- 1Department of Research and Development, Clinic of Substance Use and Addiction Medicine, St Olavs University Hospital, Trondheim, Norway
- 2Department of Mental Health, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology, Trondheim, Norway
- 3Department of Psychology, Faculty of Social and Educational Sciences, Norwegian University of Science and Technology, Trondheim, Norway
- 4Department of Global Public Health, Karolinska Institutet, Stockholm, Sweden
Introduction: Substance use disorder (SUD) is characterized by cognitive impairment, especially executive dysfunction. Executive function is recognized as an important determinant of treatment outcome as it is associated with dropout rate, attendance to therapy and potential relapse after treatment termination. Physical activity can have beneficial effects on cognitive function, but there is still a lack of knowledge regarding potential benefits of aerobic exercise for executive function in SUD treatment. The aim of this study is to examine the effect of aerobic high-intensity interval training (HIIT) on cognitive function and the subsequent effect on treatment outcome in patients with SUD.
Methods and analysis: This study is a randomized controlled trial, including men and women ≥18 years with diagnosed SUD by ICD-10. The patients will be recruited from the department for inpatient treatment at Blue Cross - Lade Addiction Treatment Center, Trondheim, Norway. Participants will be randomized 1:1 into either HIIT (3x/week) + treatment as usual (TAU), or TAU alone. Study outcomes will be assessed at baseline, after eight weeks of intervention, and at 3- and 12-months follow-up. The primary outcome is to compare the change in executive function (via altered BRIEF-A score, Behavior Rating Inventory of Executive Function-Adult) measured between the two study groups after eight weeks. Secondary outcomes include mapping of cognitive function in different subgroups (e.g. type of substance, age, fitness level), collecting self-reported information about quality of life, craving, sleep quality, etc., as well as assessing compliance to TAU and long-term treatment outcome.
Ethics and dissemination: The project was approved by the Regional Ethical Committee and will be performed in accordance with this protocol and the Declaration of Helsinki. Written informed consent will be obtained from all participants prior to inclusion. This project will explore a novel approach to how exercise can be applied in SUD treatment, beyond the well-known effects on physical health. We expect to achieve new knowledge in regard to what extent HIIT can improve cognitive abilities and subsequent treatment outcome in SUD.
Trial registration number: https://www.clinicaltrials.gov/NCT05324085.
1. Introduction
Substance use can have detrimental effects on a person's health and way of life, with negative consequences for family, friends, and participation in society. Substance use disorder (SUD) is a general term relating to harmful substance use that has evolved into abuse and addiction, and is covered by the diagnostic criteria ICD-10 and DSM-5, encompassing both illicit and licit substance use (1). According to the World Drug Report from 2021, about 275 million people worldwide used illicit drugs in 2019, whereof roughly 36 million individuals suffered from SUD (2). Almost eight times as many people (283 million) were registered having a SUD due to alcohol in 2016 (3). Patients with SUD also have high occurrence of both physical and mental comorbidities, leading to a reduced life expectancy of about 18–24 years – the most severe premature mortality gap among individuals with mental diseases (4).
A common feature in patients with SUD is impaired cognitive functioning (CF) (5–7). This particularly relates to executive functions, which for instance include decision-making, consequence analysis, impulse/self-control, and working memory (8, 9). Executive functioning is recognized as an important determinant of treatment outcome as it is associated with dropout rate, attendance to therapy sessions, and potential relapse after treatment termination (10–12). In this regard, Andersson et al. (2019) reported a relapse rate of approximately 40% three to six months after inpatient treatment in Norway (13). This constitutes a major concern and calls for new measures to overcome this challenge. Moreover, a certain degree of CF is required to benefit from verbal-based therapy forms and to learn new coping strategies, both of which are important components of SUD treatment (11). Impaired CF can not only negatively affect the treatment process itself, but also its long-term implications as for instance community integration, occupational functioning, and quality of life (14). Thus, improving CF could work as a primer for other treatment forms (e.g., psychotherapy), favoring the rehabilitation process and outcome acutely and long-term.
Physical activity (PA) can have beneficial effects on mental health and CF in healthy populations (15–17), and individuals with mental diseases (18–21), among them also patients with SUD (22, 23). A systematic review from the American College of Sports Medicine found strong evidence supporting that PA can improve CF, particularly in individuals with cognitive impairment (21). In line with this, several studies have shown that acute exercise (i.e., one single session) could improve CF in healthy individuals, an effect that was most pronounced after high-intensity aerobic exercise (24–26). Although few randomized controlled trials (RCTs) have been conducted, one RCT showed that 24 weeks of aerobic exercise improved executive function and cerebral cortical thickness in a healthy population aged 20–67 (15). Moreover, Hwang et al. (2018) reported healthy individuals with higher levels of aerobic capacity (measured as maximal oxygen uptake (V˙O2max)) performing better on neurocognitive tests than less fit individuals (27). Hence, these studies indicate that PA and exercise-induced fitness gains might contribute to improve CF.
Several physiological processes and biomarkers could be involved in improving CF. At the molecular level for instance, studies have suggested that brain-derived neurotrophic factor (BDNF) (28, 29), insulin-like growth factor 1 (IGF-1) and vascular endothelial growth factor (VEGF) (29, 30), blood lactate (29, 31, 32), fibronectin type III domain-containing protein 5/Irisin (33), Klotho (34), glycosylphosphatidylinositol (GPI)-specific phospholipase D1 (Gpld1) (35), and interleukin 6 (IL-6) (36, 37) are potential candidates favoring cognition, memory and learning. Insulin and glucose metabolism are other possible links, suggesting that improved insulin sensitivity and glucose handling by PA influence brain function and cognitive abilities (38, 39). Finally, fitness status (i.e., V˙O2max) correlated with cerebral blood flow velocity and vasomotor reactivity, and constituted a determinant of cognitive abilities (27). Those are promising findings, but the biological mechanisms underpinning the effect of exercise on CFs are still poorly understood, and warrant further exploration (40). Thus, analyzing relevant biomarkers could contribute to new knowledge to what extent they can act as physiological mediators between exercise and improved CF.
As a measure to improve somatic health in patients with SUD, aerobic high-intensity interval training (HIIT) has been implemented as mandatory part of treatment in several inpatient clinics in Norway. Aerobic HIIT has been shown to be particularly effective for improving cardiorespiratory fitness and cardiometabolic health, also in SUD inpatients (41). Additionally, higher levels of aerobic fitness have been linked to better performance on neurocognitive tests (27), and both studies with acute (24–26, 42) and chronic HIIT interventions (43) showed promising effects on CF. Since cognitive difficulties remain a critical obstacle for recovery and community integration in patients with SUD, exercise as adjunct therapy could play a vital role in restoring cognitive resources and improve treatment outcome. However, there is still a lack of RCTs examining the effects of specific training regimes on CF in this patient group, and whether such improvements can benefit other parts of the treatment. Altogether, aerobic HIIT seems to be an appropriate intervention for concurrent improvements in physical and mental health in patients with SUD.
