase strength (as measured by 1RM) (4–8), increase lean muscle mass (9, 10), and help reduce fat mass by increasing resting metabolic rate (5).
Testosterone stimulates protein synthesis and inhibits protein degradation within muscles, leading to promotion of muscle growth and increases in muscle strength (11), whereas oestrogen can exert a broadly analogous effect by increasing the anabolic response to exercise in females (12).
The herb Trigonella foenum-graecum, known as fenugreek, belongs to the Fabaceae family. Traditionally, fenugreek has been used as a food, condiment, spice, traditional medicine, and health supplement (12, 13). Based on its active constituents, including the amino acid 4-hydroxyisoleucine, saponins, and numerous other phytochemicals with varying biological and pharmacological activities (13–15), fenugreek has been shown to exert positive effects in diabetes, inflammation, and some types of cancer (15).
Studies using fenugreek, specifically in females, have mainly focused on the amelioration of menopausal symptoms. Libifem®, an extract of fenugreek, standardised to 50% of furostanol saponins, has been shown to bind to E2 receptors and induce expression of E2-responsive genes (16) and improve sexual function in both pre- and postmenopausal females (17, 18). Fenugreek has been shown to increase free oestrogen and testosterone levels in females (17, 18) and testosterone in males (19, 20), via re-partitioning mechanisms including the displacement of testosterone from relatively low-affinity binding sites on, i.e., serum albumen (21). These pharmacological effects would be expected to improve aspects of muscle function. A previous study on females undertaking resistance training with fenugreek supplementation (22) showed that completing either two or three resistance training sessions per week showed similar increases in muscle strength and lean soft tissue mass, with those in the higher-frequency group showing improvements in body mass. However, the full effects of fenugreek on females and exercise performance have not yet been studied.
The aim of this study was to examine changes in muscle strength and endurance, as well as body composition, leg power, muscle recovery, pathological markers, and quality of life in response to an 8-week bodyweight resistance training program in females aged 25–45 years, with varying doses of Libifem® or a placebo. It was hypothesised that participants on Libifem® would show an increase in muscle strength, power, and endurance, which would, in turn, positively impact body composition at a faster rate than a placebo. It was also hypothesised that participants on the higher dose of Libifem® would elicit greater improvements in performance compared with both the placebo and low-dose Libifem® group.
This study was a double-blind, randomised, placebo-controlled, multi-site (Brisbane and Gold Coast, Australia) interventional study conducted over an 8-week treatment duration, utilising two active and one placebo group. It was approved by Bellberry Human Research Ethics Committee (HREC 2016-04-307) and registered on the Australian and New Zealand Clinical Trials Registry (ACTRN12616000938404).
The participants were recruited from databases and public media outlets, and following preliminary screening via telehealth consult, potential participants attended the clinic for an information session before providing their written consent for inclusion into the study. Consenting participants underwent a health assessment that included lifestyle, current medications, physical assessment, and medical history. Muscle strength, power, endurance, body composition, and quality of life were also assessed upon enrolment. Within the week pre-treatment, the participant's blood was collected for baseline analysis. Once all baseline measures were successfully completed, the participants were enrolled in the trial and randomly allocated to the placebo comparator group or one of the two active intervention groups (300 mg of Libifem®/day or 600 mg of Libifem®/day) using Random Allocation Software (www.sealedenvelope.com). This study was conducted in a double-blind manner such that both the investigators and participants were blind to treatment allocation.
Inclusion criteria included females aged 25–45 years with a body mass index (BMI) of 18.5–29.9 kg/m2. The participants had to be undertaking low-impact cardiovascular exercise including but not limited to cycling, swimming, or walking at least once a week, but no more than five times per week, but not undertaking any resistance training exercises. The participants had to be able to provide written informed consent and willing to participate in an exercise program three times per week for 8 weeks. They were excluded based on the following criteria: currently undertaking resistance training exercise; consumed any dietary supplements within the previous 3 months; had known hypersensitivity to herbal drugs, nutritional supplements, or foods; or completed any other clinical trial within 6 months prior to their enrolment. Other exclusion criteria consisted of clinically significant medical conditions including, but not limited to, cardiovascular, neurological, psychiatric, renal, immunological, endocrine (such as uncontrolled diabetes or thyroid disease), or haematological abnormalities that were uncontrolled; prolonged (≥6 weeks) medication with corticosteroids, antidepressants, anticholinergics, or any other drugs that may have had an influence on the outcome of the study; severe pulmonary dysfunction (uncontrolled bronchial asthma and/or chronic obstruction); history of orthopaedic injuries or surgeries in the previous 6 months; active smokers; substantial alcohol consumption (≥21 drinks per week); drug use; or females that were pregnant or lactating, including those actively trying to fall pregnant.
