- 1Graduate School of Media and Governance, Keio University, Fujisawa, Japan
- 2Faculty of Environment and Information Studies, Keio University, Fujisawa, Japan
- 3Department of Rehabilitation Medicine, Keio University School of Medicine, Tokyo, Japan
Previous psychological studies using questionnaires have consistently reported that athletes have superior motor imagery ability, both for sports-specific and for sports-non-specific movements. However, regarding motor imagery of sports-non-specific movements, no physiological studies have demonstrated differences in neural activity between athletes and non-athletes. The purpose of this study was to examine the differences in sensorimotor rhythms during kinesthetic motor imagery (KMI) of sports-non-specific movements between gymnasts and non-gymnasts. We selected gymnasts as an example population because they are likely to have particularly superior motor imagery ability due to frequent usage of motor imagery, including KMI as part of daily practice. Healthy young participants (16 gymnasts and 16 non-gymnasts) performed repeated motor execution and KMI of sports-non-specific movements (wrist dorsiflexion and shoulder abduction of the dominant hand). Scalp electroencephalogram (EEG) was recorded over the contralateral sensorimotor cortex. During motor execution and KMI, sensorimotor EEG power is known to decrease in the α- (8–15 Hz) and β-bands (16–35 Hz), referred to as event-related desynchronization (ERD). We calculated the maximal peak of ERD both in the α- (αERDmax) and β-bands (βERDmax) as a measure of changes in corticospinal excitability. αERDmax was significantly greater in gymnasts, who subjectively evaluated their KMI as being more vivid in the psychological questionnaire. On the other hand, βERDmax was greater in gymnasts only for shoulder abduction KMI. These findings suggest gymnasts' signature of flexibly modulating sensorimotor rhythms with no movements, which may be the basis of their superior ability of KMI for sports-non-specific movements.
Introduction
There are two types of motor imagery, namely, kinesthetic motor imagery (KMI) and visual motor imagery (VMI). KMI involves imagining the feeling when we perform actual motor tasks, while VMI involves imagining to see ourselves or the field of vision where we perform the tasks (Hall et al., 1985; Malouin et al., 2007). In particular, KMI is regularly used by athletes to improve performance (Cumming and Hall, 2002; Mizuguchi et al., 2012). Previous studies have shown that performing KMI in training improves performance in various tasks, including sequence learning (Sobierajewicz et al., 2017; Lebon et al., 2018), jump height (Battaglia et al., 2014), and free-throw shooting (Peynircioglu et al., 2000). To explain these performance gains, several neuroscience studies have provided evidence that KMI activates some neural substrates in common with actual movement, including the primary motor cortex, supplementary motor area, and inferior parietal lobe (Decety, 1999; Hanakawa, 2002; Guillot et al., 2009; Zabicki et al., 2017), as well as inducing neural plasticity in these areas (Pascual-Leone et al., 1995; Ietswaart et al., 2011; Ruffino et al., 2017).
In the field of sports psychology, several cross-sectional questionnaire studies have consistently reported that athletes have superior motor imagery ability compared with non-athletes (Isaac and Marks, 1994; Jansen and Lehmann, 2013; Di Corrado et al., 2014). Furthermore, previous studies have indicated that athletes can perform motor imagery more vividly than non-athletes, not only for specialized movements in their own sports but also for sports-non-specific movements such as raising the arm and jumping (Isaac and Marks, 1994; Di Nota et al., 2017). The findings of these studies suggest that the neural activity underlying motor imagery ability may differ between athletes and non-athletes, not only for sports-specific imagery but also for motor imagery of sports-non-specific movements.
In the field of applied physiology, there have been many studies comparing neural activity such as sensorimotor rhythms measured using electroencephalogram (EEG) and magnetoencephalography (MEG) during motor imagery between athletes and non-athletes (Fourkas et al., 2008; Babiloni et al., 2009, 2010; Percio et al., 2010; Wolf et al., 2014; Di Nota et al., 2017; Kraeutner et al., 2018). Some studies have reported that athletes could modulate sensorimotor rhythms more greatly during sports-specific motor imagery compared with non-athletes (Wolf et al., 2014; Di Nota et al., 2017; Kraeutner et al., 2018). In addition, people with the frequent practice of manual activity (i.e., crafts, musical instruments, cooking, sports) are known to be good at modulating their sensorimotor rhythms during KMI (Rimbert et al., 2019). Although these findings lead us to expect that ability of athletes to modulate sensorimotor rhythms is also superior during motor imagery of sports-non-specific movements, to the best of our knowledge, no studies have supported such expectation. For instance, when tennis players imagined movements specifically related to tennis, their corticospinal excitability became higher than that of non-athletes, whereas such a difference between athletes and non-athletes was not observed when they imagined other movements, including non-tennis-specific movements (Fourkas et al., 2008). Thus, there is currently a gap in findings between psychological and physiological studies regarding differences in motor imagery ability, particularly for sports-non-specific movements between athletes and non-athletes.
To clarify this issue, the present study investigated differences in motor imagery ability of sports-non-specific movements between gymnasts and healthy adults (i.e., non-gymnasts) from both psychological and physiological points of view. As a psychological indicator, we evaluated the subjective vividness of motor imagery using The Kinesthetic and Visual Imagery Questionnaire (KVIQ-20) (Malouin et al., 2007). As a physiological indicator, we evaluated sensorimotor rhythms using EEG. Event-related desynchronization (ERD) is a measure of decreases in the power of the EEG sensorimotor rhythms within the α- and β-bands from the resting-state to the motor execution or KMI state (Pfurtscheller et al., 1997; Pfurtscheller and Lopes da Silva, 1999), which is known to reflect increased neuronal excitability in the corticospinal system (Takemi et al., 2013a). We chose gymnasts as a population of athletes for the following reasons: (1) Gymnasts perform motor imagery including KMI frequently as a part of their daily practices because of the high risk of serious injury in their performance; (2) gymnasts were assumed to have higher motor imagery abilities than athletes engaged in other sports because it has been reported that motor imagery is more vivid in athletes engaged in individual and/or non-contact sports compared with athletes engaged in the team and/or contact sports (Di Corrado et al., 2019); (3) to the best of our knowledge, no previous studies have measured neural activity during motor imagery in gymnasts.
Materials and Methods
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki. All experimental protocols and procedures were approved by the Research Ethics Committee in Shonan Fujisawa Campus, Keio University (Approval Number 167). The examiners provided a detailed explanation of the purpose, experimental procedures, potential benefits, and risks involved. After receiving all of the relevant information, participants provided written informed consent before participating in the experiment.
Participants
We recruited 16 gymnasts (11 men, 5 women, aged 18–24 years) and 16 healthy adults (8 men, 8 women, aged 19–22 years) as a non-gymnast group. All participants were right-handed. All gymnasts had been practicing at least for 7 years (range: 7–16 years) and had participated in an all-Japan intercollegiate gymnastic championship at least once. Note that two gymnasts were members of the Japanese national gymnastics team. The non-gymnasts group had no experience of gymnastic training. None of the participants had experienced any neurological and musculoskeletal disorders.