This project will examine a novel approach to how structured exercise can be applied in SUD treatment and could add to develop new non-pharmacological treatment options. The socioeconomic burden related to substance abuse is high (44, 45), and this study may also contribute to decrease health care costs by improving the outcome of clinical treatment and integration into society. In addition, it may contribute to improved individual health and well-being among these patients. Knowledge from this trial may, therefore, provide guidance on how supervised exercise can be used as a cost-effective and holistic approach to SUD treatment encompassing both somatic and mental health, with improved quality of life as the ultimate goal. This protocol describes the study's design, aims, methodology and clinical significance.
Aim
The overall aim of this project is to investigate the effect of HIIT on CF and associated mechanisms, and the subsequent effect on treatment outcome in patients with SUD.
Primary aim
• Assess the change in executive function between an intervention group (HIIT + treatment as usual (TAU)) and a control group (TAU) after eight weeks of intervention. Executive function will be assessed using the neuropsychological test BRIEF-A (Behavior Rating Inventory of Executive Function-Adult, self-report version). This test has been established as a valid and reliable assessment of executive functions in patients with SUD (5, 6), and was therefore chosen as the primary endpoint of this study.
Secondary aims
• Map CF in subgroups of patients with SUD (e.g. type of substance, age, fitness level).
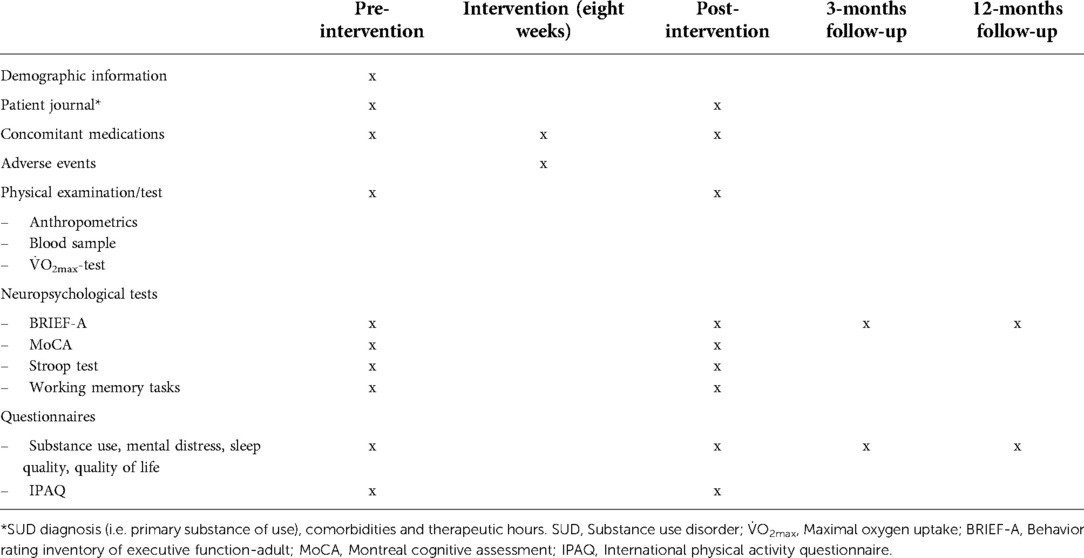
• Change in other neuropsychological assessments (i.e. Montreal Cognitive Assessment (MoCA), Stroop test, working memory tasks) between time points (Table 1).
• Change in scores on self-report questionnaires (e.g. substance use, mental distress, sleep quality, quality of life, etc.) between time points (Table 1).
• Assess compliance to TAU (e.g. therapeutic hours) and long-term outcome at follow-up (i.e. relapse rate).
• Analyze exercise-induced responses of neurocognitive biochemical markers.
2. Materials and methods
2.1. Study design and participants
This study is a parallel-group RCT (superiority trial) initiated and coordinated by the Clinic of Substance Use and Addiction Medicine (CSAM), St. Olavs Hospital, Trondheim, Norway. Patients with SUD will be recruited from the department for inpatient treatment at Blue Cross - Lade Addiction Treatment Center (Lade ATC), Trondheim, Norway (inclusion and exclusion criteria see Figure 1). Eligible patients will be stratified for primary substance of use (i.e., alcohol or other) and randomized 1:1 to either aerobic HIIT + TAU, or TAU alone (Figure 2). The Clinical Research Unit of Central Norway will provide a block randomization with the software WebCRF3. Recruitment and testing of participants will be carried out by research staff affiliated CSAM, as will the supervision of the HIIT exercise sessions. Therapists and research staff at Lade ATC will inform the patients about the study after an initial detoxification phase. The research staff will then schedule to meet with the patient to provide detailed information, and schedule baseline assessments as soon as the patient has agreed to participate and has signed the written consent form. All testing and training sessions will be performed on-site at Lade ATC. Most patients are hospitalized for 8–12 weeks and will, thus, be recruited for eight weeks. Also, eight weeks of HIIT have been shown to significantly improve aerobic fitness in patients with SUD (41), and to improve executive function in healthy individuals (43).

Figure 1. Inclusion and exclusion criteria for participants. SUD, Substance use disorder; ICD-10: F10-19 = International Classification of Diseases-10: F10-F19: Mental and behavioral disorders due to psychoactive substance use; ATC, Addiction treatment center; HIIT, High-intensity interval training.
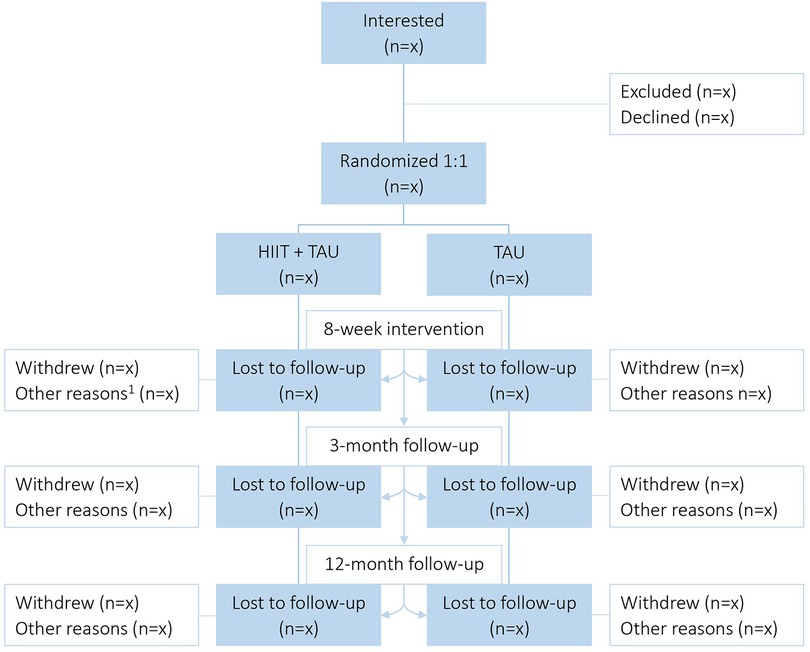
Figure 2. Study flow chart. HIIT, High-intensity interval training; TAU, Treatment as usual; 1 = for intervention: unable to complete/discontinuation (e.g. sick, relapse), missing data; for follow-up periods: unable to contact after ended treatment.
