- 1Department of Conservation of Natural Resources, NEIKER – Basque Institute for Agricultural Research and Development, Basque Research and Technology Alliance (BRTA), Derio, Spain
- 2Department of Biochemistry and Molecular Biology, University of the Basque Country (UPV/EHU), Bilbao, Spain
Approximately 20 years ago (June 11th, 2004), a highly reputable scientific journal, on a front cover much celebrated by soil scientists, printed an eye-catching phrase: “Soils: The Final Frontier”. In the introduction to that special issue, it was mentioned that “in many ways the ground beneath our feet is as alien as a distant planet”, to then state that, owing to the booming interest in soil research at that time, together with the development of advanced techniques (e.g., next-generation sequencing), subterraneana would be made “seem far less of an alien experience”. In this perspective article, using as illustrations, by way of example only, two topics of great interest nowadays – soil carbon sequestration and soil biodiversity –, it is claimed that, despite all the efforts devoted in the last 20 years to the understanding of the soil ecosystem, the “final frontier” appears to have receded, as we are discovering increasing levels of complexity that are slowing down our trip to the far reaches of the edaphic universe. Due to, among other features, its high structural and functional complexity, biodiversity, heterogeneity, opacity, and processual and dynamic nature, the soil still remains a great unknown (“a black box”). The ultimate goal of this perspective article is to draw attention to the need for further long-term investment in research into this highly complex and fascinating ecosystem.
1 Introduction
Approximately 20 years ago (June 11th, 2004), the highly regarded journal Science, on a front cover much praised by those of us dedicated to soil research, printed an attention-grabbing motto: “Soils: The Final Frontier”. Paraphrasing the quote of the Star Trek Saga, the selection of that title for a special issue on soil research was intended to convey the notion that soil is one of the most complex ecosystems on Earth and, at that time, the latest challenge for ecology. Such statement was based on the overwhelming complexity of the soil ecosystem (1). Actually, soils are extremely complex environments from a physical, chemical, and biological point of view (2), although such categorical categorization of soil characteristics may no longer make sense, since the more we know about soils the more difficult it becomes to distinctly differentiate their abiotic and biotic fractions.
At that time, in order to emphasize the fact that we hardly understood its inner workings, it was common to describe the soil as a “black box” (3). In some way, we were all in agreement with Leonardo da Vinci´s viewpoint, reflected in his quote: “We know more about the movement of celestial bodies than about the soil underfoot”. But, in tandem, we, soil scientists, were all much excited about the flourishing, rather unexpected, growing interest in soil research and, specifically, in the development of novel techniques (e.g., high-throughput sequencing technologies, omics technologies, developments in computational power, bioinformatics tools) (4, 5), which together brought the promise and hope of a new era in which new data, information, and knowledge would soon illuminate every corner of subterraneana´s black box.
Twenty years later, without a shred of doubt, it can be affirmed that, since then, soil science has advanced substantially, at a faster pace than ever, in particular, the soil microbial ecology field (6) which had been for decades hampered by its dependence on microbial cultivation. Providentially, the advent of nucleic acid sequencing technologies opened the door to the study of those microbial species that cannot be cultivated, i.e., the great majority. Importantly, in the last two decades, an influx of highly-talented young researchers have entered the soil microbial ecology field, in part stimulated by the new molecular biology tools, innovative omics technologies, and other cutting-edge techniques (7).
But, paradoxically, the more we learn about the soil ecosystem, the darker and larger the black box feels, as we are discovering increasing levels of complexity that keep us apart from “the final frontier”. Frustratingly, the more we study it, the more data we collect, the more technology we have, etc., the less we seem to understand the soil ecosystem. Our degree of perceived ignorance has, most likely, increased. We are beginning to fathom that the “soil universe” is vaster, more mysterious, and much more intricate than we imagined 20 years ago. So to speak, although we have indisputably travelled an enormous distance in our space voyage to the far reaches of the edaphic universe, the final frontier appears to be receding.