Primary measurement outcomes included a one-repetition maximum (1RM) leg press and bench press. For 1RM testing, participants stretched major muscle groups of the lower limbs followed by 10 repetitions of leg press at 50% of estimated 1RM and a 2-min rest. The weight on the leg press was then increased to approximately 70% of 1RM, and the participants completed four to six repetitions followed by a 2-min rest. Weight was added onto the leg press to approximately 90% of estimated 1RM, and the participants completed one repetition followed by a 2-min rest. Weight was then increased to 100% of estimated 1RM, and the participants attempted to complete a repetition. If successful, the weight was increased, and if unsuccessful, the weight was decreased. The participants were given up to seven attempts to achieve 1RM. They were rested for 3 min in between attempts.
Secondary measurement outcomes included 80% of 1RM leg press and bench press repetitions to fatigue. This was measured following a 5-min rest after completing the 1RM testing, where the participants completed one set of 80% of 1RM leg press for as many reps as possible. The participants again rested for further 5 min before an identical protocol was followed for bench press. Other secondary measures included body composition [lean muscle mass, fat mass, body fat percentage, BMI, total body mass, android/gynoid ratio, and visceral adipose tissue (VAT) mass] measured by dual-energy x-ray absorptiometry (DEXA); muscle recovery [creatine kinase (CK), lactate dehydrogenase (LDH)]; pathology [stress indicators (cortisol), liver and kidney safety markers, testosterone, E/LFT, C-reactive protein, homocysteine, blood glucose, and full blood count]; evaluation of dose, safety, and tolerability of Libifem®; an assessment of quality of life; and leg power as measured by a 20-min Functional Threshold Power (FTP) test. The protocol used for FTP testing was based on the existing validated protocols (23, 24). Following a 15-min warm-up, the participants completed a 20-min FTP test on a bicycle attached to a Wahoo KICKR® Power Trainer (Wahoo Fitness) and wore a heart rate monitor (Polar Electro Inc.). Each participant was instructed to perform the highest possible mean power output for the duration of the test. Standardised verbal encouragement was provided to each participant, and water could be consumed ad libitum. FTP was determined as the mean power output over the test duration.
Within a week from baseline testing, the participant's fasted (10 h) blood was collected at an accredited local pathology laboratory (Queensland Medical Laboratory) for pathology analysis [stress indicators (cortisol), liver and kidney safety markers, testosterone, E/LFT, C-reactive protein, homocysteine, blood glucose, and full blood count]. Blood samples for markers of exercise recovery (CK and LDH) were collected the following day (approximately 24 h) after the exercise test. This process was repeated at the completion of the study (week 8). All blood draws were collected in the morning, at the same time for each participant to ensure any variation due to diurnal rhythm was minimised. All blood was drawn into EDTA or serum vacutainers from a vein in the antecubital fossa. Once collected, the EDTA sample was immediately centrifuged, while the serum sample was allowed to clot for at least 30 min prior to centrifugation. Both samples were centrifuged at 1,400 × g for 10 min at 4°C. Once spun, the blood was immediately analysed or stored at −80°C until analysis.
One hundred and twenty-eight participants were enrolled and equally randomised across three treatment arms that included 600 mg of Libifem®/day (n = 29), 300 mg of Libifem®/day (n = 29), and a placebo group (n = 26) (Figure 1). The active capsules contained a standardised Trigonella foenum-graecum seed extract, Libifem®, supplied by Gencor Pacific Ltd., and the placebo product contained maltodextrin. Both treatments consisted of dose matched capsules divided into two daily doses, once with a morning meal and once with an evening meal.
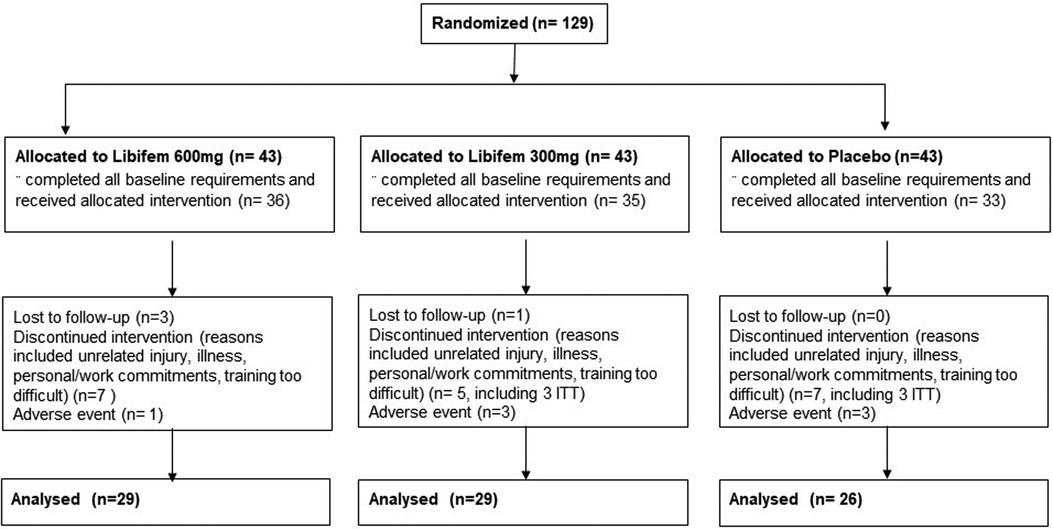
Figure 1. Participant flow diagram with designated allocations, participant withdrawal, and analysis.