Psychological Assessments
Procedures
Motor imagery ability was tested using a psychological questionnaire translated into Japanese: KVIQ-20 (Malouin et al., 2007; Nakano et al., 2018). To keep a methodological consistency with our previous study showing a correlation between KVIQ and ERD magnitude (Toriyama et al., 2018), we chose to use KVIQ as a questionnaire from a number of questionnaires to measure motor imagery ability. Briefly, the KVIQ-20 tests how vividly a person is able to imagine their own movements subjectively, using two types of motor imagery: KMI and VMI. Participants sat comfortably in a chair next to the examiner and watched the example of the examiner once. Then, they actually performed the exercise, followed by VMI or KMI of the exercise they had just performed. Participants were then asked to evaluate the vividness of the motor imagery on a 5-point ordinal scale (the more vivid the motor imagery, the higher the scale score). This procedure was repeated for 10 different simple exercises: neck flexion/extension, shoulder elevation, forward shoulder flexion, elbow flexion/extension, thumb-fingers opposition, forward trunk flexion, knee extension, hip abduction, foot tapping, and foot external rotation.
Analyses
We evaluated the vividness of motor imagery by summing all KVIQ scores for KMI and VMI, respectively. If a participant could imagine their movements perfectly, the score was 50 points.
Physiological Assessments
Recordings
Scalp EEG signals were recorded with eight passive Ag/AgCl electrodes around the sensorimotor area related to the right upper limbs (Cz, C1, C3, C5, FC1, FC3, CP1, and CP3) in accordance with the extended international 10–20 system. Electrodes with a diameter of 18 mm were mounted on an electrode cap (g.GAMMAcap 1027; Guger Technologies, Graz, Austria). Reference and ground electrodes were placed on the right and left earlobes, respectively. Surface electromyogram (EMG) signals were recorded from the right deltoid muscle (DEL) and the right extensor carpi radials muscle (ECR). Two passive Ag/AgCl electrodes with a diameter of 10 mm were placed over each muscle belly with inter-electrode distances of 20 mm. All EEG and EMG signals were amplified and bandpass-filtered (EEG, 0.5–1,000 Hz; EMG, 2–1,000 Hz) using a linked biosignal recording system (g.BSamp 0201a; Guger Technologies, Graz, Austria). All analog EEG and EMG signals were converted to digital signals at a sample rate of 1,000 Hz using an AD converter with 16-bit resolution (NI USB-6259, National Instruments, Austin, TX, United States) that was controlled by data-logger software originally designed using MATLAB software (The MathWorks, Inc., Antic, MA, United States).
Procedures
Following the psychological assessment, we performed physiological EEG and EMG measurements. The participants sat comfortably in the seat. A computer monitor for visual feedback was placed 2 m in front of eyes of the participants. First, the resting-state EEG was recorded over 60 s. Participants relaxed and fixated their eyes on a cross (+) displayed at the center of the monitor during recording. Participants then performed several practice trials of maximal voluntary contractions (MVCs) of wrist dorsiflexion and shoulder abduction. After these movements were practiced, participants performed MVC once each for wrist dorsiflexion and shoulder abduction. When performing each MVC, EMG activity of the contracting muscle was recorded. The EMG signals were full-wave-rectified. We found a 0.5-s period of stable force exertion during MVC and calculated the integrated EMG value (iEMGmax) in this period. In the following experiment, 20% of this iEMGmax value was used as a target value for visual feedback.
The physiological data recordings during motor execution and KMI were performed after several practice trials. Visual feedback was presented on the screen, with a red cursor to represent muscle contraction level as a relative value in %iEMGmax and a vertical blue line to represent a target value. Furthermore, instructions for each phase, including “Rest,” “Relax,” “Ready,” “Contraction,” or “Imagery,” were displayed on the monitor. In the wrist dorsiflexion task, participants' dominant hand was positioned on the armrest and fixed by a belt with the palm down. In the shoulder abduction task, the dominant upper limb was lowered to the side of the body while bending the elbow lightly, and the arm was fixed by a belt. In both the wrist dorsiflexion and shoulder abduction tasks, participants performed repeated motor execution and KMI according to the procedure used in our previous study (Toriyama et al., 2018).
The experimental paradigm is shown in Figure 1. In detail, each trial was started from the rest phase, and the word “Rest” was displayed on the monitor for 7 s. During the resting phase, participants were able to adjust their posture freely and/or blink their eyes strongly. After the rest phase, the word “Relax” was displayed on the monitor for 3 s. During the relaxing phase, participants were instructed to relax as much as possible, without performing any movement. The word “Ready” was then displayed for 3 s, accompanied by a short sound presented every second. During the ready phase, participants prepared for the next instruction. After the ready phase, the word “Contraction” was displayed for 5 s. During the contraction phase, participants performed isometric voluntary contraction (wrist dorsiflexion or shoulder abduction) at 20% of iEMGmax by their dominant hand. In the contraction phase, the participants were instructed to contract their muscles so that the cursor could follow the target line as accurately as possible. After the contraction phase, the word “Relax” was displayed for 2 s. In this relaxing phase, participants were instructed to relax as much as possible, without any movement. After the rest, relax, and ready phases, the imagery phase was started, and participants performed KMI of the preceding contraction for 5 s with their eyes open and without any movement. During the imagery phase, we checked that no EMG activity occurred. When the imagery phase finished, the relax phase was presented again for 2 s. This flow was conducted for each trial, including motor execution and KMI, and five trials were repeated within each set. Six sets were performed for each task. Thus, a total of 30 trials were performed for both wrist flexion and shoulder abduction tasks. We set the wrist dorsiflexion and shoulder abduction tasks in a randomized order across participants. The duration of the set interval was longer than 2 min, to provide sufficient rest for participants.
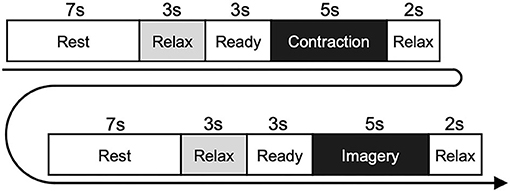
Figure 1. Experimental paradigm of the physiological experiment. Participants performed isometric contraction in the contraction phase and performed motor imagery of the same movement in the imagery phase. The diagram shows the flow in each trial, which was repeated five times within each set. Six sets were performed for each of the wrist dorsiflexion and shoulder abduction tasks.
Analyses
To remove noise arising from the electric power, the EEG and EMG signals were notch-filtered at 50 Hz. The EEG signals over C1 and C3 were derived with a four-neighbor Laplacian spatial filter. For example, in the case of C3, the EEG signal over C3 was subtracted by an average of C1, C5, FC3, and CP3. The Laplacian derivation method is known to strongly emphasize cortical activity originating below the electrode of interest (McFarland et al., 1997). If Laplacian-derived EEG included potentials exceeded 50 μV, we considered the trial to contain an artifact and excluded the data from future analyses. Additionally, visual inspection was performed to reject additional artifacts missed by the automatic inspection. As a result, we removed up to five trials from each task for each participant because of large noise due to strong blinking or body movements (1.75 ± 1.82 trials, on average).