In this perspective article, using as illustrations, by way of example only, two topics of great relevance nowadays – soil carbon sequestration and soil biodiversity –, a few cases in point are briefly presented to support our claim that the “final frontier recedes”. Thus, our methodological approach is based on the review of two topics of maximum interest, to which much effort has been devoted over the last two decades, as proof that, despite all the resources and work put into understanding them, there are still many substantial gaps in their understanding.
The selection of these two topics is not accidental. On the contrary, in the last two decades, three crucial facts have become blatantly clear: (i) we are degrading our planet to such an extent that we are crossing several of the planetary boundaries that define the “safe operating space for humanity” (8, 9); in this respect, climate change and biosphere integrity (the latter focused on genetic and functional biodiversity) have been identified as the two core planetary boundaries because either one, on its own, could change the course of the Earth’s trajectory (10); (ii) soil degradation is interrelated with the nine Earth-system processes included in the planetary boundaries framework; in fact, soil degradation has been proposed as the 10th Earth-system process for the planetary boundaries framework (11); and (iii) soil biodiversity is not only essential for soil functioning and the provision of ecosystem services, but it also provides benefits to both human and planetary health, and, relevantly, to climate change mitigation (12). These crucial facts provide ample justification for the choice of these two topics, which are briefly discussed in the following sections.
In relation to soil carbon sequestration, within the context of climate change mitigation and, particularly, as a result of carbon farming and carbon offset markets and initiatives (13), much attention is being paid to the capacity of soils to sequester carbon. These days, many soil scientists are being asked, by decision-takers, entrepreneurs, land managers, foresters, farmers and so on, the following questions: How much carbon can this soil sequester in the next “x” years? What practices should I apply to enhance soil carbon sequestration in my property or territory? But the sad truth is that, despite all the remarkable research being done in the last two decades in soil carbon dynamics and sequestration, there is still no straightforward answer to those questions, owing in great part to the complexity and dynamic character of soils.
2 Soil carbon sequestration
Soil is the largest terrestrial ecosystem carbon pool (ca. 2,500 Pg C) (14). Soil carbon sequestration can potentially remove between 0.79 and 1.54 Gt C yr-1 from the atmosphere, pointing out to the great potential of soils in stabilizing the climate (15). Importantly, due to its being a complex system, the soil carbon pool is influenced by many factors, such as, climate change, soil management, land use change, etc. (14). The soil microbiome contributes to the decomposition of organic matter through various metabolic pathways, thus playing a critical role in carbon cycling and the stabilization of organic carbon, thereby affecting soil carbon storage and turnover (16). It is possible to boost soil carbon sequestration by means of manipulating the microbiome to favor specific microbial taxa or traits, but strategies to do so must be carefully evaluated, since, due to its complexity and our lack of knowledge on soil functioning, there is the risk that the manipulation of the soil microbiome could have undesirable consequences (17). Some groups of microorganisms can play a key role in the restoration of degraded soils, such as, for instance, plant growth-promoting rhizobacteria, nitrogen-fixing bacteria, mycorrhizal fungi, and contaminant-degrading microbes (18). Then, it is not surprising that soil microbial properties have long been used as bioindicators of soil health and ecosystem restoration (19, 20).
As a rule, the stock of soil carbon can fluctuate, to a non-trivial extent, in reaction to a range of interactions between natural and anthropogenic drivers, thus increasing the level of uncertainty in carbon sequestration estimations. In the last years, and after the existence of large-molecular-size and persistent humic substances was questioned (21), the soil carbon sequestration debate has been dominated by different topics as complex as they are fascinating: the contribution of particulate organic matter vs. mineral associated organic matter (POM vs. MAOM) (22), the mineral sink vs. the microbial sink (16, 23), the influence of microbial composition and necromass (24), and the importance of element stoichiometry (25), among other aspects.