The participants were asked to take the allocated product and complete a resistance training program consisting of both upper and lower body exercises focusing on all major muscle groups. They completed three training sessions per week during weeks 1–3 and 5–7 and two sessions during assessment weeks 4 and 8. Testing sessions during weeks 4 and 8 constituted their third weekly session.
At the mid-point (week 4), the participants were assessed on resting heart rate (HR) and blood pressure (BP); anthropometric measurements including height, weight, waist, and hip circumference; 1RM leg press and bench press; and 80% of 1RM bench leg press and bench press repetitions to fatigue.
Upon completion of the study (week 8), an assessment identical to baseline was completed. At both the mid-point and endpoint of the study (weeks 4 and 8), the participants were asked to provide details regarding any lifestyle changes (diet, exercise, medication) and any adverse effects. It should be noted that the phase of each participant's menstrual cycle at the time of testing was not recorded for this study.
Statistical analyses were conducted using SPSS software. All data were initially assessed for normality, and statistical differences were assessed by ANOVA and RMANOVA for change over time and treatment. Post hoc analyses were undertaken to assess the differences between treatment groups. Significance was assumed when p < 0.05. A sample size of 26 per group was calculated based on the power to detect a 5% change in 1RM leg/bench press (effect size of 0.56, alpha error probability of 0.05, power of 0.95) (25).
One hundred and twenty-nine participants were recruited and enrolled into the study, with 74 completing the full requirements. Of the 54 participants that did not complete the study, 26 did not complete the baseline requirements, 7 dropped out due to adverse events, 5 were lost to follow-up, 5 withdrew due to personal reasons, and 12 withdrawals were due to COVID-19 restrictions. Of the participants that did not complete the study, 10 provided data for at least the week 4 time-point, and these were included in modified intention to treat analysis that included all randomised participants who had at least one post-baseline measurement for the primary outcome. Data were therefore analysed for 84 participants (Figure 1).