We intend to store participant data for analyses until 2025 and will keep them anonymized for another five years. We will handle all participant data coded with an identification-number, which links the participant to a list of names. This list will be stored as an encrypted data file on a data server belonging to St. Olavs University Hospital, and only the project leader and the responsible research coordinator will have access to this list. We will use the identification-number for all assessments, tests, and analyses of the data throughout the whole project. The Regional Committee for Medical Research Ethics, Norway, has approved the protocol and procedures. We registered the study at ClinicalTrials.gov in April 2022 (Identifier: NCT05324085).
2.2. Assessments
The participants will be assessed at baseline, after eight weeks of intervention, and at 3- and 12-months follow-up (Table 1). We will perform most assessments on two consecutive days: neuropsychological tests at test day one, and the fitness test and questionnaires at test day two. Blood samples will be collected on a separate day.
2.2.1. Neuropsychological tests
Many patients with SUD suffer from impaired cognitive functioning, especially executive dysfunction, reduced response inhibition, attention, and working memory (9, 14, 46), which can influence treatment outcome negatively (10–12). A selection of standardized and validated neuropsychological tests and work tasks will be conducted to measure the extent of these impairments at baseline and follow-up time points. The following tests were chosen due to their applicability (easy to administer, time-efficient) and established validity and reliability in this patient population (5–7, 47).
2.2.1.1. BRIEF-A
The BRIEF-A is a 75-item standardized questionnaire appraising executive functions in real-life situations (48). It incorporates self-reported cognitive characteristics and collects subjective information about the ability to maintain appropriate control of emotional responses and behavior. The BRIEF-A encompasses nine sub-scales in three indexes/composite scores: inhibit, shift, self-monitor, emotional control (Behavioral Regulation Index, BRI); initiate, plan/organize, working memory, organization of materials, and task monitor (Metacognition Index, MI); BRI and MI are also combined to an overall summary score (Global Executive Composite, GEC). It additionally includes three validity scales (i.e., negativity, inconsistency, and infrequency). Conducting BRIEF-A takes 10–15 min. All items are rated with a 3-point scale, whereas a total score ≥65 points is considered as a clinically significant executive function deficiency (48, 49). The BRIEF-A has been shown to be particularly sensitive for assessing executive functions in the SUD population, and is considered more relevant for real-life situations than performance-oriented measures of executive functions (e.g., memory tasks) (5, 50).
2.2.1.2. MoCA
The MoCA is a brief screening tool to detect mild cognitive impairment (51), and is used in populations with different mental and cognitive health challenges, including SUD (6, 52). It is a performance-based test that can be administered in approximately ten minutes and assesses the following CF categories: visuospatial/executive abilities, naming, memory, attention, language, abstraction, and orientation (51). All categories range from 3 to 5 points each, leading to a maximal score of 30 points, whereas a score of <26 points is considered as having impaired CF (51).
2.2.1.3. Stroop test and working memory tasks
We will use commercially available tests developed by Cambridge Brain Science (https://www.cambridgebrainsciences.com/). This web-based cognitive assessment platform delivers digital cognitive tests based on validated traditional neuropsychological tasks (e.g., original Stroop test, 1935 (53)). These computer-based tests are widely used and cited in scientific literature (54, 55). As opposed to more traditional pen-and-paper tasks, they have the advantage of being more interactive and dynamic due to the task's difficulty level automatically adjusting to the participant's performance (55).
We will conduct the tests “Double Trouble” (manual Stroop task), “Digit Span” and “Token Search” to assess response inhibition and selective attention, verbal short-term/working memory, and spatial working memory, respectively. Performing these tests takes approximately five minutes each. Outcome measures are number or accuracy of correct answers and response time (56).
2.2.2. Physical examination/tests
2.2.2.1. Anthropometrics
Before physical testing, body weight and height will be measured with a standard scale and a stadiometer (standing without shoes), respectively. Medical staff at the clinic will perform a general health check (e.g., assess blood pressure and resting heart rate) as a standardized part of patient care. The research staff will also retrieve this information from patient journals.
2.2.2.2. Exercise test
Cardiorespiratory fitness will be measured as V˙O2max (or V˙O2peak, if criteria for V˙O2max are not fulfilled, see below) by cardiopulmonary exercise testing, which is regarded as the gold standard to determine aerobic fitness level (57). Maximal oxygen uptake will be assessed on a treadmill (TX200, Gymleco, Norway) using a metabolic gas analyzer (MetaLyzer 3B, Cortex, Germany). Participants will start with a 10-min warm-up at 5% incline, followed by an individualized test protocol. Here, we will increase either incline (1%–2%) or velocity (0.5–1 km · h−1) every minute until exhaustion. The mean of the highest oxygen uptake values measured during a 30 s interval is defined as V˙O2max. At least one of the following criteria will be used to verify V˙O2max: plateau in oxygen uptake despite increasing workload; respiratory exchange ratio > 1.05; blood lactate concentration >7 mmol (58). Blood lactate will be measured within one minute after the test (Biosen C_line, EKF Diagnostics GmbH, Barleben, Germany). Maximal heart rate (HRmax) will be assessed by adding two beats per minute to the highest HR attained in the test (59), and will be used as a basis to calculate the workload for the HIIT sessions. Moreover, maximal effort during the test will be evaluated by Borg Rating of Perceived Exertion (RPE) scale (60).