But the more we delve into the mechanisms of carbon sequestration, the more we realize that many unforeseen variables might have a key role in soil carbon dynamics. For instance, viruses, and in particular RNA viruses, may contribute to soil carbon dynamics to a much greater extent than previously anticipated (26). Compared to bacteria and fungi, soil viruses have been much less investigated, a somewhat surprising circumstance taking into consideration the well-known relevance of bacteriophages as major players in global biogeochemical cycling, as reflected by the fact that bacteriophages can lyse up to one-third of bacteria in ocean waters per day (27), thus releasing an enormous amount of carbon, with substantial consequences for global carbon cycling and sequestration. Another example comes from the finding that, besides microbial growth, microbial death (i.e., the different microbial death pathways) might be a key driver of the soil microbial carbon pump, as it can affect soil microbial necromass composition and its subsequent fate (24). Furthermore, the long-known progressively slower soil carbon dynamics observed in the subsoil vs. the topsoil appears to depend on bioenergetic constraints, affecting the variety of organic matter decomposers over a range of mineral reactivity contexts, resulting in an unfavorable “return-on-energy-investment” for those decomposers (traditionally, the persistence of deep soil organic carbon has been largely attributed to stabilization mechanisms) (28). We are discovering hitherto unexpected links between soil organic carbon contents and plant-microorganism interactions with crucial significance for nutrient dynamics. In this regard, soil organic matter content can attenuate the efficacy of flavonoid-based plant-microbe communication with concomitant consequences for nitrogen fixation by legumes (organic carbon was found to interrupt the signaling between Medicago sativa and Ensifer meliloti, leading to a 75% decrease in nodule formation) and, concurrently, potential effects on carbon storage (29).
On the other hand, in order to increase soil organic carbon content and fertility (nutrient content), organic amendments of animal (manure, slurry, compost) and urban (sewage sludge) origin have traditionally been applied to agricultural soils as fertilizers (30, 31). Also, organic amendments are applied to agricultural soils for phytopathogen control purposes (32). An unintended adverse effect of great concern is the introduction of antibiotic residues and antibiotic-resistant bacteria, harboring antibiotic resistance genes, to soils via the application of those amendments (33, 34). Antibiotics are widely used in livestock farming and, since a considerable percentage of the antibiotic administered to the animals is excreted directly, or as transformation products, in the feces and urine, the addition of animal manure or slurry to agricultural soils is leading to the emergence and dissemination of antibiotic resistance in the environment (the environmental resistome) (34). Similarly, when applying sewage sludge to agricultural fields, antibiotic residues are released and antibiotic-resistant bacteria are incorporated to the environmental resistome (35, 36). In the last years, this topic has become one of the main issues in organic fertilization forums, questioning the practice of applying organic amendments to increase soil carbon content.
3 Soil biodiversity
As far as soil biodiversity is concerned, in the last two decades, the application of next-generation sequencing techniques (37) for soil metabarcoding, metagenomic (shotgun metagenomics, targeted metagenomics), and metatranscriptomic analyses has revolutionized the in-depth study of soil structural and functional biodiversity. In particular, the study of soil prokaryotes and fungi (i.e., the two dominant taxonomic groups in the soil), in terms of both composition and function, has greatly benefited from the advent and development of sequencing technologies, together with molecular biology tools. Importantly, these novel techniques have opened the door to the investigation of non-culturable microorganisms.
Nonetheless, soil microbiome studies have still important limitations that remind us of the imperative need for extreme caution when interpreting data and drawing conclusions. In this respect, apart from well-known limitations and biases coming from sampling procedures (disruption of the soil matrix, sample representativeness), sample storage, nucleic acid extraction methods, PCR amplifications, sequencing protocols and errors, functional annotations, imperfect databases, bioinformatics tools, snapshot analyses, etc., it is important to emphasize that, even if these problems were solved, significant conceptual challenges would remain, e.g., the presence of a gene is not evidence of its activity; the reduction of the relative abundance of a functional gene does not imply a reduction in the associated process rate; absolute gene abundance values are not reliable indicators of activity or function because enzyme activity per gene might not be constant; and metagenomic data often do not provide information on ecologically important activities (38).