No significant differences between the active treatment and placebo groups at baseline for age, anthropometric measures, pathology, lifestyle factors, quality of life, or exercise measurements were noted (Tables 1–3). No significant differences between groups for any biochemistry safety parameter measured were found. All biochemical safety markers were within the normal range at baseline and remained stable and within normal reference ranges at week 8 (Table 3). No significant differences between groups for exercise session compliance were reported, with compliance of approximately 82% for all three trial groups. All data were normally distributed.
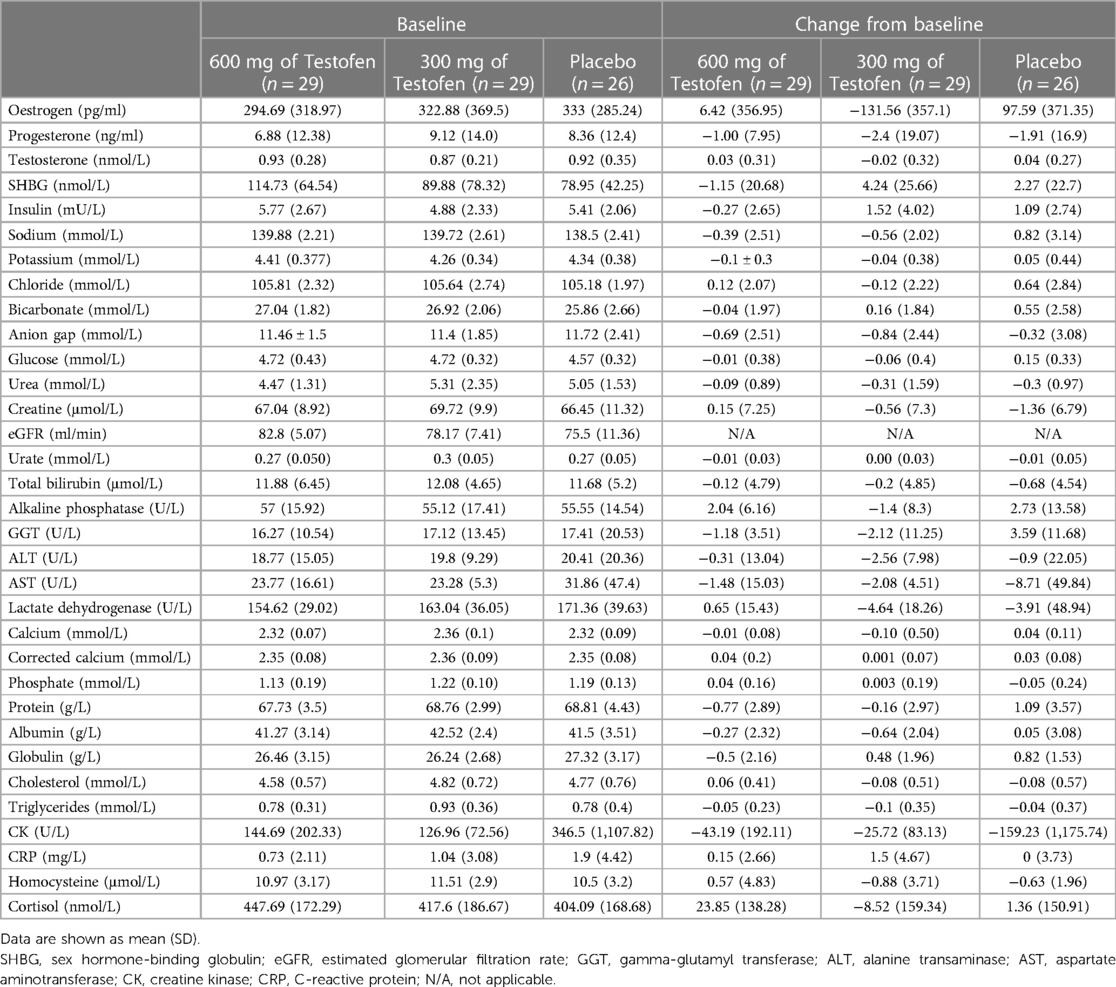
Table 3. Biochemical blood safety markers at baseline of the study population and the change following 8 weeks of supplementation and training.
A significant difference of between-group treatment effect was seen for leg press at week 8 [F (2, 82) = 0.122, p = 0.045]. All three groups improved their 1RM leg press from baseline to week 8 (22.17 kg, 17.68 kg, and 10.12 kg for the 600 mg, 300 mg, and placebo groups, respectively). The 600 mg Libifem® group significantly improved (p = 0.014) from baseline compared with the placebo at week 8 (Table 4 and Figure 2).
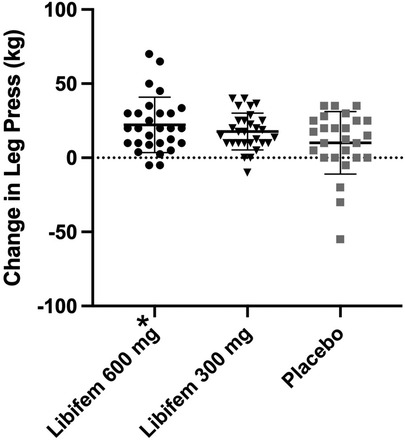
Figure 2. Change in 1RM leg press (kg) following 8 weeks of resistance training and supplementation with either 600 mg of Libifem®, 300 mg of Libifem®, or placebo.
Significant increases (p < 0.05) from baseline in 1RM bench press were observed for all three groups (5.12 kg 600 mg Libifem®, 5.98 kg 300 mg Libifem®, 6.15 kg placebo); however, no significant differences between the groups were found. No differences were observed over time or between groups for the 80% max bench or leg press repetitions.
A significant between-group treatment effect was seen for the change in total fat mass at week 8 [F (2, 70) = 3.90, p = 0.025] (Table 5). The 600 mg Libifem® group had a significant decrease from baseline total fat mass compared with placebo, losing on average 0.96 kg over the 8 weeks, compared with a 0.23 kg loss in the 300 mg group and a 0.09 kg gain in the placebo group.
A significant between-group treatment effect was observed for the change in trunk mass fat at week 8 [F (2, 70) = 3.45, p = 0.037] (Table 5). The 600 mg Libifem® group had a significant decrease from baseline trunk fat mass compared with placebo, losing on average 0.59 kg over the 8 weeks, compared with 0.14 kg loss in the 300 mg group and a 0.07 kg gain in the placebo group (Figure 3).