2.2.2.3. Blood samples and immunoassays
To add biological insight, we will analyze relevant biochemical markers of neuroplasticity and cognition. Our aim is to explore possible alterations in serum concentration of relevant biomarkers of neurocognition throughout the intervention. Qualified staff at Lade ATC will draw blood samples following an overnight fast, before and after the intervention, applying well-established and validated procedures. Serum samples will be stored at −80°C until further analysis. The serum concentrations of relevant proteins (e.g., BDNF, Klotho and Gpld1) will be assessed using enzyme-linked immunosorbent assay (ELISA) kits. Additionally, we will use multianalyte profiling (Milliplex MAP) assays to assess larger panels' neurocognitive inflammatory/metabolic markers. For storage and management of serum samples, we will use the services of Biobank1 (Regional Research Biobank St. Olavs Hospital, https://biobank1.no/nb/).
2.2.3. Other assessments
2.2.3.1. Questionnaires
We have developed a questionnaire that acquires information concerning substance use, craving, motivation, self-esteem, sleep quality, etc. with items put together from a carefully selected battery of well-established and validated questionnaires (61–73). For instance, the 10-item short form of the Hopkins Symptoms checklist (SCL-10) has been incorporated to measure psychological distress (63), and several items of World Health organization's Quality of Life questionnaire (WHOQOL-BREF) have been added to assess quality of life (73). Additionally, we will monitor PA level by administering the International Physical Activity Questionnaire (IPAQ, short version) (74).
2.2.3.2. Clinical status therapeutic hours and concomitant medication
The research staff will register demographic data and medical history. Information concerning SUD diagnosis, comorbidities, therapeutic hours and previous treatment for SUD (if applicable) will be obtained from the patient journal by authorized staff at Lade ATC.
2.3. Intervention
2.3.1. Control group
Participants will receive standard TAU by therapists at Lade ATC with specific competence in working with patients with SUD. The content of TAU is broadly individualized, but also includes various forms of group therapy, psychotherapy, psychoeducation and PA. The PA schedule for the patients typically includes gym-based exercises (e.g., yoga, different types of circuit training, team sports) and various outdoor activities (e.g., walks, hikes, bonfire), four times per week all together.
2.3.2. Exercise group
Participants will undergo eight weeks of supervised aerobic HIIT, three times a week. This type of training is chosen since it earlier has been shown to be effective for improving somatic health in patients with SUD (41), and to have promising effects on CF as well (43, 75). The HIIT sessions will substitute most of the PA sessions included in TAU, and will be performed as 4 × 4 min intervals, running (or walking on steep incline) on a treadmill (see Figure 3 for illustration). The session will start with a 10-min warm-up at 60%–70% of HRmax, followed by 4 × 4-min intervals at ∼90% of HRmax, with 3-min active rest periods at approximately 60%–70% of HRmax between each interval, as described previously (41). The workload is relative to the maximal exercise capacity of each patient, and will be controlled using HR monitors (Polar Vantage M multisport watch and HR sensor H10, Polar Electro, Finland) and Borg scale derived RPE (60). We will instruct the participants to follow a RPE score of 16–18 (hard–very hard) for the interval period and 11–13 (fairly light–somewhat hard) for the warm-up, active rest periods, and cool-down. Total duration of each HIIT session is approximately 40–45 min.
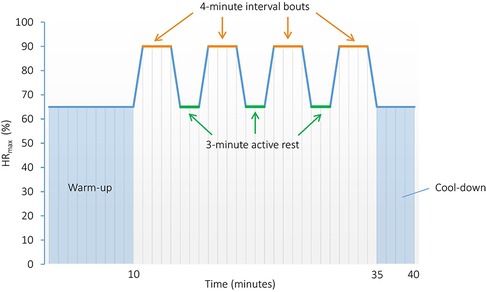
Figure 3. High-intensity interval training protocol performed as 4 × 4 min intervals. HRmax, Maximal heart rate.
2.4. Feasibility and adherence
Intervention periods of 8–12 weeks can be considered as a normal duration for this type of training intervention (16, 41, 76). The participants will only perform three short sessions per week for eight weeks, and the training facilities are situated on-site at the clinic, with immediate access to medical assistance if needed. In addition, qualified staff will supervise all training sessions, ensuring close monitoring and facilitating adherence to training. It has already been shown good adherence to HIIT sessions in this patient population (41), confirming the feasibility of this project. Moreover, participants will exercise together two by two, providing a social aspect for training which may enhance motivation to perform and adhere to the sessions. Sport therapists at Lade ATC will facilitate the implementation of the training sessions. The exercise tests and blood sampling may cause discomfort, but these assessments are usually well tolerated when performed by experienced staff following standardized protocols. However, adverse events as fatigue, increased stress, and anxiety may occur. Patient safety is highly prioritized and in case of suspicion of adverse events or reactions, we will terminate the intervention immediately and the patient will be taken care of by medical personnel.
2.5. Sample size and statistical analyses
The primary comparison is altered BRIEF-A score in aerobic HIIT + TAU vs. TAU alone after eight weeks of intervention. Based on previous studies highlighting the pronounced effect of aerobic exercise, preferably using high intensity (15, 77), we anticipate a feasible effect size of ≥ 0.6 (Cohen's d) on BRIEF-A score favoring the HIIT intervention. With a statistical power of 0.8 and a significance level of 0.05, it will be sufficient with 45 participants in each group. To compensate for an expected dropout rate of approximately 20%, we aim to include 55 participants in each arm of the study (n = 110 patients in total). This sample size is feasible to recruit within 18 months, considering the annual patient flow at Lade ATC. It will also be possible to expand the recruitment phase, and subsequent intervention period and follow-up for another six months if necessary.
We will present descriptive data as mean ± standard deviation, and medians with 25th to 75th percentiles where appropriate. To compare mean change in BRIEF-A score between the two study groups, we will apply a two-level linear mixed model (LMM), with BRIEF-A score as dependent variable, time point as level 1 and participant as level 2. Group allocation and an interaction term between group allocation and time point will be included as covariates. The LMM analyses will also produce unbiased estimate when data is missing for some time points under the assumption that the data is missing at random (78). We will perform the analysis according to the intention-to-treat principle, including all randomized study participants regardless of adherence. A per-protocol analysis of the primary outcome will also be performed, including all participants adherent to at least 70% of training sessions (i.e., 17 out of 24). We will perform similar analyses for other biological and neuropsychological parameters, as well as self-report questionnaires (secondary endpoints). In sensitivity analyses we will adjust for baseline values of cardiorespiratory fitness (27), and psychological distress (i.e., SCL-10 total score) (6). Executive dysfunction is a common feature in attention-deficit/hyperactivity disorder (ADHD), anxiety and mood disorders (79, 80), and due to high co-occurrence of those disorders among patients with SUD (81, 82), these will also be adjusted for. Statistical significance will be set to an α-level of p < 0.05, and effect sizes (i.e., Cohen's d (83)) will be presented together with 95% confidence intervals. We will use the software program SPSS (version 25.0 or higher) and GraphPad Prism (version 5.0 or higher) for all statistical analyses.