On the other hand, amplicon sequencing data, using 16S rRNA or ITS as phylogenetic markers for prokaryotes and fungi, respectively, frequently do not allow full taxonomic identification at the species level, not to mention at the strain level. Moreover, it is often overlooked that relic-extracellular DNA from dead microorganisms (39) is abundant in soil where it can persist for weeks to years. Actually, by studying a wide range of soils using viability PCR based on the photoreactive DNA-intercalating dye propidium monoazide, it was reported (40) that, on average, 40% of both prokaryotic and fungal DNA might be extracellular or from cells that are no longer intact, and this extracellular DNA inflated the prokaryotic and fungal richness by up to 55%, causing significant misestimations of taxon relative abundances. Another limitation present in soil microbiome investigations is the dearth of soil-specific reference databases and a lack of in-vitro mock communities derived from soil microbial strains that could be used for taxonomic classification faithfulness (41). Compared to microbes, the application of omics technologies for the study of the biodiversity of soil fauna is still in its infancy for many taxonomic groups.
Although molecular and omics technologies have certainly brought much light to the microbial ecology field, they have also engendered an excess of technique-driven, descriptive studies, without a proper question-driven and hypothesis-driven approach, possibly hampering the progress of microbial ecology theory (42). High-throughput techniques have allowed us to study in detail the structure and dynamics of soil microbial communities, microbial interspecies co-occurrence networks, metabolic and functional aspects of soil microbial communities, etc. but we must not forget that these techniques (e.g., omics analysis) cannot completely grasp all the elements of soil microbial ecosystems, and, above all, that we still need sound theoretical frameworks to be able to generalize microbial ecology observations and, particularly, to properly interpret big data from, for example, multi-omics approaches (43).
Interestingly, innovative molecular tools (e.g., meta-omics, gene editing, CRISPR-Cas), together with new technological advances (e.g., artificial intelligence, satellite technologies) are being developed to increase the success of microbiome-based strategies to boost soil health and restoration, by means of, for instance, microbial inoculants, microbiome in situ manipulations, plant-microbe products, etc. (44).
4 Discussion
As illustrated by these two topics (carbon sequestration and biodiversity), although much progress has undoubtedly been made in the last 20 years, soil researchers are constantly discovering increasing levels of complexity in the study of the soil ecosystem and its biota. The more we study the soil the more we realize that it is an amazingly complex and awe-inspiring ecosystem that shelters a vast biodiversity (soil is likely home to 59 ± 15% of the species on Earth) (45) responsible for a number of processes that underpin the integrity and sustainable functioning of soils.
The soil is not a static matrix, composed of sand, silt, and clay, but a processual and dynamic entity that displays an ontological interdependence between activity and existence. There is such a multitude of interconnections, interactions, and interdependencies among its abiotic and biotic components that it is often very difficult, if not impossible, to unambiguously differentiate them. As an example, the criticality of the soil´s microbiological component for soil structure and functioning is so large that it has been purported (46) that soil behaves as an extended composite phenotype of the resident microbiome, a proposition that supports the theory that soil-microbe systems are self-organizing states. To some extent, this is not a surprising proposal as scientists have long been aware of the biotic nature of many soil forming processes and debated the idea that soils are biotic constructs (47).
Inevitably, we must embrace the overwhelming complexity of the soil ecosystem and accept the associated uncertainty. As an example of this complexity, after decades of discussion, the definition and concept of soil health are still a matter of much debate and controversy (48, 49). Luckily, the lack of an agreed definition is not an insurmountable obstacle to developing a field of knowledge, in both its theoretical and practical aspects, as illustrated by the fact that, in the absence of an agreed definition of life, the field of biology has successfully studied for centuries the living organisms that inhabit our planet.
From all of the above, it can be concluded that soil is still a largely unknown ecosystem, in which the limitations of our analytical tools and the lack of solid theoretical foundations prevent us from understanding key aspects of the soil system, such as its biodiversity (a pillar of its sustainable functioning) or its capacity to sequester carbon. Therefore, and given the criticality of the soil resource, we must strongly support theoretical and practical soil research, especially that characterized by its multidisciplinary character, creativity, critical thinking, and holistic approach, far from reductionist visions and focused on the myriad of interconnections, interdependencies, and interrelationships among the biotic and abiotic components of the soil matrix.