ERDs during motor execution and KMI of each task were calculated as follows. After separating the data into motor execution and KMI periods, we extracted the 30 1-s data windows in the same period from the data for each trial. Then, fast Fourier transformation was performed using Welch's method for the data (window length, 1 s; window function, Hanning window; overlap, 0), and the power spectrum densities (PSDs) of the EEG signal were calculated. This process was repeated by sliding the 1 s data window in 50 ms steps. The ERDs were calculated using the following equation:
where A is the EEG PSDs at time t, frequency f, and R is the mean PSDs of the baseline period (last 1 s in the relax phase). This equation indicates that the positively greater the ERD value, the larger the decrease in EEG PSD during motor execution or KMI compared with the relax phase. Because the most reactive frequency band of ERD was slightly different across participants (Pfurtscheller and Neuper, 2006), we determined an electrode and the 3-Hz frequency width showing the largest ERD in each of the α-band (8–15 Hz) and β-band (16–35 Hz) during motor execution. Because it has been suggested that the functional roles played by ERD differ between the α- and β-bands (Brinkman et al., 2014; Stolk et al., 2019), we analyzed ERDs from these two frequency bands separately. The magnitude of ERD in the α-band (αERDmax) and β-band (βERDmax) was measured by calculating the peak value of ERD for motor execution and KMI of each task, respectively (Takemi et al., 2013b; Toriyama et al., 2018).
To compare the features of EEG during the relax phase in the task with continuous resting state for a prolonged period, we also analyzed the α-band or β-band PSDs for both data sets. For the relax phase EEG, the final 1-s periods in the relax phase, which were used as the baseline periods for ERD analyses, were extracted from all trials and combined to create a 60 s relax phase EEG signal. For the resting-state EEG, a continuous 60-s period with few artifacts was extracted. We then calculated the ratio of the sum of EEG power within the α-band or β-band PSD to that of the entire frequency range (4–50 Hz) (named EEGα-PSD and EEGβ-PSD) for both data sets, and compared these values between relax-phase EEG during tasks and resting-state EEG.
Statistical Analyses
Two-sided unpaired t-tests were performed on VMI and KMI scores of KVIQ between groups (non-gymnasts vs. gymnasts), to confirm differences in the subjective vividness of motor imagery between them. To test the differences in αERDmax and βERDmax during motor execution or KMI, we performed a two-way mixed-model ANOVA between participant groups (gymnasts and non-gymnasts) and tasks (wrist dorsiflexion and shoulder abduction). If the interaction was significant, we performed a two-sided unpaired t-test for groups (gymnasts vs. non-gymnasts) and a two-sided paired t-test for tasks (wrist dorsiflexion vs. shoulder abduction). To test the differences in the EEGα-PSD or EEGβ-PSD between resting-state EEG and relax-phase EEG during tasks, we also performed two-way ANOVA between participant groups (gymnasts and non-gymnasts) and conditions (resting sate and relax phase). The p-values of 0.05 were used to indicate statistical significance. All statistical analyses were performed using SPSS statistics software (IBM SPSS Statistics 25, IBM developerWorks, Tokyo, Japan).
Results
Kinesthetic and Visual Imagery Questionnaire
Figure 2 shows group data (mean ± S.D.) for KVIQ scores obtained from VMI and KMI tasks between gymnasts and non-gymnasts. The KVIQ scores were significantly greater in gymnasts, both in VMI (gymnasts, 42.68 ± 6.22; non-gymnasts, 35.44 ± 8.73, p = 0.011) (Figure 2A) and in KMI (gymnasts, 43.50 ± 6.78; non-gymnasts, 35.94 ± 8.24, p = 0.008) (Figure 2B). The KVIQ results indicate that gymnasts subjectively evaluated how vividly they could imagine their own movements.
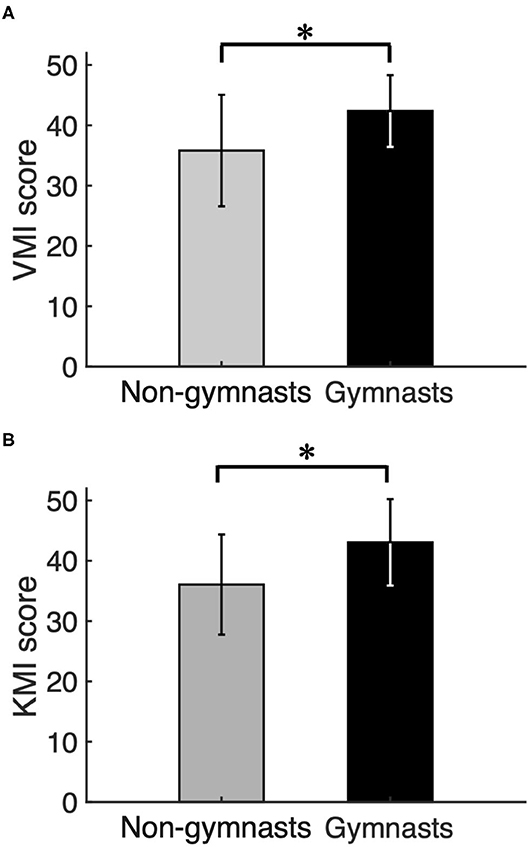
Figure 2. Results of psychological experiment. Group data (mean ± S.D.) for visual motor imagery (VMI) (A) and kinesthetic motor imagery (KMI) (B) scores obtained from the Kinesthetic and Visual Imagery Questionnaire (KVIQ) are shown for both groups. The gray bars represent the data for non-gymnasts, while the black bars represents the data for gymnasts. *P < 0.05.
ERD Magnitude
Typical examples of EEG signals, EEG time-frequency maps, and ERD time courses during wrist dorsiflexion from a non-gymnast and a gymnast are shown in Figures 3A,B, respectively. From these time-frequency maps, a decrease in EEG power can be observed around 12 and 22 Hz in the contraction phase (0 to 5 s) compared with the relax phase (−6 to −3 s) in both participants when performing motor execution.
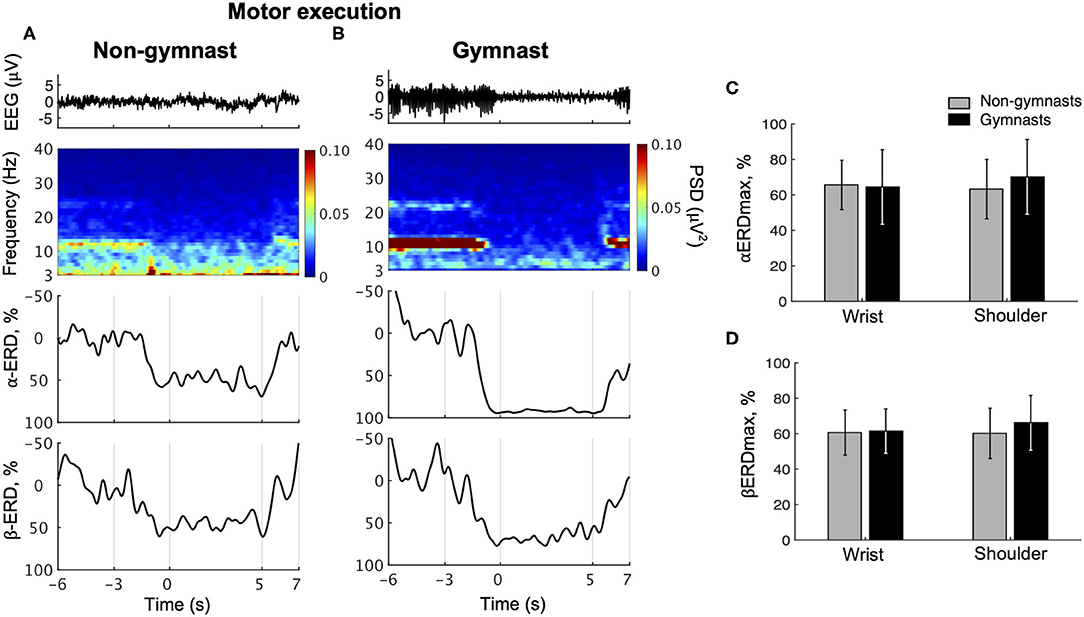
Figure 3. Results from physiological experiments for motor execution. Typical time courses of single-trial EEG, time-frequency map, α-band event-related desynchronization (ERD), and β-band in wrist dorsiflexion motor execution are shown for non-gymnast (A) and gymnast (B) participants. Note that participants performed wrist dorsiflexion motor execution from 0 to 5 s. Group data (mean ± S.D.) for the maximal peak of ERD both in the α- (αERDmax) (C) and in the β-bands (βERDmax) (D) during motor execution are shown across groups and tasks. The gray bars represent the data for non-gymnasts, while the black bars represent the data for gymnasts. No significant differences were observed across groups and tasks.