2.6. Blinding
Due to the nature of the study being an interventional trial with supervised training sessions, the study investigators and participants will not be blinded to group allocation. However, we will undertake baseline assessments prior to randomization.
2.7. Patient and public involvement
The project has been presented for the user committees at Lade ATC and St. Olavs University Hospital, who have commented on the study design and research questions; they have given full support for the project to be carried out. There is also one user representative in the steering group of the project, along with other representatives from CSAM and Lade ATC. To obtain valuable feedback and improve compliance to the exercise intervention and follow-up assessments, we will continue to meet and discuss with the user committees throughout the study period.
We will encourage participants to ask questions about the trial; it is of high importance to provide them with clear information, hereby communicating that personal data will be handled and stored coded, participation is voluntary, and withdrawal is possible at any point of time. Also, we will make individual test results available for each participant after testing (e.g., V˙O2max test results).
3. Discussion
Substance use disorder is one of the most common mental disorders (84), and accounted for more than 700 substance-use-related deaths in Norway in 2020 (85). Physical activity as an adjunct to cognitive and pharmaceutical therapy in SUD treatment has been introduced to clinics about 40 years ago, with walking, games, sports, and weight training being the most reported PA programs (86, 87). However, the lack of a detailed description of interventions (e.g., frequency, volume, intensity) makes the PA programs seem unstructured and inconsistent (41, 87). Additionally, different methodological approaches, training regimes and outcome measures of trials examining PA for patients with SUD make them difficult to compare and draw clear and compelling conclusions.
The relationship between HIIT and mental health/CF is inconclusive. One RCT by Flemmen et al. (2014) looked into the effects of HIIT on somatic and mental health in patients with SUD (41). The HIIT group had significantly higher cardiorespiratory fitness than the control group after eight weeks of intervention, but the authors could not find significant between-group differences in mental health aspects such as anxiety, depression and insomnia. As stated by Flemmen and colleagues, one reason could be that the study was underpowered in order to investigate changes in mental health. Moreover, they did not include any assessments of CF. A systematic review by Ai et al. (2021) analyzed effects of HIIT on executive function and found facilitating effects, but the authors only included studies performing one acute bout of exercise in healthy participants (42). Those results are promising, but there is still limited research available when it comes to effects of aerobic high-intensity exercise on CF, especially in patients with SUD.
Data from this trial will contribute to new knowledge to what extent HIIT can have a positive impact on CF in patients with SUD specifically. If proven beneficial, HIIT can be used as a non-pharmaceutical, cost-effective and holistic approach to SUD treatment, encompassing both mental and somatic health. Knowledge from this trial can thereby contribute to reduce the economic burden on the society and health care system and improve the individual's quality of life substantially. Finally, new insight regarding exercise-induced responses of relevant hormones and cytokines involved in neurocognitive processes can also be expected, potentially broadening the mechanistic understanding of exercise-mediated alterations in brain function on the physiological level.
4. Ethics and dissemination
The Regional Ethical Committee approved this project (REK# 171845), and the research will be performed in accordance with this protocol and the Declaration of Helsinki. Important protocol amendments will be communicated after approval by the Regional Ethical Committee. We will obtain written informed consent from all participants prior to inclusion. All data collected will be stored in anonymized case report forms. General Data Protection Regulation (GDPR) guidelines will be followed, and an appropriate Data Protection Impact Assessment (DPIA) has been performed. Data from the project will be stored and managed according to regulations by the research team affiliated CSAM. Access to detailed study protocols and participant-level data may be granted on request.
The control group will not have access to supervised HIIT, but will be offered PA sessions that are part of the standard treatment policy at Lade ATC. Inadequate SUD recovery throughout the intervention will be carefully monitored, and all participants will, independent of group allocation, receive standard TAU.
We plan to publish several scientific papers in well-acknowledged international peer-reviewed journals. The project has a distinct clinical value, and results will be communicated to patients, families, the public, and clinicians through several routes. Examples are educational groups at addiction clinics, meetings at the users' interest associations and communication through media. Additionally, we will present results at national and international scientific meetings.
Ethics statement
The studies involving human participants were reviewed and approved by Magnus Alm. Regional Comittee for Medical Research Ethics Central Norway. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All ICMJE criteria for authorship are fulfilled. CH and MPM: drafted and wrote the manuscript. MPM: conceived the study. MPM, SW, HL, GF, MH and HWA: contributed to the design of the study, revised the manuscript critically and provided feedback. All authors contributed to the article and approved the submitted version.
Funding
This project is supported by Helse-Midt Norge RHF (Central Norway Regional Health Authority), and the Clinic of Substance Use and Addiction Medicine, St. Olavs University Hospital, Norway.
Acknowledgments
The research staff thanks the Clinical Research Unit of Central Norway for providing the randomization. We would also like to acknowledge Lade ATC for their collaboration and providing facilities for testing and training of participants, research nurses at Lade ATC for collecting blood samples, Biobank1 for allocating and storing blood samples, and finally, Melanie Rae Simpson for giving advice on statistical procedures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rounsaville BJ. Experience with Icd-10/Dsm-Iv substance use disorders. Psychopathol. (2002) 35(2–3):82–8. doi: 10.1159/000065124
3. World Health Organization. Global status report on alcohol and health 2018. Geneva: World Health Organization (2018). 9241565632.