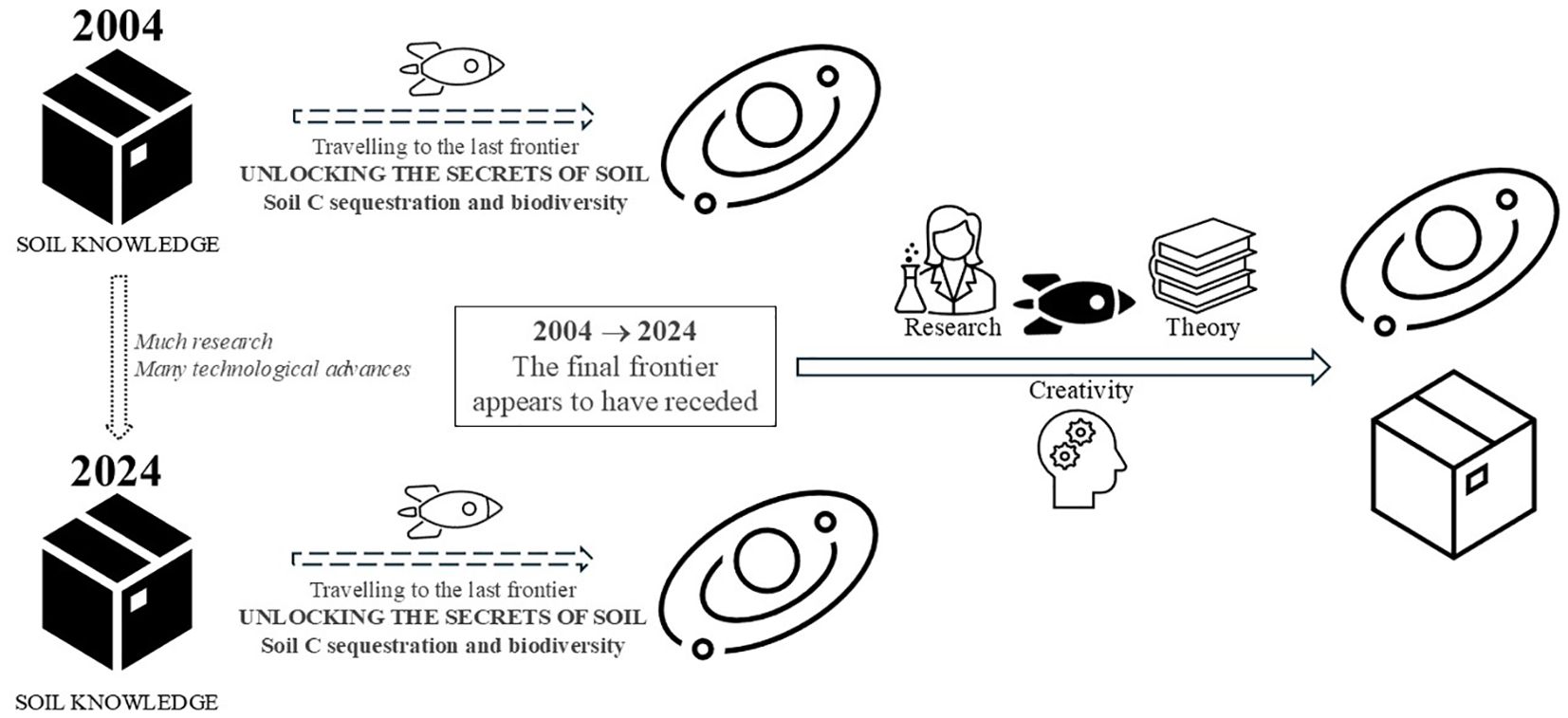
Despite all the efforts, resources, money, time, and hopes put in the last 20 years into reaching “the final frontier”, we, crew members of the Soil Research Enterprise, must accept the fact that the final frontier has receded during this time (Figure 1). But our mission is to explore the strangest of worlds, subterraneana, and to boldly go where no human has gone before, i.e., to the deepest corners of the belowground universe.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
CG: Conceptualization, Writing – original draft, Writing – review & editing. IA: Conceptualization, Writing – original draft, Writing – review & editing. OU: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vogel H-J, Betancur-Corredor B, Franke L, König S, Lang B, Lucas M, et al. The soil knowledge library (KLIB) – a structured literature database on soil process research. Soil. (2023) 9:533–43. doi: 10.1046/j.1462-2920.2003.00539.x
2. Turner BL. Soil as an archetype of complexity: a systems approach to improve insights, learning, and management of coupled biogeochemical processes and environmental externalities. Soil Syst. (2021) 5:39. doi: 10.3390/soilsystems5030039
3. Barot S, Blouin M, Fontaine S, Jouquet P, Lata J-C, Mathieu J. A tale of four stories: soil ecology, theory, evolution and the publication system. PLoS One. (2007) 2:e1248. doi: 10.1371/journal.pone.0001248
4. Lagos L, Maruyama F, Nannipieri P, Mora ML, Ogram A, Jorquera MA. Current overview on the study of bacteria in the rhizosphere by modern molecular techniques: a mini−review. J Soil Sci Plant Nutr. (2015) 15:504–23. doi: 10.4067/S0718-95162015005000042
5. Nannipieri P. Soil is still an unknown biological system. Appl Sci. (2020) 10:3717. doi: 10.3390/app10113717
6. Eisenhauer N, Franz Bender S, Calderón-Sanou I, de Vries FT, Lembrechts JL, Thuiller W, et al. Frontiers in soil ecology—Insights from the World Biodiversity Forum 2022. J Sustain Agric Environ. (2022) 1:245–61. doi: 10.1002/sae2.12031
7. Falkenberg R, Sigl L, Fochler M. From 'making lists' to conducting 'well-rounded' studies: Epistemic re-orientations in soil microbial ecology. Soc Stud Sci. (2024) 54:78–104. doi: 10.1177/03063127231179700
8. Rockström J, Steffen W, Noone K, Persson A, Chapin FS III, Lambin EF, et al. A safe operating space for humanity. Nature. (2009) 461:472–5. doi: 10.1038/461472a
9. Rockström J, Steffen W, Noone K, Persson A, Chapin FS III, Lambin E, et al. Planetary boundaries: exploring the safe operating space for humanity. Ecol Soc. (2009) 14:1–33. http://www.ecologyandsociety.org/vol14/iss2/art32/.
10. Steffen W, Richardson K, Rockström J, Cornell SE, Fetzer I, Bennett EM, et al. Planetary boundaries: guiding human development on a changing planet. Science. (2015) 347:1–10. doi: 10.1126/science.1259855