Figure 3C shows group data for αERDmax during wrist dorsiflexion and shoulder abduction motor execution. An ANOVA on αERDmax during motor execution revealed no significant effects of group [F(1, 30) = 0.209, p = 0.651] and task [F(1, 30) = 0.831, p = 0.369], while a significant interaction was obtained [F(1, 30) = 4.654, p = 0.0391]. An unpaired t-test for group revealed no significant difference in αERDmax between gymnasts and non-gymnasts for wrist dorsiflexion (gymnasts, 64.41 ± 20.96; non-gymnasts, 65.58 ± 13.97, p = 0.855) and shoulder abduction task (gymnasts, 70.13 ± 21.07; non-gymnasts, 63.26 ± 16.77, p = 0.316). A paired t-test for task revealed no significant difference in αERDmax between wrist dorsiflexion and shoulder abduction execution both for non-gymnasts (wrist dorsiflexion, 65.58 ± 13.97; shoulder abduction, 63.26 ± 16.77, p = 0.303) and for gymnasts (wrist dorsiflexion, 64.41 ± 20.96; shoulder abduction, 70.13 ± 21.07, p = 0.078). Figure 3D shows the group data for βERDmax during wrist dorsiflexion and shoulder abduction motor execution. An ANOVA on the βERDmax during motor execution with groups and task revealed no significant effects of group [F(1, 30) = 0.571, p = 0.456] and task [F(1, 30) = 1.249, p = 0.273], and interaction [F(1, 30) = 1.815, p = 0.188]. The results revealed no effects of sports experience and body part on ERD magnitude during motor execution.
Typical examples of EEG signals, EEG time-frequency maps, and ERD time courses during wrist dorsiflexion KMI from a non-gymnast and a gymnast are shown in Figures 4A,B, respectively. From the time-frequency map for a non-gymnast participant, we did not observe clear ERD in the imagery phase (0–5 s) compared with the relax phase (−6 to −3 s) in both the α-band and β-band (Figure 4A). Conversely, clear ERD can be observed in the time-frequency map for the gymnast participant around 12 and 22 Hz (Figure 4B).
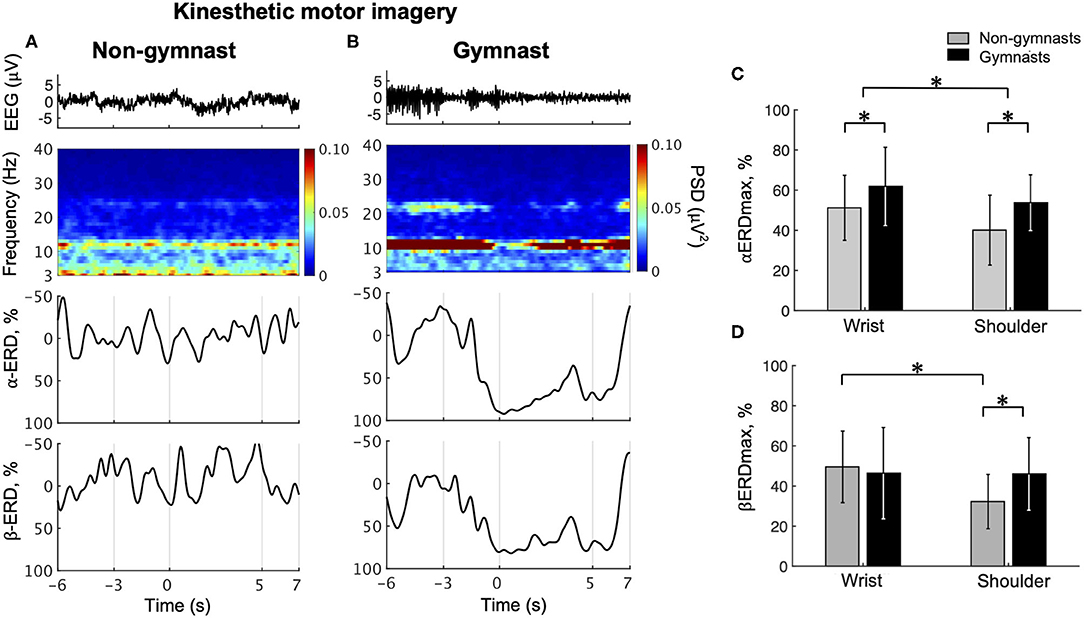
Figure 4. Results from physiological experiments for KMI. Typical time courses of single-trial EEG, time-frequency map, α-band ERD, and β-band in wrist dorsiflexion KMI are shown for non-gymnast (A) and gymnast (B) participants. Note that participants performed wrist dorsiflexion KMI from 0 to 5 s. Group data (mean ± S.D.) for αERDmax (C) and βERDmax (D) during KMI are shown across groups and tasks. The gray bars represent the data for non-gymnasts, while the black bars represent the data for gymnasts. *P < 0.05.
Figure 4C shows the group data for the αERDmax during wrist dorsiflexion and shoulder abduction KMI. An ANOVA on αERDmax during KMI showed significant effects of group [F(1, 30) = 5.437, p = 0.027] and task [F(1, 30) = 10.975, p = 0.002]. No significant interaction effect [F(1, 30) = 0.266, p = 0.610] was observed. Figure 4D shows the group data for βERDmax during wrist dorsiflexion and shoulder abduction KMI. An ANOVA on βERDmax during KMI showed no significant effects of group [F(1, 30) = 0.876, p = 0.357], but significant effects of task [F(1, 30) = 8.019, p = 0.008] and a significant interaction [F(1, 30) = 7.421, p = 0.010]. An unpaired t-test for group revealed a significant difference in βERDmax between gymnasts and non-gymnasts for the shoulder abduction task (gymnasts, 46.08 ± 18.09; non-gymnasts, 32.27 ± 17.84, p = 0.021) but not for the wrist dorsiflexion task (gymnasts, 46.42 ± 22.76; non-gymnasts, 49.56 ± 17.84, p = 0.666). A paired t-test for task revealed a significant difference in βERDmax between the wrist dorsiflexion and shoulder abduction KMI conditions for non-gymnasts (wrist dorsiflexion, 49.56 ± 17.84; shoulder abduction, 32.27 ± 17.84, p = 0.002), but not for gymnasts (wrist dorsiflexion, 46.42 ± 22.76; shoulder abduction, 46.08 ± 18.09, p = 0.938). These results indicated that gymnastics experience affected ERD magnitude during KMI of sports-non-specific movements.