4. Nordentoft M, Wahlbeck K, Hällgren J, Westman J, Osby U, Alinaghizadeh H, et al. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS One. (2013) 8(1):e55176. doi: 10.1371/journal.pone.0055176
5. Hagen E, Erga AH, Hagen KP, Nesvåg SM, McKay JR, Lundervold AJ, et al. Assessment of executive function in patients with substance use disorder: a comparison of inventory- and performance-based assessment. J Subst Abuse Treat. (2016) 66:1–8. doi: 10.1016/j.jsat.2016.02.010
6. Hagen E, Sømhovd M, Hesse M, Arnevik EA, Erga AH. Measuring cognitive impairment in young adults with polysubstance use disorder with moca or brief-a - the significance of psychiatric symptoms. J Subst Abuse Treat. (2019) 97:21–7. doi: 10.1016/j.jsat.2018.11.010
7. Fernández-Serrano MJ, Pérez-García M, Perales JC, Verdejo-García A. Prevalence of executive dysfunction in cocaine, heroin and alcohol users enrolled in therapeutic communities. Eur J Pharmacol. (2010) 626(1):104–12. doi: 10.1016/j.ejphar.2009.10.019
8. Bechara A. Decision making impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. (2005) 8(11):1458–63. doi: 10.1038/nn1584
9. Verdejo-García A, Bechara A, Recknor EC, Pérez-García M. Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc. (2006) 12(3):405–15. doi: 10.1017/s1355617706060486
10. Bates ME, Pawlak AP, Tonigan JS, Buckman JF. Cognitive impairment influences drinking outcome by altering therapeutic mechanisms of change. Psychol Addict Behav. (2006) 20(3):241–53. doi: 10.1037/0893-164x.20.3.241
11. Brorson HH, Ajo Arnevik E, Rand-Hendriksen K, Duckert F. Drop-out from addiction treatment: a systematic review of risk factors. Clin Psychol Rev. (2013) 33(8):1010–24. doi: 10.1016/j.cpr.2013.07.007
12. Stevens L, Verdejo-García A, Goudriaan AE, Roeyers H, Dom G, Vanderplasschen W. Impulsivity as a vulnerability factor for poor addiction treatment outcomes: a review of neurocognitive findings among individuals with substance use disorders. J Subst Abuse Treat. (2014) 47(1):58–72. doi: 10.1016/j.jsat.2014.01.008
13. Andersson HW, Wenaas M, Nordfjærn T. Relapse after inpatient substance use treatment: a prospective cohort study among users of illicit substances. Addict Behav. (2019) 90:222–8. doi: 10.1016/j.addbeh.2018.11.008
14. Fernández-Serrano MJ, Pérez-García M, Verdejo-García A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neurosci Biobehav Rev. (2011) 35(3):377–406. doi: 10.1016/j.neubiorev.2010.04.008
15. Stern Y, MacKay-Brandt A, Lee S, McKinley P, McIntyre K, Razlighi Q, et al. Effect of aerobic exercise on cognition in younger adults: a randomized clinical trial. Neurology. (2019) 92(9):e905–16. doi: 10.1212/WNL.0000000000007003
16. Martland R, Korman N, Firth J, Vancampfort D, Thompson T, Stubbs B. Can high-intensity interval training improve mental health outcomes in the general population and those with physical illnesses? a systematic review and meta-analysis. Br J Sports Med. (2022) 56(5):279–91. doi: 10.1136/bjsports-2021-103984
17. Hoy S, Östh J, Pascoe M, Kandola A, Hallgren M. Effects of yoga-based interventions on cognitive function in healthy older adults: a systematic review of randomized controlled trials. Complement Ther Med. (2021) 58:102690. doi: 10.1016/j.ctim.2021.102690
18. Nuzum H, Stickel A, Corona M, Zeller M, Melrose RJ, Wilkins SS. Potential benefits of physical activity in mci and dementia. Behav Neurol. (2020) 2020:7807856. doi: 10.1155/2020/7807856
19. Nebiker L, Lichtenstein E, Minghetti A, Zahner L, Gerber M, Faude O, et al. Moderating effects of exercise duration and intensity in neuromuscular vs. Endurance exercise interventions for the treatment of depression: a meta-analytical review. Front Psychiatry. (2018) 9:305. doi: 10.3389/fpsyt.2018.00305
20. Martland R, Mondelli V, Gaughran F, Stubbs B. Can high intensity interval training improve health outcomes among people with mental illness? a systematic review and preliminary meta-analysis of intervention studies across a range of mental illnesses. J Affect Disord. (2020) 263:629–60. doi: 10.1016/j.jad.2019.11.039
21. Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, et al. Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc. (2019) 51(6):1242–51. doi: 10.1249/mss.0000000000001936
22. Costa KG, Cabral DA, Hohl R, Fontes EB. Rewiring the addicted brain through a psychobiological model of physical exercise. Front Psychiatry. (2019) 10:600. doi: 10.3389/fpsyt.2019.00600
23. Cabé N, Lanièpce A, Pitel AL. Physical activity: a promising adjunctive treatment for severe alcohol use disorder. Addict Behav. (2021) 113:106667. doi: 10.1016/j.addbeh.2020.106667
24. Chang Y-K, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res. (2012) 1453:87–101. doi: 10.1016/j.brainres.2012.02.068
25. Tsukamoto H, Suga T, Takenaka S, Tanaka D, Takeuchi T, Hamaoka T, et al. Greater impact of acute high-intensity interval exercise on post-exercise executive function compared to moderate-intensity continuous exercise. Physiol Behav. (2016) 155:224–30. doi: 10.1016/j.physbeh.2015.12.021
26. Kao SC, Wang CH, Kamijo K, Khan N, Hillman C. Acute effects of highly intense interval and moderate continuous exercise on the modulation of neural oscillation during working memory. Int J Psychophysiol. (2021) 160:10–7. doi: 10.1016/j.ijpsycho.2020.12.003
27. Hwang J, Kim K, Brothers RM, Castelli DM, Gonzalez-Lima F. Association between aerobic fitness and cerebrovascular function with neurocognitive functions in healthy, young adults. Exp Brain Res. (2018) 236(5):1421–30. doi: 10.1007/s00221-018-5230-6
28. Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, et al. Exercise induces hippocampal bdnf through a Pgc-1α/Fndc5 pathway. Cell Metab. (2013) 18(5):649–59. doi: 10.1016/j.cmet.2013.09.008
29. Kujach S, Olek RA, Byun K, Suwabe K, Sitek EJ, Ziemann E, et al. Acute sprint interval exercise increases both cognitive functions and peripheral neurotrophic factors in humans: the possible involvement of lactate. Front Neurosci. (2019) 13:1455. doi: 10.3389/fnins.2019.01455
30. Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. (2001) 21(15):5678–84. doi: 10.1523/jneurosci.21-15-05678.2001
31. Morland C, Andersson KA, Haugen Ø P, Hadzic A, Kleppa L, Gille A, et al. Exercise induces cerebral vegf and angiogenesis via the lactate receptor Hcar1. Nat Commun. (2017) 8:15557. doi: 10.1038/ncomms15557
32. Hayek L E, Khalifeh M, Zibara V, Abi Assaad R, Emmanuel N, Karnib N, et al. Lactate mediates the effects of exercise on learning and memory through Sirt1-dependent activation of hippocampal brain-derived neurotrophic factor (Bdnf). J Neurosci. (2019) 39(13):2369–82. doi: 10.1523/jneurosci.1661-18.2019.30692222