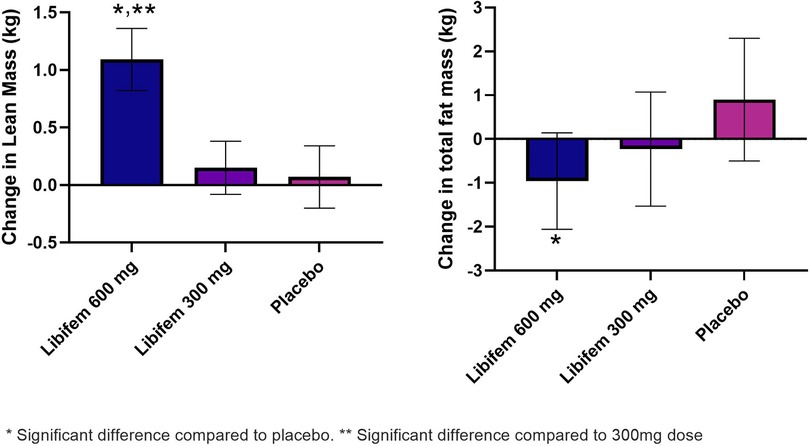
Figure 3. Change in lean mass (kg) and total fat mass (g) as measured by DEXA following 8 weeks of resistance training and supplementation with either 600 mg of Libifem®, 300 mg of Libifem®, or placebo.
A significant between-group treatment effect was reported for the change in total lean mass at week 8 [F (2, 70) = 4.73, p = 0.012] (Table 5). The 600 mg Libifem® group had a significant increase in lean mass compared with both the 300 mg and placebo groups (p = 0.011 and 0.009, respectively), gaining 1.09 kg compared with 0.152 kg and 0.079 kg in the 300 mg and placebo groups, respectively, over 8 weeks (Figure 3).
Complementing an increase in total lean mass, the 600 mg group had a significant increase in total lean mass in the legs (280 g) compared with a reduction in the placebo group (−70 g) at week 8 (p = 0.022).
No other significant changes in body composition were determined.
One serious adverse event, a bowel obstruction, was reported, which was not related to the trial product (placebo). The participant has since fully recovered. Six other adverse events occurred, and each participant withdrawn from the study. Reasons for dropout in the placebo group (n = 1) included joint pain and tingling and, in the active groups (n = 5), bloating, knee pain, acne breakout, and reflux (two participants).
This study examined the effect of Libifem® on body composition and muscle strength, power, endurance, and recovery in females undertaking resistance training. Daily supplementation with 600 mg dose of Libifem® in conjunction with resistance training significantly increased 1RM values and lean mass compared with resistance training alone (placebo) in females. The 600 mg Libifem® group decreased total fat mass (−0.96 kg) and trunk fat mass (−0.59 kg), increased lean mass (+1.09 kg), and improved 1RM leg press compared with the placebo group. In both the 300 mg and 600 mg Libifem® groups, significant intra-group changes for total fat mass, trunk fat mass, and lean mass from baseline to week 8 that did not occur in the placebo group were identified.
Supporting the findings of this study, a study conducted by Poole and colleagues (2010) on 49 resistance trained men found that supplementation with 500 mg of fenugreek for 8 weeks resulted in reduced body fat percentage and increased 1RM leg press and bench press (26). Other studies conducted by Rao et al. (25) and Wankhede et al. (27) used a similar protocol to Poole and the current study, with a dose of 300 mg of fenugreek twice daily finding that fenugreek improved 1RM leg press and body fat percentage (27). Although the present study focuses on females, the results of fenugreek on resistance training and body composition appear transferable between genders, with similar changes being seen across studies.
A study by Taylor and colleagues (2011) combining the fenugreek extract with creatine found that the combination supplement increased 1RM bench press and leg press and significantly increased lean mass. However, some of these changes were also seen in the placebo group and creatine/dextrose group (28). While the study by Taylor and colleagues was also conducted on resistance-trained males and had an additional component in the treatment group, similar improvements were seen to the current study. Based on this evidence, it can be assumed that fenugreek can significantly improve factors associated with both males and females who are resistance trained or not.