Comparison of EEGα-PSD and EEGβ-PSD Between Resting-State EEG and Relax-Phase EEG During Tasks
Figure 5A shows the group data for the EEGα-PSD in resting-state EEG and relax-phase EEG during the tasks. An ANOVA examining EEGα-PSD data revealed no significant effects of group [F(1, 30) = 0.486, p = 0.491] or condition [F(1, 30) = 4.135, p = 0.051]; however, a significant interaction [F(1, 30) = 6.382, p = 0.017] was observed. An unpaired t-test for group revealed no significant difference in EEGα-PSD during resting-state EEG (gymnasts, 0.473 ± 0.155; non-gymnasts, 0.482 ± 0.166, p = 0.878) and that during the relax phase (gymnasts, 0.482 ± 0.155; non-gymnasts, 0.403 ± 0.125, p = 0.878; p = 0.122) between gymnasts and non-gymnasts. In non-gymnasts, a paired t-test revealed significant differences in the EEGα-PSD between conditions (resting state, 0.482 ± 0.166; relax phase, 0.403 ± 0.125, p = 0.008). However, in gymnasts, no significant differences in EEGα-PSD were observed between conditions (resting state, 0.473 ± 0.155; relax phase, 0.481 ± 0.155, p = 0.715). Figure 5B shows the group data for EEGβ-PSD. An ANOVA on EEGβ-PSD showed no significant effects of group [F(1, 30) = 0.106, p = 0.747] and condition [F(1, 30) = 1.787, p = 0.191], and no significant interaction [F(1, 30) = 0.063, p = 0.804]. These results indicate that in non-gymnasts, the EEGα-PSD was smaller in the relax-phase EEG than during resting-state EEG, while such a difference was not observed in gymnasts. Conversely, EEGβ-PSD did not differ between resting-state EEG and relax-phase EEG in both gymnasts and non-gymnasts.
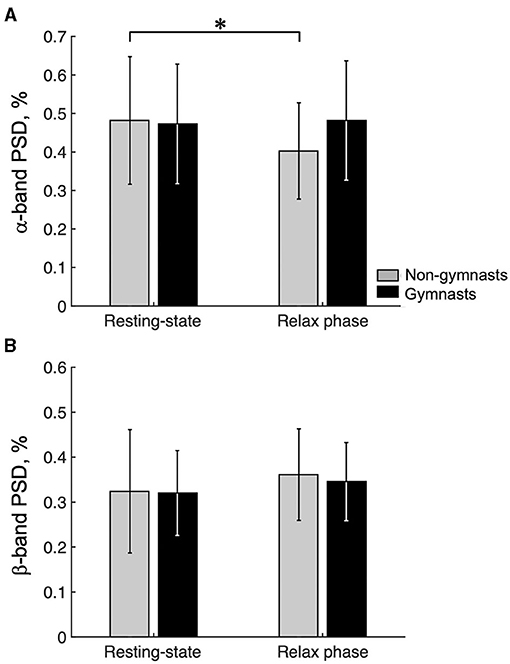
Figure 5. Results of the ratio of the α-band and the β-band power spectrum densities. Group data (mean ± S.D.) for the ratio of the α-band (A) and β-band power spectrum densities (PSD) (B) are shown for both groups. The gray bars represent the data for non-gymnasts, while the black bars represent the data for gymnasts. *P < 0.05.
Discussion
The purpose of this study was to clarify differences in EEG sensorimotor rhythms during KMI of sports-non-specific movements between gymnasts and non-gymnasts. The results revealed that when required to repeatedly switch between relaxing and motor execution or KMI of sports-non-specific movements, the ERD magnitude during KMI was significantly greater in gymnasts, who subjectively evaluated their imagery including KMI as more vivid, while no difference between groups was observed during motor execution. In particular, the ERD magnitude in the α-band was greater in gymnasts compared with non-gymnasts, both in wrist dorsiflexion and in shoulder abduction KMIs, whereas the ERD magnitude in the β-band was greater in gymnasts only in shoulder abduction KMI.
The Difference in KMI Ability of Sports-non-specific Movements Between Gymnasts and Non-gymnasts
We evaluated ERD as a physiological indicator of KMI ability in the present study because it is considered to reflect changes in corticospinal excitability (Takemi et al., 2013a) and is associated with the subjective vividness of KMI measured by KVIQ (Toriyama et al., 2018). It should be noted that the present results revealed greater ERD magnitude during KMI of sports-non-specific movements in gymnasts than in non-gymnasts, although differences in neural activity between athletes and non-athletes have not been reported in motor imagery of sports-non-specific movements in previous studies using EEG (Di Nota et al., 2017), MEG (Kraeutner et al., 2018), TMS (Fourkas et al., 2008), or fMRI (Wei and Luo, 2010). This may be related to the fact that gymnasts perform motor imagery including KMI frequently as a part of their daily practice to reduce the risk of serious injury in their practice. Furthermore, a previous psychological study showed that the vividness of motor imagery of sports-non-specific movements was higher in athletes engaged in individual and/or non-contact sports compared with athletes engaged in the team and/or contact sports (Di Corrado et al., 2019). Thus, as gymnasts have superior motor imagery ability among athletes, they may provide a particularly suitable population for highlighting differences in neural activity during KMI of sports-non-specific movements compared with non-athletes.
It is possible that the present task protocol, in which participants performed KMI following motor execution repeatedly in the order of seconds, led to the current finding of greater ERD magnitude in gymnasts. In psychological questionnaires, the conventional procedure for measuring motor imagery ability is to examine participants while they perform motor execution, then motor imagery in one trial, and subjectively evaluate the vividness of the motor imagery of the preceding movement (Malouin et al., 2007). In physiological experiments, however, the conventional procedure involves evaluating neural activity while participants perform only motor imagery (Fourkas et al., 2008; Wei and Luo, 2010; Di Nota et al., 2017; Kraeutner et al., 2018). Thus, there has been a methodological gap in the approach for examining motor imagery between psychological questionnaire studies and experimental physiological studies. In response to direct questioning in the current study, gymnasts reported that they usually perform actual movements and KMIs alternately in their daily practice. Therefore, the method for measuring KMI in the present physiological experiment was designed in accord with the procedure of psychological questionnaire measurement. We provided no special prior training or instruction to the participants to remove its effect on motor imagery (Wriessnegger et al., 2018; Meng and He, 2019; Corsi et al., 2020). Participants were asked to imagine the sensation of the motor execution they had performed just before, similar to the way in the KVIQ. The current physiological findings may have been due to differences between gymnasts and non-gymnasts in the ability to flexibly modulate corticospinal excitability when imagining their own movements, by referring to the actual movement.