33. Huuha AM, Norevik CS, Moreira JBN, Kobro-Flatmoen A, Scrimgeour N, Kivipelto M, et al. Can exercise training teach us how to treat Alzheimer's disease? Ageing Res Rev. (2022) 75:101559. doi: 10.1016/j.arr.2022.101559
34. Amaro-Gahete FJ, De-la OA, Jurado-Fasoli L, Espuch-Oliver A, de Haro T, Gutierrez A, et al. Exercise training increases the s-Klotho plasma levels in sedentary middle-aged adults: a randomised controlled trial. The fit-ageing study. J Sports Sci. (2019) 37(19):2175–83. doi: 10.1080/02640414.2019.1626048
35. Horowitz AM, Fan X, Bieri G, Smith LK, Sanchez-Diaz CI, Schroer AB, et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science. (2020) 369(6500):167–73. doi: 10.1126/science.aaw2622.32646997
36. Funk JA, Gohlke J, Kraft AD, McPherson CA, Collins JB, Jean Harry G. Voluntary exercise protects hippocampal neurons from trimethyltin injury: possible role of interleukin-6 to modulate tumor necrosis factor receptor-mediated neurotoxicity. Brain Behav Immun. (2011) 25(6):1063–77. doi: 10.1016/j.bbi.2011.03.012
37. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. (2012) 8(8):457–65. doi: 10.1038/nrendo.2012.49
38. Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev. (2016) 96(4):1169–209. doi: 10.1152/physrev.00032.2015
39. Evans PL, McMillin SL, Weyrauch LA, Witczak CA. Regulation of skeletal muscle glucose transport and glucose metabolism by exercise training. Nut. (2019) 11(10):2432. doi: 10.3390/nu11102432
40. Mahalakshmi B, Maurya N, Lee SD, Bharath Kumar V. Possible neuroprotective mechanisms of physical exercise in neurodegeneration. Int J Mol Sci. (2020) 21(16):5895. doi: 10.3390/ijms21165895
41. Flemmen G, Unhjem R, Wang E. High-intensity interval training in patients with substance use disorder. Biomed Res Int. (2014) 2014:616935. doi: 10.1155/2014/616935
42. Ai JY, Chen FT, Hsieh SS, Kao SC, Chen AG, Hung TM, et al. The effect of acute high-intensity interval training on executive function: a systematic review. Int J Environ Res Public Health. (2021) 18(7):3539. doi: 10.3390/ijerph18073593
43. Mekari S, Earle M, Martins R, Drisdelle S, Killen M, Bouffard-Levasseur V, et al. Effect of high intensity interval training compared to continuous training on cognitive performance in young healthy adults: a pilot study. Brain Sci. (2020) 10(2):81. doi: 10.3390/brainsci10020081
44. Vossius C, Testad I, Skjæveland R, Nesvåg S. The use and costs of health and social services in patients with longstanding substance abuse. BMC Health Serv Res. (2013) 13:185. doi: 10.1186/1472-6963-13-185
45. Andlin-Sobocki P, Rehm J. Cost of addiction in Europe. Eur J Neurol. (2005) 12(Suppl 1):28–33. doi: 10.1111/j.1468-1331.2005.01194.x
46. Verdejo-Garcia A, Lorenzetti V, Manning V, Piercy H, Bruno R, Hester R, et al. A roadmap for integrating neuroscience into addiction treatment: a consensus of the neuroscience interest group of the international society of addiction medicine. Front Psychiatry. (2019) 10:877. doi: 10.3389/fpsyt.2019.00877
47. Copersino ML, Fals-Stewart W, Fitzmaurice G, Schretlen DJ, Sokoloff J, Weiss RD. Rapid cognitive screening of patients with substance use disorders. Exp Clin Psychopharmacol. (2009) 17(5):337–44. doi: 10.1037/a0017260
48. Gioia GA, Isquith PK, Roth RM. Behavior rating inventory for executive function. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of clinical neuropsychology. Cham: Springer International Publishing (2018). p. 532–8.
49. Isquith P, Roth R, Gioia G, Par S. Behavior rating inventory of executive function–adult version (brief-a). Interpretive Report. (2006).
50. Isquith PK, Roth RM, Gioia G. Contribution of rating scales to the assessment of executive functions. Appl Neuropsychol Child. (2013) 2(2):125–32. doi: 10.1080/21622965.2013.748389
51. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, moca: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x
52. Ridley N, Batchelor J, Draper B, Demirkol A, Lintzeris N, Withall A. Cognitive screening in substance users: diagnostic accuracies of the Mini-mental state examination, addenbrooke's cognitive examination-revised, and Montreal cognitive assessment. J Clin Exp Neuropsychol. (2018) 40(2):107–22. doi: 10.1080/13803395.2017.1316970
53. Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. (1935) 18(6):643. doi: 10.1037/h0054651
54. Hampshire A, Highfield RR, Parkin BL, Owen AM. Fractionating human intelligence. Neuron. (2012) 76(6):1225–37. doi: 10.1016/j.neuron.2012.06.022
55. Brenkel M, Shulman K, Hazan E, Herrmann N, Owen AM. Assessing capacity in the elderly: comparing the moca with a novel computerized battery of executive function. Dement Geriatr Cogn Dis Extra. (2017) 7(2):249–56. doi: 10.1159/000478008
56. Battista M. Explore the science [online document]. Cambridge Brain Sciences, Resource Center (2021). Available from: http://help.cambridgebrainsciences.com/en/collections/258738-explore-the-science (cited 8.10.2021).
57. Beltz NM, Gibson AL, Janot JM, Kravitz L, Mermier CM, Dalleck LC. Graded exercise testing protocols for the determination of vo(2)Max: historical perspectives, progress, and future considerations. J Sports Med. (2016) 2016:3968393. doi: 10.1155/2016/3968393
58. Edvardsen E, Hem E, Anderssen SA. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: a cross-sectional study. PLoS One. (2014) 9(1):e85276. doi: 10.1371/journal.pone.0085276
59. Berglund IJ, Sørås SE, Relling BE, Lundgren KM, Kiel IA, Moholdt T. The relationship between maximum heart rate in a cardiorespiratory fitness test and in a Maximum heart rate test. J Sci Med Sport. (2019) 22(5):607–10. doi: 10.1016/j.jsams.2018.11.018
60. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. (1982) 14(5):377–81.7154893
61. McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the addiction severity Index. J Subst Abuse Treat. (1992) 9(3):199–213. doi: 10.1016/0740-5472(92)90062-s
62. Lauritzen G, Ravndal E. Introduction of the europasi in Norway: clinical and research experiences from a cost-effectiveness study. J Subst Use. (2004) 9(3–4):141–6. doi: 10.1080/14659890410001697415
63. Strand BH, Dalgard OS, Tambs K, Rognerud M. Measuring the mental health Status of the Norwegian population: a comparison of the instruments Scl-25, Scl-10, Scl-5 and mhi-5 (sf-36). Nord J Psychiatry. (2003) 57(2):113–8. doi: 10.1080/08039480310000932
64. De Leon G, Melnick G, Kressel D, Circumstances JN, Motivation R. And suitability (the Cmrs scales): predicting retention in therapeutic community treatment. Am J Drug Alcohol Abuse. (1994) 20(4):495–515. doi: 10.3109/00952999409109186