Following 8 weeks of fenugreek supplementation, no significant changes in testosterone levels in any group were seen, with all values remaining within the normal range. This is consistent with other studies of resistance training that have shown increases in muscle mass but no increase in testosterone or free testosterone levels (9, 10). The lack of change in testosterone could be due to the timing of the blood sampling. Other studies have indicated that testosterone and free testosterone trainings are only temporarily raised during exercise once the participants have reached exhaustion, with levels returning to baseline within 24 h (29). Therefore, it is possible that change in testosterone due to the exercise could have been missed. There could also be a gender differentiation with respect to fenugreek's effects on testosterone. An identical study of the Trigonella foenum-graecum extract in males similarly showed positive effects on 1RM values and body composition, with significant increases in testosterone in the 600 mg group (19). Males self-evidently produce more testosterone than females, especially during resistance training (30). This is thought to be due to adaptive changes in the synthesis of testosterone and/or secretory capacity of Leydig cells, adrenergic stimulation, plasma volume reductions, and lactate-stimulated secretion (30). It is unlikely that the differences are due to testosterone in females being used in the conversion to oestradiol, as oestradiol circulates in picomolar concentrations compared with nanomolar concentrations of testosterone (31). It could be dependent upon the stage of the menstrual cycle, as testosterone significantly fluctuates throughout the cycle (32). The amount of free circulating testosterone is determined by testosterone production, which, when compared with males, is significantly less in females (31). A previous research has shown that Libifem® can increase levels of free oestradiol (17) and may help explain the resulting enhanced anabolic response to exercise (12). Future research using Libifem® in females may benefit by having a closer focus on oestradiol rather than testosterone.
Exercise studies in females present a number of challenges not typically encountered in male-only studies. The most confounding factor in female-only studies is the potential for hormone shifts linked to the menstrual cycle and menopause. Hormonal changes throughout a study period have the potential to influence cardiovascular and respiratory systems, thermoregulation, and injury/repair mechanisms (33–35), all of which can impact exercise outcomes. A limitation of this study was the lack of evaluation of the participant's menstrual cycles and the effect hormone fluctuation may have had on the results. However, as menstrual cycles were not monitored in this study, further testing is required on this. Testing of the hormone levels or monitoring the menstrual cycle phases may have assisted in evaluating the true effects of Libifem® and how testosterone levels may have improved or affected the measured outcomes. Further research including variables to account for these changes in hormones may be beneficial to assist in the understanding of this phenomenon.
Overall, this study was able to find that supplementation with Libifem® improved 1RM measurements and body composition. The major outcomes from this study can be applied to women's resistance training, as there is a need for natural products that benefit body composition. Despite the potential variables involved in female-only exercise studies, the beneficial effects of Libifem® following resistance training were evident, and the product was well tolerated. Therefore, this study has the potential to increase the exercising capabilities and alter the physique of females partaking in resistance-based exercise—an area of increasing popularity, but typically under targeted by both science and commercial products.
The developing science of phyto- and nutrimodulation of multiple metabolic sequences using natural and often food-based products opens a new chapter in sporting enhancement. The enhancement effects of any one product are generally slight, but the safety of these compounds permits concomitant use. Given the different mechanisms of action of natural and food-based products (36–40), it is possible that multiple supplements may have an additive effect offering greater advantages to those who know the science. The effect that combining multiple supplements together may have is an area of increasing interest and a potential focus for future studies.
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Bellberry Limited. The patients/participants provided their written informed consent to participate in this study.
The authors confirm their contribution to the paper as follows: DB and AR: study conception and design, data collection, and analysis and interpretation of the results. DB, PC, and AR: draft of the manuscript preparation. All authors reviewed the results and approved the final version. All authors contributed to the article and approved the submitted version.