As shown in equation (1), we were able to confirm that the ERD was determined by both the degree of synchronization during the relax phase [R(f)] and the degree of desynchronization during KMI [A(f, t)]. As shown in Figure 5, first, gymnasts could return their sensorimotor α-rhythm during the relax phase in the task to the same power level as during the resting state for 60 s, whereas non-gymnasts could not. Thus, gymnasts appeared to be good at relaxing deeply by making their sensorimotor rhythms more synchronized within a short period of time. However, higher EEGα-PSD during the relax phase does not appear to be the only factor involved in gymnasts' greater ERD magnitude in the α-band. As shown in Figure 2, second, differences in αERDmax between groups were not observed in motor execution but were found in KMI. Thus, gymnasts also appeared to be good at increasing corticospinal excitability by making their sensorimotor rhythms more desynchronized, even in KMI. Overall, the present results demonstrate that gymnasts have the ability to generate a clear contrast in the state of the sensorimotor cortex, when required to repeatedly switch across relaxing, motor execution, and KMI conditions. On the basis of the current findings, we believe that the ability to modulate the brain state without any movement is a core aspect of superior KMI ability in gymnasts.
The Difference in the Functional Role of ERD Between α- and β-Bands
Interestingly, this study demonstrated different results between ERD magnitude in the α-band and β-band. Several previous studies reported that functional roles played by the sensorimotor rhythms are different between frequency bands. During actual muscle contraction with weak-to-moderate intensity, the sensorimotor rhythm is known to be coherent with EMG activity only in the β-band, with no significant coherence in the α-band (Ushiyama et al., 2010, 2017; Suzuki and Ushiyama, 2020). In addition, sensorimotor β-oscillations have been proposed to be the basis for large-scale communication across sensorimotor, other areas, and the periphery (Kilavik et al., 2013). When focusing on oscillatory power itself, the EEG spectral power in the sensorimotor area contralateral to the contracted/imagined limb was decreased in both the α- and β-bands, while that in task-irrelevant cortical regions was increased in the α-band (Pfurtscheller, 1992; Pfurtscheller and Lopes da Silva, 1999), but not in the β-band (Stolk et al., 2019). During KMI, the ERD magnitude was increased by increasing task demand in the β-band, but not in the α-band (van Elk et al., 2010; Brinkman et al., 2014). Taken together, these findings suggest that the functional roles of sensorimotor rhythms for movement/imagery should be distinguished between the α-band and β-band.
First, αERDmax was larger in gymnasts than in non-gymnasts during both KMI tasks (i.e., wrist dorsiflexion and shoulder abduction). In task-relevant cortical regions, neural populations are assumed to be disinhibited by the ERD of the sensorimotor area in the α-band, which would allow reallocation of computational resources (Brinkman et al., 2014). However, task-irrelevant cortical regions are assumed to be inhibited by enhancing their α-oscillations (Pfurtscheller et al., 1997). As gymnasts are required to perform skilled movements successively, they are trained to quickly switch their attention across their body parts by facilitating task-relevant regions and inhibiting task-irrelevant regions. The present results regarding ERD in the α-band would reflect such an ability of gymnasts.
Second, βERDmax was larger in gymnasts only during shoulder abduction KMI, but not during wrist dorsiflexion KMI. This task specificity in βERDmax may be caused by ERD in the β-band playing a role in the calculation of specific motor commands. In general, wrist movement is used frequently in daily life, which makes it easy for most people to perform wrist dorsiflexion KMI. Thus, βERDmax would not differ between gymnasts and non-gymnasts in the wrist dorsiflexion KMI task. However, as isometric shoulder abduction is a movement rarely used in daily life, it may be difficult for most people to perform this KMI. Conversely, gymnasts are well-trained to move their upper limbs, including the shoulder joints, both dynamically (i.e., giant swing) and statically (i.e., handstand and rings). Therefore, it would be easy for gymnasts to imagine shoulder abduction because they are skilled at adjusting the movement parameters of their shoulder joints. We assume that ERD in the β-band is an indicator for how precisely a person can imagine their own movement kinesthetically.
Limitations
In the present study, only upper limb movements (i.e., wrist dorsiflexion and shoulder abduction) were examined. Gymnasts use their upper limb muscles specifically as anti-gravity muscles for postural control, such as handstand and pommel horse. This usage of the upper limbs is unique relative to the movements of non-gymnasts. The uniqueness of gymnasts' upper limb usage may lead to superior KMI ability regarding upper limb movements. Thus, we cannot clearly predict whether similar results would be obtained when performing similar experiments for other body parts. However, the KVIQ results demonstrated that gymnasts tended to show higher scores for all movements. In addition, most gymnasts perform motor imagery of various body parts in their daily practice. On the basis of these findings, we speculate that gymnasts have superior KMI ability irrespective of body parts, although confirming this possibility will require further investigation.
The present study is the first to observe differences in physiological indices between athletes and non-athletes during KMI of sports-non-specific movements. This means that the present study can bridge the gap between psychology and physiology studies regarding differences in KMI ability of sports-non-specific movements between athletes and non-athletes. However, because only gymnasts participated in this study, it is unclear whether the present results are specific to gymnasts or apply generally to athletes performing any sports. Because differences in motor imagery ability of sports-non-specific movements would be expected among athletes, further investigation is needed to elucidate sports-specific differences in motor imagery ability for sports-non-specific movements.
In the field of brain–computer interfaces (BCIs), conflicting results have been reported for the relationship between BCI performance and psychological questionnaire score. In particular, some studies have demonstrated a significant correlation between them (Vuckovic and Osuagwu, 2013; Marchesotti et al., 2016), while the other has not (Rimbert et al., 2019). The present study supports the former one because gymnasts reported higher subjective vividness of KMI with a greater magnitude of ERD compared to non-gymnasts. This result might be due to the consistency of imagery method between physiological and psychological measurements of this study (i.e., imagining the sensation of the movement performed just before). Thus, it is still unclear whether gymnasts always excel in the ability to modulate sensorimotor rhythms in other imagery methods such as repeating only motor imageries and/or imagery with online neurofeedback. In any case, the present study indicated the importance of comparing corticospinal excitability measured by ERD for evaluating KMI ability. In future studies, imagery training using bioelectrical signals may provide a useful tool for improving the motor imagery ability of athletes.
Conclusion
The present study demonstrated that, during KMI of sports-non-specific movements, the corticospinal excitability measured by ERD magnitude was significantly greater in gymnasts compared with non-gymnasts. These results are consistent with the higher subjective vividness of KMI in gymnasts measured using the KVIQ psychological questionnaire. The observed signature of flexibly modulating sensorimotor rhythms with no movement would be the basis of their superior KMI ability of sports-non-specific movements in gymnasts.
Data Availability Statement
The datasets presented in this article are not readily available because the authors restrict applications to the dataset. Requests to access the datasets should be directed to Junichi Ushiyama, dXNoaXlhbWFAc2ZjLmtlaW8uYWMuanA=.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee in Shonan Fujisawa Campus, Keio University. The participants provided their written informed consent to participate in this study.