65. Rosenberg M. Society and the adolescent self-image. Princeton, N.J: Princeton University Press (1965).
66. Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J Pers. (2004) 72(2):271–324. doi: 10.1111/j.0022-3506.2004.00263.x
67. Ilgen M, McKellar J, Tiet Q. Abstinence self-efficacy and abstinence 1 year after substance use disorder treatment. J Consult Clin Psychol. (2005) 73(6):1175–80. doi: 10.1037/0022-006x.73.6.1175
68. Haugum M, Iversen HH, Bjertnaes O, Lindahl AK. Patient experiences questionnaire for interdisciplinary treatment for substance dependence (Peq-Itsd): reliability and validity following a national survey in Norway. BMC Psychiatry. (2017) 17(1):73. doi: 10.1186/s12888-017-1242-1
69. Horvath AO, Greenberg LS. Development and validation of the working alliance inventory. J Couns Psychol. (1989) 36(2):223. doi: 10.1037/0022-0167.36.2.223
70. Marsden J, Stewart D, Gossop M, Rolfe A, Bacchus L, Griffiths P, et al. Assessing client satisfaction with treatment for substance use problems and the development of the treatment perceptions questionnaire (Tpq). Addict Res Theory. (2000) 8(5):455–70. doi: 10.3109/16066350009005590
71. Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. (2011) 34(5):601–8. doi: 10.1093/sleep/34.5.601
72. Ware JE, Kosinski M, Keller S. Sf-36 Physical and Mental Health Summary Scales: A User's Manual: Health Assessment Lab. (1994).
73. Skevington SM, Lotfy M, O'Connell KA. The world health organization’s whoqol-bref quality of life assessment: psychometric properties and results of the international field trial. A report from the whoqol group. Qual Life Res. (2004) 13(2):299–310. doi: 10.1023/b:Qure.0000018486.91360.00
74. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35(8):1381–95. doi: 10.1249/01.Mss.0000078924.61453.Fb
75. Hsieh S-S, Chueh T-Y, Huang C-J, Kao S-C, Hillman CH, Chang Y-K, et al. Systematic review of the acute and chronic effects of high-intensity interval training on executive function across the lifespan. J Sports Sci. (2021) 39(1):10–22. doi: 10.1080/02640414.2020.1803630
76. Chapman JJ, Coombes JS, Brown WJ, Khan A, Chamoli S, Pachana NA, et al. The feasibility and acceptability of high-intensity interval training for adults with mental illness: a pilot study. Ment Health Phys Act. (2017) 13:40–8. doi: 10.1016/j.mhpa.2017.09.007
77. Moreau D, Chou E. The acute effect of high-intensity exercise on executive function: a meta-analysis. Perspect Psychol Sci. (2019) 14(5):734–64. doi: 10.1177/1745691619850568
78. Krueger C, Tian L. A comparison of the general linear mixed model and repeated measures anova using a dataset with multiple missing data points. Biol Res Nurs. (2004) 6(2):151–7. doi: 10.1177/1099800404267682
79. Silverstein MJ, Faraone SV, Leon TL, Biederman J, Spencer TJ, Adler LA. The relationship between executive function deficits and dsm-5-defined adhd symptoms. J Atten Disord. (2020) 24(1):41–51. doi: 10.1177/1087054718804347
80. Warren SL, Heller W, Miller GA. The structure of executive dysfunction in depression and anxiety. J Affect Disord. (2021) 279:208–16. doi: 10.1016/j.jad.2020.09.132
81. van Emmerik-van Oortmerssen K, van de Glind G, van den Brink W, Smit F, Crunelle CL, Swets M, et al. Prevalence of attention-deficit hyperactivity disorder in substance use disorder patients: a meta-analysis and meta-regression analysis. Drug Alcohol Depend. (2012) 122(1–2):11–9. doi: 10.1016/j.drugalcdep.2011.12.007
82. Lai HM, Cleary M, Sitharthan T, Hunt GE. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: a systematic review and meta-analysis. Drug Alcohol Depend. (2015) 154:1–13. doi: 10.1016/j.drugalcdep.2015.05.031
84. Folkehelseinsituttet. Mental illness among adults in Norway [online document]. Norwegian Institute of Public Health (2016) [updated 04.09.2019; cited 20.07.2021]. Available from: https://www.fhi.no/en/op/hin/mental-health/psykisk-helse-hos-voksne.
85. Dødårsaksregisteret. D7: Dødsfall Som Skyldes Bruk Av Alkohol, Narkotika Etter Bofylke Og Dødsårsak, Antall Og Rater [Death Registry. D7: Death Due to Use of Alcohol, Illicit Drugs by County and Cause of Death, Number and Rates] [Internet]. Norwegian Institute of Public Health (2021). Available from: http://statistikkbank.fhi.no/dar/(cited 31.08.2021)
86. Kremer D, Malkin MJ, Benshoff JJ. Physical activity programs offered in substance abuse treatment facilities. J Subst Abuse Treat. (1995) 12(5):327–33. doi: 10.1016/0740-5472(95)02008-3
Keywords: physical activity, exercise, high-intensity interval training (HIIT), maximal oxygen uptake, cognitive function, executive function, BRIEF-A, substance use disorder (SUD)
Citation: Haberstroh C, Weider S, Flemmen G, Loe H, Andersson HW, Hallgren M and Mosti MP (2022) The effect of high-intensity interval training on cognitive function in patients with substance use disorder: Study protocol for a two-armed randomized controlled trial. Front. Sports Act. Living 4:954561. doi: 10.3389/fspor.2022.954561
Received: 2 June 2022; Accepted: 21 November 2022;
Published: 9 December 2022.
Edited by:
Jan H. Rosenvinge, UiT The Arctic University of Norway, NorwayReviewed by:
Olivier Dupuy, University of Poitiers, FranceCarlos Márquez, University of Chile, Chile
© 2022 Haberstroh, Weider, Flemmen, Loe, Andersson, Hallgren and Mosti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carolin Haberstroh Y2Fyb2xpbi5oYWJlcnN0cm9oQHN0b2xhdi5ubw== Mats Peder Mosti bWF0cy5wZWRlci5tb3N0aUBzdG9sYXYubm8=
Specialty Section: This article was submitted to Physical Activity in the Prevention and Management of Disease, a section of the journal Frontiers in Sports and Active Living
 Carolin Haberstroh
Carolin Haberstroh Siri Weider
Siri Weider Grete Flemmen
Grete Flemmen Henrik Loe1
Henrik Loe1 Helle Wessel Andersson
Helle Wessel Andersson Mats Hallgren
Mats Hallgren