11. Kraamwinkel CT, Beaulieu A, Dias T, Howison RA. Planetary limits to soil degradation. Commun Earth Environ. (2021) 2:249. doi: 10.1038/s43247-021-00323-3
12. Nielsen UN, Wall DH, Six J. Soil biodiversity and the environment. Annu Rev Environ Resour. (2015) 40:4.1–4.28. doi: 10.1146/annurev-environ-102014-021257
13. Raina N, Zavalloni M, Davide Viaggi D. Incentive mechanisms of carbon farming contracts: A systematic mapping study. J Environ Manage. (2024) 352:120126. doi: 10.1016/j.jenvman.2024.120126
14. Xu S, Sheng C, Tian C. Changing soil carbon: influencing factors, sequestration strategy and research direction. Carbon Balance Manage. (2020) 15:2. doi: 10.1186/s13021-020-0137-5
15. Amelung W, Bossio D, de Vries W, Kögel-Knabner I, Lehmann J, Amundson R, et al. Towards a global-scale soil climate mitigation strategy. Nat Commun. (2020) 11:5427. doi: 10.1038/s41467-020-18887-7garbisu
16. Wu H, Cui H, Fu C, Li R, Qi F, Liu Z, et al. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci Total Environ. (2024) 909:168627. doi: 10.1016/j.scitotenv.2023.168627
17. Fierer N, Walsh CM. Can we manipulate the soil microbiome to promote carbon sequestration in croplands? PLoS Biol. (2023) 21:e3002207. doi: 10.1371/journal.pbio.3002207
18. Coban O, De Deyn GB, van der Ploeg M. Soil microbiota as game-changers in restoration of degraded lands. Science. (2022) 375:abe0725. doi: 10.1126/science.abe0725
19. Gómez-Sagasti MT, Hernández A, Artetxe U, Garbisu C, Becerril JM. How valuable are organic amendments as tools for the phytomanagement of degraded soils? The knowns, known unknowns, and unknowns. Front Sustain Food Syst. (2018) 2:68. doi: 10.3389/fsufs.2018.00068
20. Bhaduri D, Sihi D, Bhowmik A, Verma BC, Munda S, Dari B. A review on effective soil health bio-indicators for ecosystem restoration and sustainability. Front Microbiol. (2022) 13:938481. doi: 10.3389/fmicb.2022.938481
21. Lehmann J, Kleber M. The contentious nature of soil organic matter. Nature. (2015) 528:60–8. doi: 10.1038/nature16069
22. Yu W, Huang W, Weintraub-Leff SR, Hall SJ. Where and why do particulate organic matter (POM) and mineral-associated organic matter (MAOM) differ among diverse soils? Soil Biol Biochem. (2022) 172:108756. doi: 10.1016/j.soilbio.2022.108756
23. Wu S, Konhauser KO, Chen B, Huang L. Reactive Mineral Sink” drives soil organic matter dynamics and stabilization. NPJ Mater Sustain. (2023) 1:3. doi: 10.1038/s44296-023-00003-7
24. Camenzind T, Mason-Jones K, Mansour I, Rillig MC, Lehmann J. Formation of necromass-derived soil organic carbon determined by microbial death pathways. Nat Geosci. (2023) 16:115–22. doi: 10.1038/s41561-022-01100-3