Author Contributions
HS and JU conceptualized and designed the study, interpreted data, wrote the manuscript, and acquired funding. HS acquired and analyzed data. JU supervised the study. Both authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Grant-in-Aid for Scientific Research (B) (Japan Society for the Promotion of Science, JSPS) (Grant Number: 20H04091) to JU, a designated donation from Living Platform, Ltd, Japan to JU, and Taikichiro Mori Memorial Research Grants to HS.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Ms. Tomomi Hamaoka, Ms. Kana Iijima, and Ms. Chieko Matsuda for their secretarial assistance, and Mr. Hisato Toriyama, Mr. Ryoichiro Yamazaki, Mr. Takuya Ideriha, Ms. Rina Suzuki, and all other members of our laboratory for their useful comments on the work. We thank Mr. Hisashi Mizutori for his practical comments on the work. We thank Benjamin Knight, M.Sc., from Edanz (https://jp.edanz.com/), for editing a draft of this manuscript.
References
Babiloni, C., Del Percio, C., Rossini, P. M., Marzano, N., Iacoboni, M., Infarinato, F., et al. (2009). Judgment of actions in experts: A high-resolution EEG study in elite athletes. Neuroimage 45, 512–521. doi: 10.1016/j.neuroimage.2008.11.035
Babiloni, C., Marzano, N., Iacoboni, M., Infarinato, F., Aschieri, P., Buffo, P., et al. (2010). Resting state cortical rhythms in athletes: a high-resolution EEG study. Brain Res. Bull. 81, 149–156. doi: 10.1016/j.brainresbull.2009.10.014
Battaglia, C., D'Artibale, E., Fiorilli, G., Piazza, M., Tsopani, D., Giombini, A., et al. (2014). Use of video observation and motor imagery on jumping performance in national rhythmic gymnastics athletes. Hum. Mov. Sci. 38, 225–234. doi: 10.1016/j.humov.2014.10.001
Brinkman, L., Stolk, A., Dijkerman, H. C., de Lange, F. P., and Toni, I. (2014). Distinct roles for alpha- and beta-band oscillations during mental simulation of goal-directed actions. J. Neurosci. 34, 14783–14792. doi: 10.1523/JNEUROSCI.2039-14.2014
Corsi, M. C., Chavez, M., Schwartz, D., George, N., Hugueville, L., Kahn, A. E., et al. (2020). Functional disconnection of associative cortical areas predicts performance during BCI training. Neuroimage 209:116500. doi: 10.1016/j.neuroimage.2019.116500
Cumming, J., and Hall, C. (2002). Deliberate imagery practice: the development of imagery skills in competitive athletes. J. Sports Sci. 20, 137–145. doi: 10.1080/026404102317200846
Decety, J. (1999). Neural mechanisms subserving the perception of human actions. Trends Cogn. Sci. 3, 172–178. doi: 10.1016/S1364-6613(99)01312-1
Di Corrado, D., Guarnera, M., and Quartiroli, A. (2014). Vividness and transformation of mental images in karate and ballet. Percept. Mot. Skills. 119, 764–773. doi: 10.2466/22.24.PMS.119c30z6
Di Corrado, D., Guarnera, M., Vitali, F., Quartiroli, A., and Coco, M. (2019). Imagery ability of elite level athletes from individual vs. Team and contact vs. No-contact sports. PeerJ. 7, 1–14. doi: 10.7717/peerj.6940
Di Nota, P. M., Chartrand, J. M., Levkov, G. R., Montefusco-Siegmund, R., and DeSouza, J. F. X. (2017). Experience-dependent modulation of alpha and beta during action observation and motor imagery. BMC Neurosci. 18:28. doi: 10.1186/s12868-017-0349-0
Fourkas, A. D., Bonavolont, V., Avenanti, A., and Aglioti, S. M. (2008). Kinesthetic imagery and tool-specific modulation of corticospinal representations in expert tennis players. Cereb. Cortex. 18, 2382–2390. doi: 10.1093/cercor/bhn005
Guillot, A., Collet, C., Nguyen, V. A., Malouin, F., Richards, C., and Doyon, J. (2009). Brain activity during visual versus kinesthetic imagery: an fMRI study. Hum. Brain Mapp. 30, 2157–2172. doi: 10.1002/hbm.20658
Hall, C., Pongrac, J., and Buckholz, E. (1985). The measurement of imagery ability. Hum. Mov. Sci. 4, 107–118. doi: 10.1016/0167-9457(85)90006-5
Hanakawa, T. (2002). Functional properties of brain areas associated with motor execution and imagery. J Neurophysiol. 89, 989–1002. doi: 10.1152/jn.00132.2002
Ietswaart, M., Johnston, M., Dijkerman, H. C., Joice, S., Scott, C. L., MacWalter, R. S., et al. (2011). Mental practice with motor imagery in stroke recovery: Randomized controlled trial of efficacy. Brain. 134, 1373–1386. doi: 10.1093/brain/awr077
Isaac, A. R., and Marks, D. F. (1994). Individual differences in mental imagery experience: Developmental changes and specialization. Br. J. Psychol. 85, 479–500. doi: 10.1111/j.2044-8295.1994.tb02536.x
Jansen, P., and Lehmann, J. (2013). Mental rotation performance in soccer players and gymnasts in an object-based mental rotation task. Adv. Cogn. Psychol. 9, 92–98. doi: 10.5709/acp-0135-8
Kilavik, B. E., Zaepffel, M., Brovelli, A., MacKay, W. A., and Riehle, A. (2013). The ups and downs of beta oscillations in sensorimotor cortex. Exp. Neurol. 245, 15–26. doi: 10.1016/j.expneurol.2012.09.014
Kraeutner, S. N., McWhinney, S. R., Solomon, J. P., Dithurbide, L., and Boe, S. G. (2018). Experience modulates motor imagery-based brain activity. Eur. J. Neurosci. 47, 1221–1229. doi: 10.1111/ejn.13900
Lebon, F., Horn, U., Domin, M., and Lotze, M. (2018). Motor imagery training: kinesthetic imagery strategy and inferior parietal fMRI activation. Hum. Brain Mapp. 39, 1805–1813. doi: 10.1002/hbm.23956
Malouin, F., Richards, C. L., Jackson, P. L., Lafleur, M. F., Durand, A., and Julien, D. (2007). The kinesthetic and visual imagery questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: a reliability and construct validity study. J. Neurol. Phys. Ther. 31, 20–29. doi: 10.1097/01.NPT.0000260567.24122.64
Marchesotti, S., Bassolino, M., Serino, A., Bleuler, H., and Blanke, O. (2016). Quantifying the role of motor imagery in brain-machine interfaces. Sci. Rep. 6, 1–12. doi: 10.1038/srep24076
McFarland, D. J., McCane, L. M., David, S. V., and Wolpaw, J. R. (1997). Spatial filter selection for EEG-based communication. Electroencephalogr. Clin. Neurophysiol. 103, 386–394. doi: 10.1016/S0013-4694(97)00022-2
Meng, J., and He, B. (2019). Exploring training effect in 42 human subjects using a non-invasive sensorimotor rhythm based online BCI. Front. Hum. Neurosci. 13, 1–19. doi: 10.3389/fnhum.2019.00128
Mizuguchi, N., Nakata, H., Uchida, Y., and Kanosue, K. (2012). Motor imagery and sport performance. J. Phys. Fit Sport Med. 1, 103–111. doi: 10.7600/jpfsm.1.103