This study was funded by Gencor Pacific Ltd. The funder was not involved in the study design, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
AR and DB were employed by RDC Clinical. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med. (2010) 40:1037–53. doi: 10.2165/11536910-000000000-00000
2. Foulds HJ, Bredin SS, Charlesworth SA, Ivey AC, Warburton DE. Exercise volume and intensity: a dose-response relationship with health benefits. Eur J Appl Physiol. (2014) 114:1563–71. doi: 10.1007/s00421-014-2887-9
3. Kobayashi E, Sato Y, Umegaki K, Chiba T. The prevalence of dietary supplement use among college students: a nationwide survey in Japan. Nutrients. (2017) 9(11):1250. doi: 10.3390/nu9111250
4. Arazi H, Salek L, Nikfal E, Izadi M, Tufano JJ, Elliott BT, et al. Comparable endocrine and neuromuscular adaptations to variable vs. constant gravity-dependent resistance training among young women. J Transl Med. (2020) 18:239. doi: 10.1186/s12967-020-02411-y
5. Ahtiainen JP, Walker S, Peltonen H, Holviala J, Sillanpaa E, Karavirta L, et al. Heterogeneity in resistance training-induced muscle strength and mass responses in men and women of different ages. Age. (2016) 38:10. doi: 10.1007/s11357-015-9870-1
6. Wikström-Frisén L, Boraxbekk CJ, Henriksson-Larsén K. Effects on power, strength and lean body mass of menstrual/oral contraceptive cycle-based resistance training. J Sports Med Phys Fitness. (2017) 57:43–52. doi: 10.23736/s0022-4707.16.05848-5
7. Cholewa JM, Rossi FE, MacDonald C, Hewins A, Gallo S, Micenski A, et al. The effects of moderate-versus high-load resistance training on muscle growth, body composition, and performance in collegiate women. J Strength Cond Res. (2018) 32:1511–24. doi: 10.1519/JSC.0000000000002048
8. Schoenfeld BJ, Grgic J, Ogborn D, Krieger JW. Strength and hypertrophy adaptations between low- vs. high-load resistance training: a systematic review and meta-analysis. J Strength Cond Res. (2017) 31:3508–23. doi: 10.1519/JSC0000000000002200
9. Westcott WL. Resistance training is medicine: effects of strength training on health. Curr Sports Med Rep. (2012) 11:209–16. doi: 10.1249/JSR.0b013e31825dabb8
10. Linnamo V, Pakarinen A, Komi PV, Kraemer WJ, Häkkinen K. Acute hormonal responses to submaximal and maximal heavy resistance and explosive exercises in men and women. J Strength Cond Res. (2005) 19:566–71. doi: 10.1519/r-15404.1
11. Kramer A. An overview of the beneficial effects of exercise on health and performance. Adv Exp Med Biol. (2020) 1228:3–22. doi: 10.1007/978-981-15-1792-1_1
12. Nagulapalli Venkata KC, Swaroop A, Bagchi D, Bishayee A. A small plant with big benefits: Fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol Nutr Food Res. (2017) 61(6). doi: 10.1002/mnfr.201600950
13. Yadav UC, Baquer NZ. Pharmacological effects of Trigonella foenum-graecum L. in health and disease. Pharm Biol. (2014) 52:243–54. doi: 10.3109/13880209.2013.826247
14. Goyal S, Gupta N, Chatterjee S. Investigating therapeutic potential of Trigonella foenum-graecum L. as our defense mechanism against several human diseases. J Toxicol. (2016) 2016:1250387. doi: 10.1155/2016/1250387
15. Rao A, Steels E, Beccaria G, Inder WJ, Vitetta L. Influence of a specialized Trigonella foenum-graecum seed extract (Libifem), on testosterone, estradiol and sexual function in healthy menstruating women, a randomised placebo controlled study. Phytother Res. (2015) 29:1123–30. doi: 10.1002/ptr.5355
16. Smith SJ, Lopresti AL, Teo SYM, Fairchild TJ. Examining the effects of herbs on testosterone concentrations in men: a systematic review. Adv Nutr. (2021) 12:744–65. doi: 10.1093/advances/nmaa134
17. Steels E, Steele ML, Harold M, Coulson S. Efficacy of a proprietary Trigonella foenum-graecum L. de-husked seed extract in reducing menopausal symptoms in otherwise healthy women: a double-blind, randomized, placebo-controlled study. Phytother Res. (2017) 31:1316–22. doi: 10.1002/ptr.5856
18. Sreeja S, Anju VS, Sreeja S. In vitro estrogenic activities of fenugreek Trigonella foenum-graecum seeds. Indian J Med Res. (2010) 131:814–9. PMID: 20571172.20571172
19. Rao A, Steels E, Inder WJ, Abraham S, Vitetta L. Testofen, a specialised Trigonella foenum-graecum seed extract reduces age-related symptoms of androgen decrease, increases testosterone levels and improves sexual function in healthy aging males in a double-blind randomised clinical study. Aging Male. (2016) 19:134–42. doi: 10.3109/13685538.2015.1135323
20. Palacios S, Soler E, Ramirez M, Lilue M, Khorsandi D, Losa F. Effect of a multi-ingredient based food supplement on sexual function in women with low sexual desire. BMC Womens Health. (2019) 19:58. doi: 10.1186/s12905-019-0755-9
21. Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev. (2017) 38:302–24. doi: 10.1210/er.2017-00025