25. Fan R, Du J, Liang A, Lou J, Li J. Carbon sequestration in aggregates from native and cultivated soils as affected by soil stoichiometry. Biol Fertil. Soils. (2020) 56:1109–20. doi: 10.1007/s00374-020-01489-2
26. Starr EP, Nuccio EE, Pett-Ridge J, Banfield JF, Firestone MK. Metatranscriptomic reconstruction reveals RNA viruses with the potential to shape carbon cycling in soil. Proc Natl Acad Sci. (2019) 116:25900–8. doi: 10.1073/pnas.190829111
27. Weinbauer MG, Rassoulzadegan F. Are viruses driving microbial diversification and diversity? Environ Microbiol. (2004) 6:1-11. doi: 10.1046/j.1462-2920.2003.00539.x
28. Henneron L, Balesdent J, Alvarez G, Barré P, Baudin F, Basile-Doelsch I, et al. Bioenergetic control of soil carbon dynamics across depth. Nat Commun. (2022) 13:7676. doi: 10.1038/s41467-022-34951-w
29. Del Valle I, Webster TM, Cheng H-Y, Thies JE, Kessler A, Miller MK, et al. Soil organic matter attenuates the efficacy of flavonoid-based plant-microbe communication. Sci Adv. (2020) 6:eaax8254. doi: 10.1126/sciadv.aax8254
30. Epelde L, Jauregi L, Urra J, Ibarretxe L, Romo J, Goikoetxea I, et al. Characterization of composted organic amendments for agricultural use. Front Sustain Food Syst. (2018) 2:44. doi: 10.3389/fsufs.2018.00044
31. Urra J, Alkorta I, Garbisu C. Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy. (2019) 9:542. doi: 10.3390/agronomy9090542
32. Núñez-Zofío M, Larregla S, Garbisu C. Application of organic amendments followed by soil plastic mulching reduces the incidence of Phytophthora capsici in pepper crops under temperate climate. Crop Protect. (2011) 30:1563–772. doi: 10.1016/j.cropro.2011.08.020
33. Urra J, Alkorta I, Lanzén A, Mijangos I, Garbisu C. The application of fresh and composted horse and chicken manure affects soil quality, microbial composition and antibiotic resistance. Appl Soil Ecol. (2019) 135:73–84. doi: 10.1016/j.apsoil.2018.11.005
34. Jauregi L, Epelde L, Alkorta I, Garbisu C. Antibiotic resistance in agricultural soil and crops associated to the application of cow manure-derived amendments from conventional and organic livestock farms. Front Vet Sci. (2021) 8:633858. doi: 10.3389/fvets.2021.633858
35. Urra J, Alkorta I, Mijangos I, Epelde L, Garbisu C. Application of sewage sludge to agricultural soil increases the abundance of antibiotic resistance genes without altering the composition of prokaryotic communities. Sci Total Environ. (2019) 647:1410–20. doi: 10.1016/j.scitotenv.2018.08.092
36. Jauregi L, Epelde L, Alkorta I, Garbisu C. Agricultural soils amended with thermally-dried anaerobically-digested sewage sludge showed increased risk of antibiotic resistance dissemination. Front Microbiol. (2021) 12:666854. doi: 10.3389/fmicb.2021.666854
37. Garg D, Patel N, Rawat A, Soares Rosado A. Cutting edge tools in the field of soil microbiology. Curr Res Microb Sci. (2024) 6:100226. doi: 10.1016/j.crmicr.2024.100226