Nakano, H., Kodama, T., Ukai, K., Kawahara, S., Horikawa, S., and Murata, S. (2018). Reliability and validity of the Japanese version of the kinesthetic and visual imagery questionnaire (KVIQ). Brain Sci. 8:79. doi: 10.3390/brainsci8050079
Pascual-Leone, A., Dang, N., Cohen, L. G., Brasil-Neto, J. P., Cammarota, A., and Hallett, M. (1995). Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J. Neurophysiol. 74, 1037–1045. doi: 10.1152/jn.1995.74.3.1037
Percio, C., Del, I.nfarinato F., Iacoboni, M., Marzano, N., Soricelli, A., Aschieri, P., et al. (2010). Movement-related desynchronization of alpha rhythms is lower in athletes than non-athletes: a high-resolution EEG study. Clin. Neurophysiol. 121, 482–491. doi: 10.1016/j.clinph.2009.12.004
Peynircioglu, Z. F., Thompson, J. L. W., Tanielian, T. B., and Tanielian, T. B. (2000). Improvement strategies in free-throw shooting and grip-strength tasks. J. Gen. Psychol. 127, 145–156. doi: 10.1080/00221300009598574
Pfurtscheller, G. (1992). Event-related synchronization (ERS): an electrophysiological correlate of cortical areas at rest. Electroencephalogr. Clin. Neurophysiol. 83, 62–69. doi: 10.1016/0013-4694(92)90133-3
Pfurtscheller, G., and Lopes da Silva, F. H. (1999). Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 110, 1842–1857. doi: 10.1016/S1388-2457(99)00141-8
Pfurtscheller, G., and Neuper, C. (2006). Future prospects of ERD/ERS in the context of brain–computer interface (BCI) developments. Prog. Brain Res. 159, 433–437. doi: 10.1016/S0079-6123(06)59028-4
Pfurtscheller, G., Neuper, C., Andrew, C., and Edlinger, G. (1997). Foot and hand area mu rhythms. Int. J. Psychophysiol. 26, 121–135. doi: 10.1016/S0167-8760(97)00760-5
Rimbert, S., Gayraud, N., Bougrain, L., Clerc, M., and Fleck, S. (2019). Can a subjective questionnaire be used as brain-computer interface performance predictor? Front. Hum. Neurosci. 12:529. doi: 10.3389/fnhum.2018.00529
Ruffino, C., Papaxanthis, C., and Lebon, F. (2017). Neural plasticity during motor learning with motor imagery practice: review and perspectives. Neuroscience 341, 61–78. doi: 10.1016/j.neuroscience.2016.11.023
Sobierajewicz, J., Przekoracka-Krawczyk, A., Jaśkowski, W., Verwey, W. B., and van der Lubbe, R. (2017). The influence of motor imagery on the learning of a fine hand motor skill. Exp. Brain Res. 235, 305–320. doi: 10.1007/s00221-016-4794-2
Stolk, A., Brinkman, L., Vansteensel, M. J., Aarnoutse, E., Leijten, F. S., Dijkerman, C. H., et al. (2019). Electrocorticographic dissociation of alpha and beta rhythmic activity in the human sensorimotor system. Elife 8, 1–24. doi: 10.7554/eLife.48065
Suzuki, R., and Ushiyama, J. (2020). Context-dependent modulation of corticomuscular coherence in a series of motor initiation and maintenance of voluntary contractions. Cereb. Cortex Commun. 1, 1–14. doi: 10.1093/texcom/tgaa074
Takemi, M., Masakado, Y., Liu, M., and Ushiba, J. (2013a). Event-related desynchronization reflects downregulation of intracortical inhibition in human primary motor cortex. J. Neurophysiol. 110, 1158–1166. doi: 10.1152/jn.01092.2012
Takemi, M., Masakado, Y., Liu, M., and Ushiba, J. (2013b). Is event-related desynchronization a biomarker representing corticospinal excitability? Proc. Annu Int Conf IEEE Eng Med Biol Soc EMBS 2013, 281–4. doi: 10.1109/EMBC.2013.6609492
Toriyama, H., Ushiba, J., and Ushiyama, J. (2018). Subjective vividness of kinesthetic motor imagery is associated with the similarity in magnitude of sensorimotor event-related desynchronization between motor execution and motor imagery. Front. Hum. Neurosci. 12:295. doi: 10.3389/fnhum.2018.00295
Ushiyama, J., Takahashi, Y., and Ushiba, J. (2010). Muscle dependency of corticomuscular coherence in upper and lower limb muscles and training-related alterations in ballet dancers and weightlifters. J. Appl. Physiol. 109, 1086–1095. doi: 10.1152/japplphysiol.00869.2009
Ushiyama, J., Yamada, J., Liu, M., and Ushiba, J. (2017). Individual difference in β-band corticomuscular coherence and its relation to force steadiness during isometric voluntary ankle dorsiflexion in healthy humans. Clin. Neurophysiol. 128, 303–311. doi: 10.1016/j.clinph.2016.11.025
van Elk, M., van Schie, H. T., van den Heuvel, R., and Bekkering, H. (2010). Semantics in the motor system: motor-cortical beta oscillations reflect semantic knowledge of end-postures for object use. Front. Hum. Neurosci. 4:8. doi: 10.3389/neuro.09.008.2010
Vuckovic, A., and Osuagwu, B. A. (2013). Using a motor imagery questionnaire to estimate the performance of a Brain-Computer Interface based on object oriented motor imagery. Clin. Neurophysiol. 124, 1586–1595. doi: 10.1016/j.clinph.2013.02.016
Wei, G., and Luo, J. (2010). Sport expert's motor imagery: functional imaging of professional motor skills and simple motor skills. Brain Res. 1341, 52–62. doi: 10.1016/j.brainres.2009.08.014
Wolf, S., Brölz, E., Scholz, D., Ramos-Murguialday, A., Keune, P. M., Hautzinger, M., et al. (2014). Winning the game: brain processes in expert, young elite and amateur table tennis players. Front. Behav. Neurosci. 8:370. doi: 10.3389/fnbeh.2014.00370
Wriessnegger, S. C., Brunner, C., and Müller-Putz, G. R. (2018). Frequency specific cortical dynamics during motor imagery are influenced by prior physical activity. Front Psychol. 9:1976. doi: 10.3389/fpsyg.2018.01976
Keywords: athletes, kinesthetic motor imagery (KMI), electroencephalogram (EEG), event-related desynchronization (ERD), the Kinesthetic and Visual Imagery Questionnaire (KVIQ)
Citation: Sugino H and Ushiyama J (2021) Gymnasts' Ability to Modulate Sensorimotor Rhythms During Kinesthetic Motor Imagery of Sports Non-specific Movements Superior to Non-gymnasts. Front. Sports Act. Living 3:757308. doi: 10.3389/fspor.2021.757308
Received: 12 August 2021; Accepted: 30 September 2021;
Published: 04 November 2021.
Edited by:
Hamdi Chtourou, University of Sfax, TunisiaReviewed by:
Sébastien Rimbert, Inria Nancy - Grand-Est Research Centre, FranceToshiyuki Kondo, Tokyo University of Agriculture and Technology, Japan
Copyright © 2021 Sugino and Ushiyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junichi Ushiyama, dXNoaXlhbWFAc2ZjLmtlaW8uYWMuanA=
 Hirotaka Sugino
Hirotaka Sugino Junichi Ushiyama
Junichi Ushiyama