22. Pina FLC, Pedro Nunes J, Schoenfeld BJ, Nascimento MA, Gerage AM, Januario RSB, et al. Effects of different weekly sets-equated resistance training frequencies on muscular strength, muscle mass, and body fat in older women. J Strength Cond Res. (2020) 34:2990–5. doi: 10.1519/jsc.0000000000003130
23. Denham J, Scott-Hamilton J, Hagstrom AD, Gray AJ. Cycling power outputs predict functional threshold power and maximum oxygen uptake. J Strength Cond Res. (2020) 34:3489–97. doi: 10.1519/JSC.0000000000002253
24. Sorenson A, Aune TK, Rangul V, Dalen T. The validity of functional threshold power and maximal oxygen uptake for cycling performance in moderately trained cyclists. Sports. (2019) 7:217. doi: 10.3390/sports7100217
25. Rao AJ, Mallard AR, Grant R. Testofen® (Fenugreek extract) increases strength and muscle mass compared to placebo in response to calisthenics. A randomized control trial. Transl Sports Med. (2020) 3:374–80. doi: doi: 10.1002/tsm2.153
26. Poole C, Bushey B, Foster C, Campbell B, Willoughby D, Kreider R, et al. The effects of a commercially available botanical supplement on strength, body composition, power output, and hormonal profiles in resistance-trained males. J Int Soc Sports Nutr. (2010) 7:34. doi: 10.1186/1550-2783-7-34
27. Wankhede S, Mohan V, Thakurdesai P. Beneficial effects of fenugreek glycoside supplementation in male subjects during resistance training: a randomized controlled pilot study. J Sport Health Sci. (2016) 5:176–83. doi: 10.1016/j.shs.2014.09.005
28. Taylor L, Poole C, Pena E, Lewing M, Kreider R, Foster C, et al. Effects of combined creatine plus fenugreek extract vs. creatine plus carbohydrate supplementation on resistance training adaptations. J Sports Sci Med. (2011) 10:254–60. PMID: 24149869.24149869
29. Consitt LA, Copeland JL, Tremblay MS. Endogenous anabolic hormone responses to endurance versus resistance exercise and training in women. Sports Med. (2002) 32:1–22. doi: 10.2165/00007256-200232010-00001
30. Hirschberg AL, Knutsson JE, Helge T, Godhe M, Ekblom M, Bermon S, et al. Effects of moderately increased testosterone concentration on physical performance in young women: a double blind, randomised, placebo-controlled study. Br J Sports Med. (2020) 54:599–604. doi: 10.1136/bjsports-2018-100525
31. Davis SR, Wahlin-Jacobsen S. Testosterone in women – the clinical significance. Lancet Diabetes Endocrinol. (2015) 3:980–92. doi: 10.1016/s2213-8587(15)00284-3
32. Sokoloff N C, Misra M, Ackerman KE. Exercise, training, and the hypothalamic-pituitary-gonadal axis in men and women. Front Horm Res. (2016) 47:27–43. doi: 10.1159/000445154
33. Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. (2005) 35:339–61. doi: 10.2165/00007256-200535040-00004
34. Huebner M, Meltzer D, Ma W, Arrow H. The masters athlete in Olympic weightlifting: training, lifestyle, health challenges, and gender differences. PLoS One. (2020) 15:e0243652. doi: 10.1371/journal.pone.0243652
35. Romero-Parra N, Cupeiro R, Alfaro-Magallanes VM, Rael B, Rubio-Arias JA, Peinado AB, et al. Exercise-induced muscle damage during the menstrual cycle: a systematic review and meta-analysis. J Strength Cond Res. (2021) 35:549–61. doi: 10.1519/JSC.0000000000003878
36. Fernández-Lázaro D, Mielgo-Ayuso J, Seco Calvo J, Córdova Martínez A, Caballero García A, Fernandez-Lazaro CI. Modulation of exercise-induced muscle damage, inflammation, and oxidative markers by curcumin supplementation in a physically active population: a systematic review. Nutrients. (2020) 12(2):501. doi: 10.3390/nu12020501
37. Mallard A, Briskey D, Richards A, Mills D, Rao A. The effect of orally dosed levagen+™ (palmitoylethanolamide) on exercise recovery in healthy males-A double-blind, randomized, placebo-controlled study. Nutrients. (2020) 12(3):596. doi: 10.3390/nu12030596
38. Ji J, Damschroder D, Bessert D, Lazcano P, Wessells R, Reynolds CA, et al. NAD supplementation improves mitochondrial performance of cardiolipin mutants. Biochim Biophys Acta Mol Cell Biol Lipids. (2022) 1867(4):159094. doi: 10.1016/j.bbalip.2021.159094
39. Williams M. Dietary supplements and sports performance: herbals. J Int Soc Sports Nutr. (2006) 3(1):1. doi: 10.1186/1550-2783-3-1-1
Keywords: muscle strength, female exercise, body composition, fenugreek, resistance training
Citation: Rao A, Clayton P and Briskey D (2023) Libifem® (Trigonella foenum-graecum) in conjunction with exercise on muscle strength, power, endurance, and body composition in females aged between 25 and 45 years. Front. Sports Act. Living 5:1207013. doi: 10.3389/fspor.2023.1207013
Received: 16 April 2023; Accepted: 3 July 2023;
Published: 11 August 2023.
Edited by:
Matthew Cooke, Swinburne University of Technology, AustraliaReviewed by:
Geoffrey Hudson, University of South Alabama, United States© 2023 Rao, Clayton and Briskey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda Rao YW1hbmRhQHJkY2dsb2JhbC5jb20uYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.