38. Prosser JI. Dispersing misconceptions and identifying opportunities for the use of 'omics' in soil microbial ecology. Nat Rev Microbiol. (2015) 13:439–46. doi: 10.1038/nrmicro3468
39. Lennon JT, Muscarella ME, Placella SA, Lehmkuhl BK. How, when, and where relic DNA affects microbial diversity. mBio. (2018) 9:1128. doi: 10.1128/mbio.00637-00618
40. Carini P, Marsden PJ, Leff JW, Morgan EE, Michael S. Strickland MS, Fierer N. Relic DNA is abundant in soil and obscures estimates of soil microbial diversity. Nat Microbiol. (2017) 2:16242. doi: 10.1038/nmicrobiol.2016.242
41. Edwin NR, Fitzpatrick AH, Brennan F, Abram F, O’Sullivan O. An in-depth evaluation of metagenomic classifiers for soil microbiomes. Environ Microbiome. (2024) 19:19. doi: 10.1186/s40793-024-00561-w
42. Prosser JI. Putting science back into microbial ecology: a question of approach. Phil Trans R Soc B. (2020) 375:20190240. doi: 10.1098/rstb.2019.0240
43. Haruta S, Saito Y, Futamata H. Editorial: development of microbial ecological theory: stability, plasticity, and evolution of microbial ecosystems. Front Microbiol. (2016) 7:2069. doi: 10.3389/fmicb.2016.02069
44. Sáez-Sandino T, Delgado-Baquerizo M, Egidi E, Singh BK. New microbial tools to boost restorationand soil organic matter. Microb Biotechnol. (2023) 16:2019-25. doi: 10.1111/1751-7915.14325
45. Anthony MA, Bender SF, van der Heijden MGA. Enumerating soil biodiversity. Proc Natl Acad Sci USA. (2023) 120:e2304663120. doi: 10.5167/uzh-236224
46. Neal AL, Bacq-Labreuil A, Zhang X, Clark IM, Coleman K, Mooney SJ, et al. Soil as an extended composite phenotype of the microbial metagenome. Sci Rep. (2020) 10:10649. doi: 10.1038/s41598-020-67631-0
47. van Breemen N. Soils as biotic constructs favouring net primary productivity. Geoderma. (1993) 57:183–211. doi: 10.1016/0016-7061(93)90002-3
48. Lehmann J, Bossio DA, Kögel-Knabner I, Rillig MC. The concept and future prospects of soil health. Nat Rev Earth Environ. (2020) 1:544–53. doi: 10.1038/s43017-020-0080-8
Keywords: biodiversity, carbon sequestration, microbial ecology, soil ecosystem, soil research, soil science
Citation: Garbisu C, Alkorta I and Unamunzaga O (2024) Soils: the final frontier recedes. Front. Soil Sci. 4:1495941. doi: 10.3389/fsoil.2024.1495941
Received: 13 September 2024; Accepted: 29 October 2024;
Published: 18 November 2024.
Edited by:
Luiz Fernando Wurdig Roesch, University of Florida, United StatesReviewed by:
Knight Nthebere, National University of Lesotho, LesothoCopyright © 2024 Garbisu, Alkorta and Unamunzaga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olatz Unamunzaga, b3VuYW11bnphZ2FAbmVpa2VyLmV1cw==
 Carlos Garbisu
Carlos Garbisu Itziar Alkorta
Itziar Alkorta Olatz Unamunzaga
Olatz Unamunzaga